Quantitative Analysis of Signal Heterogeneity in the Hepatobiliary Phase of Pretreatment Gadoxetic Acid-Enhanced MRI as a Prognostic Imaging Biomarker in Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Transarterial Chemoembolization (TACE) Procedure
2.3. Gadoxetic Acid-Enhanced MRI and Image Analysis
2.4. Evaluation of Cutoff for Coefficient of Variation
2.5. Follow-Up and Evaluation
2.6. Statistical Analysis
3. Results
3.1. Patients
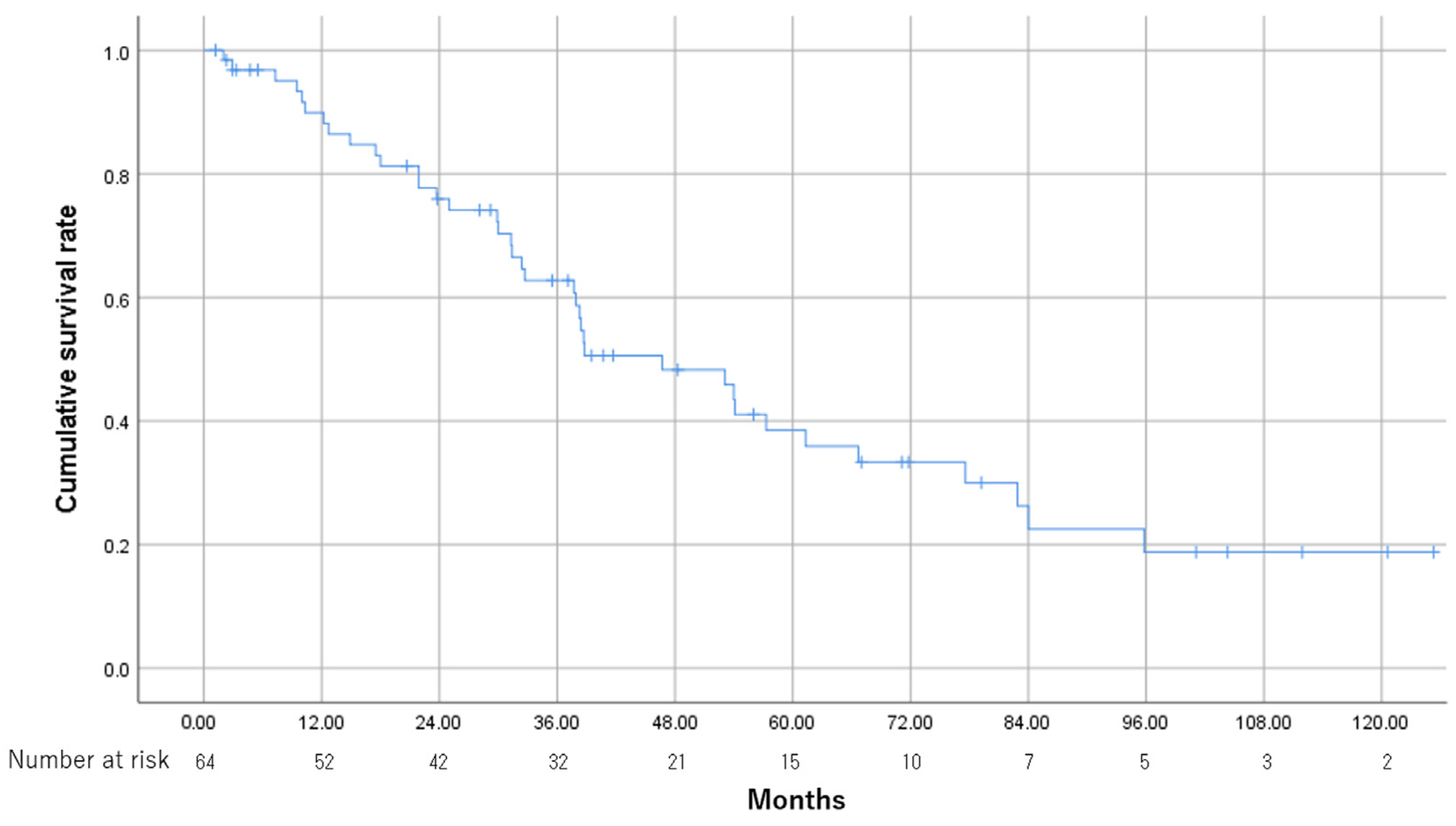
3.2. Treatment Outcome
3.3. CV Cutoff Value
3.4. Univariate and Multivariate Analyses of Prognostic Factors
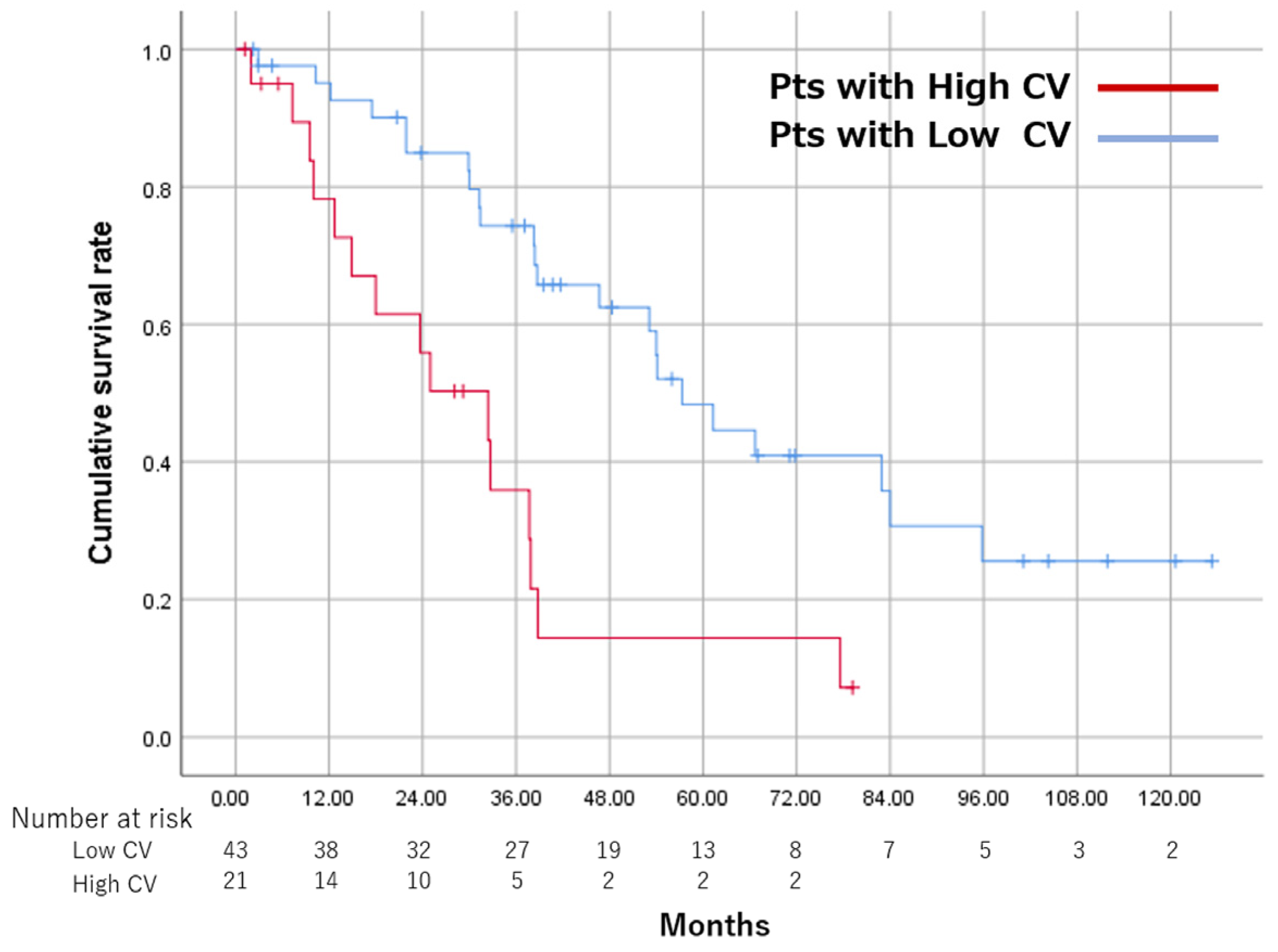
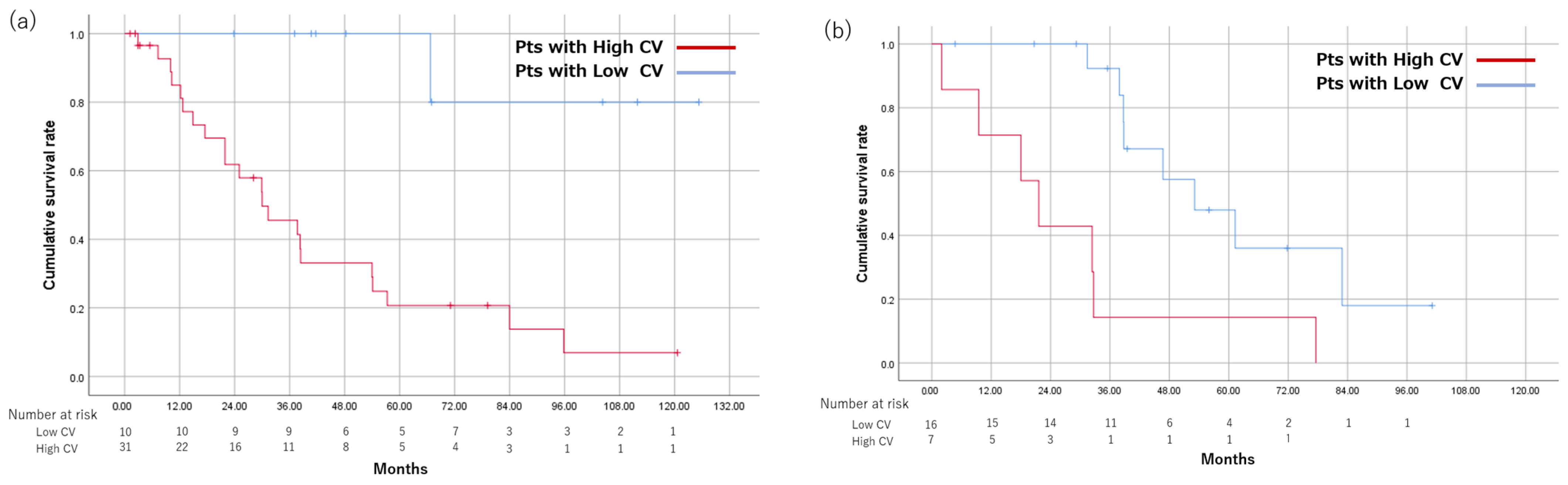
3.5. Overall Survival and CR Rate between the High CV and Low CV Groups
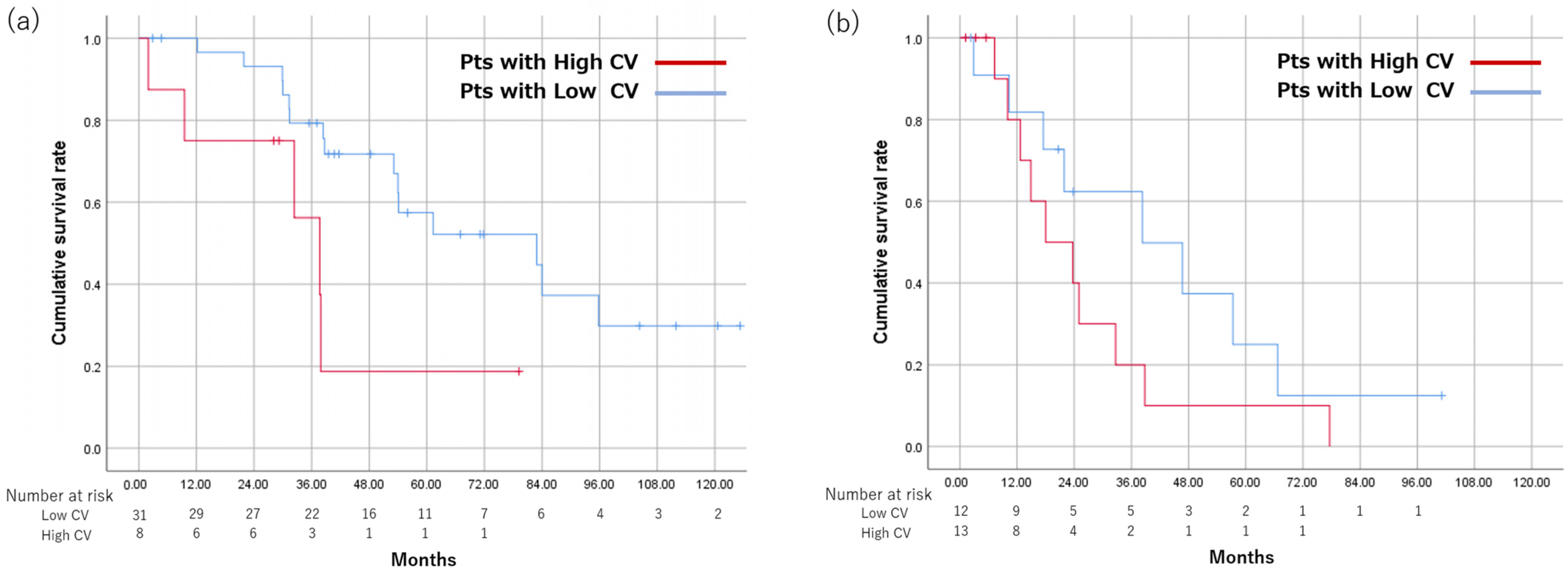
3.6. Subgroup Analysis by Up-To-7 Criteria
3.7. Overall Survival between the High CV and Low CV Groups, Respectively, Scanned at 1.5 T and 3.0 T
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021, 73 (Suppl. S1), 158–191. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. A paradigm change in the treatment strategy for hepatocellular carcinoma. Liver Cancer 2020, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Recent advances in systemic therapy for hepatocellular carcinoma in an aging society: 2020 Update. Liver Cancer 2020, 9, 640–662. [Google Scholar] [CrossRef]
- Kudo, M. A Novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: Upfront systemic therapy followed by curative conversion. Liver Cancer 2021, 10, 539–544. [Google Scholar] [CrossRef]
- Miyayama, S. Treatment strategy of transarterial chemoembolization for hepatocellular carcinoma. Appl. Sci. 2020, 10, 7337. [Google Scholar] [CrossRef]
- Charoenvisal, C.; Tanaka, T.; Nishiofuku, H.; Anai, H.; Sato, T.; Matsumoto, T.; Marugami, N.; Kichikawa, K. Feasibility and techniques of securing 3D-safety margin in superselective transarterial chemoembolization to improve local tumor control for small hepatocellular carcinoma: An intend-to-treat analysis. Liver Cancer 2021, 10, 63–71. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Sugimori, N.; Ikeda, R.; Okimura, K.; Sakuragawa, N. Outcomes of patients with hepatocellular carcinoma treated with conventional transarterial chemoembolization using guidance software. J. Vasc. Interv. Radiol. 2019, 30, 10–18. [Google Scholar] [CrossRef]
- Takaki, S.; Sakaguchi, H.; Anai, H.; Tanaka, T.; Yamamoto, K.; Morimoto, K.; Nishiofuku, H.; Inoue, M.; Sueyoshi, S.; Nagata, T.; et al. Long-term outcome of transcatheter subsegmental and segmental arterial chemoemobolization using lipiodol for hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2012, 35, 544–554. [Google Scholar] [CrossRef]
- Yamakado, K.; Miyayama, S.; Hirota, S.; Mizunuma, K.; Nakamura, K.; Inaba, Y.; Maeda, H.; Matsuo, K.; Nishida, N.; Aramaki, T.; et al. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn. J. Radiol. 2014, 32, 260–265. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Lee, H.C.; Sung, K.B.; Ko, H.K.; Ko, G.Y.; Gwon, D.I.; Kim, J.W.; Lim, Y.S.; Park, S.H. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017, 37, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Tanaka, T.; Nishiohuku, H.; Sato, T.; Masada, T.; Matsumoto, T.; Anai, H.; Sakaguchi, H.; Sueyoshi, S.; Marugami, N.; et al. Transarterial- chemoembolization remains an effective therapy for intermediate-stage hepatocellular carcinoma with preserved liver function. Hepatol. Res. 2020, 50, 1176–1185. [Google Scholar] [CrossRef]
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: Proposal of modified bolondi’s subclassification (Kinki criteria). Dig. Dis. 2015, 33, 751–758. [Google Scholar] [CrossRef]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.W.; Lee, I.C.; Chi, C.T.; Lee, R.C.; Liu, C.A.; Chiu, N.C.; Hwang, H.E.; Chao, Y.K.; Hou, M.C.; Huang, Y.H. Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: The seven-eleven criteria. Liver Cancer 2021, 10, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Boyault, S.; Rickman, D.S.; de Reyniès, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Hérault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Ueno, A.; Masugi, Y.; Yamazaki, K.; Komuta, M.; Effendi, K.; Tanami, Y.; Tsujikawa, H.; Tanimoto, A.; Okuda, S.; Itano, O.; et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J. Hepatol. 2014, 61, 1080–1087. [Google Scholar] [CrossRef]
- Audard, V.; Grimber, G.; Elie, C.; Radenen, B.; Audebourg, A.; Letourneur, F.; Vacher-Lavenu, M.C.; Perret, C.; Cavard, C.; Terris, B. Cholestasis is a marker for hepatocellular carcinomas displaying beta-catenin mutations. J. Pathol. 2007, 212, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Ogawa, R.; Ojima, H.; Kanai, Y. Expression of SLCO1B3 is associated with intratumoral cholestasis and CTNNB1 mutations in hepatocellular carcinoma. Cancer Sci. 2011, 102, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, C.H.; Park, Y.S.; Seo, T.S.; Song, M.G.; Kim, J.H.; Kim, K.A.; Park, C.M. The value of paradoxical uptake of hepatocellular carcinoma on the hepatobiliary phase of gadoxetic acid-enhanced liver magnetic resonance imaging for the prediction of lipiodol uptake after transcatheter arterial chemoembolization. Eur. J. Radiol. 2017, 89, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, N.; Matsui, O.; Kitao, A.; Kita, R.; Kozaka, K.; Koda, W.; Kobayashi, S.; Gabata, T.; Ikeda, H.; Nakanuma, Y. Hypervascular hepatocellular carcinomas showing hyperintensity on hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging: A possible subtype with mature hepatocyte nature. Jpn. J. Radiol. 2013, 31, 480–490. [Google Scholar] [CrossRef]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Kobayashi, S.; Sanada, J.; Koda, W.; Minami, T.; Inoue, D.; Yoshida, K.; et al. Hepatocellular carcinoma with β-catenin mutation: Imaging and pathologic characteristics. Radiology 2015, 275, 708–717. [Google Scholar] [CrossRef]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Kobayashi, S.; Koda, W.; Gabata, T.; Yamashita, T.; Kaneko, S.; Nakanuma, Y.; et al. Hypervascular hepatocellular carcinoma: Correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 2012, 265, 780–789. [Google Scholar] [CrossRef]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Kobayashi, S.; Koda, W.; Minami, T.; Inoue, D.; Yoshida, K.; Yamashita, T.; et al. Gadoxetic acid-enhanced magnetic resonance imaging reflects co-activation of β-catenin and hepatocyte nuclear factor 4α in hepatocellular carcinoma. Hepatol. Res. 2018, 48, 205–216. [Google Scholar] [CrossRef]
- Fujita, N.; Nishie, A.; Kubo, Y.; Asayama, Y.; Ushijima, Y.; Takayama, Y.; Moirta, K.; Shirabe, K.; Aishima, S.; Honda, H. Hepatocellular carcinoma: Clinical significance of signal heterogeneity in the hepatobiliary phase of gadoxetic acid-enhanced MR imaging. Eur. Radiol. 2015, 25, 211–220. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, B.C.; Kim, H.O.; Heo, S.H.; Shin, S.S.; Jeong, Y.Y. Liver MRI and clinical findings to predict response after drug eluting bead transarterial chemoembolization in hepatocellular carcinoma. Sci. Rep. 2021, 11, 24076. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Lewis, R.J. (Ed.) An introduction to classification and regression tree (CART) analysis. In Proceedings of the Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, 22–25 May 2000; pp. 1–14. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees, 1st ed.; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tovoli, F.; Renzulli, M.; Negrini, G.; Brocchi, S.; Ferrarini, A.; Andreone, A.; Benevento, F.; Golfieri, R.; Morselli-Labate, A.M.; Mastroroberto, M.; et al. Inter-operator variability and source of errors in tumour response assessment for hepatocellular carcinoma treated with sorafenib. Eur. Radiol. 2018, 28, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, S.I.; Ban, D.; Abe, Y.; Kumamoto, T.; Koizumi, S.; Tanabe, M.; Shinoda, M.; Endo, I.; Otsubo, T.; Yamamoto, M. High-signal-intensity MR image in the hepatobiliary phase predicts long-term survival in patients with hepatocellular carcinoma. Anticancer Res. 2019, 39, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Kim, S.Y.; Kim, J.H.; Kim, M.J.; Yoo, C.; Shim, J.H.; Lee, S.S. Prediction of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma using imaging features of gadoxetic acid-enhanced magnetic resonance imaging. Acta Radiol. 2021, 62, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Hatano, E.; Arizono, S.; Miyagawa-Hayashino, A.; Isoda, H.; Kitamura, K.; Taura, K.; Yasuchika, K.; Nitta, T.; Ikai, I.; et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J. Gastroenterol. 2009, 44, 793–798. [Google Scholar] [CrossRef]
- Kitao, A.; Zen, Y.; Matsui, O.; Gabata, T.; Kobayashi, S.; Koda, W.; Kozaka, K.; Yoneda, N.; Yamashita, T.; Kaneko, S.; et al. Hepatocellular carcinoma: Signal intensity at gadoxetic acid-enhanced MR imaging--correlation with molecular transporters and histopathologic features. Radiology 2010, 256, 817–826. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.N.; Anjum, K.M.; Gui, L.X.; Zhu, S.S.; Liu, J.; Chen, J.K.; Liu, Q.F.; Ye, G.D.; Wang, W.J.; et al. A double-negative feedback loop between Wnt-β-catenin signaling and HNF4α regulates epithelial-mesenchymal transition in hepatocellular carcinoma. J. Cell Sci. 2013, 126 Pt 24, 5692–5703. [Google Scholar] [CrossRef]
- van Zijl, F.; Zulehner, G.; Petz, M.; Schneller, D.; Kornauth, C.; Hau, M.; Machat, G.; Grubinger, M.; Huber, H.; Mikulits, W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009, 5, 1169–1179. [Google Scholar] [CrossRef]
- Xu, X.; Gao, D.; Yuan, X.; Liu, L.I.; Zhang, X.; Liang, X.; Chen, S.; Ai, M.; Chen, B.O.; Shi, D.; et al. β-catenin expression correlates with prognosis in hepatocellular carcinoma patients treated with transcatheter arterial chemoembolization. Anticancer Res. 2019, 39, 1129–1134. [Google Scholar] [CrossRef]
- Chen, E.; Mar, W.A.; Horowitz, J.M.; Allen, A.; Jha, P.; Cantrell, D.R.; Cai, K. Texture analysis of placental MRI: Can it aid in the prenatal diagnosis of placenta accreta spectrum? Abdom. Radiol. 2019, 44, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Iyama, Y.; Nakaura, T.; Kidoh, M.; Katahira, K.; Namimoto, T.; Morishita, S.; Yamashita, Y. Fat suppressed contrast-enhanced T1-weighted dynamic magnetic resonance imaging at 3T: Comparison of image quality between spectrally adiabatic iversion recovery and the multiecho dixon technique in imaging of the prostate. J. Comput. Assist. Tomogr. 2017, 41, 382–387. [Google Scholar] [CrossRef]
- Bowen, S.R.; Yuh, W.T.C.; Hippe, D.S.; Wu, W.; Partridge, S.C.; Elias, S.; Jia, G.; Huang, Z.; Sandison, G.A.; Nelson, D.; et al. Tumor radiomic heterogeneity: Multiparametric functional imaging to characterize variability and predict response following cervical cancer radiation therapy. J. Magn. Reson. Imaging 2018, 47, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, S.; Hippe, D.S.; Liu, H.; Wang, Y.; Mayr, N.A.; Yuh, W.T.C.; Xia, L.; Bowen, S.R. Whole-lesion DCE-MRI intensity histogram analysis for diagnosis in patients with suspected lung cancer. Acad. Radiol. 2021, 28, e27–e34. [Google Scholar] [CrossRef]
- Kimura, H.; Ohkawa, K.; Miyazaki, M.; Sakakibara, M.; Imanaka, K.; Tamura, T.; Sueyoshi, H.; Takada, R.; Fukutake, N.; Uehara, H.; et al. Subclassification of patients with intermediate-stage (Barcelona Clinic Liver Cancer stage-B) hepatocellular carcinoma using the up-to-seven criteria and serum tumor markers. Hepatol. Int. 2017, 11, 105–114. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mariani, L.; Sposito, C.; Spreafico, C.; Bongini, M.; Morosi, C.; Cascella, T.; Marchianò, A.; Camerini, T.; Bhoori, S.; et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh A liver function: A proof-of-concept study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Kudo, M. Gd-EOB-DTPA-MRI Could Predict WNT/β-Catenin mutation and resistance to immune checkpoint inhibitor therapy in hepatocellular carcinoma. Liver Cancer 2020, 9, 479–490. [Google Scholar] [CrossRef]
- Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; et al. Higher enhancement intrahepatic nodules on the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI as a poor responsive marker of Anti-PD-1/PD-L1 monotherapy for unresectable hepatocellular carcinoma. Liver Cancer 2021, 10, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Suda, G.; Kimura, M.; Maehara, O.; Tokuchi, Y.; Kitagataya, T.; Ohara, M.; Yamada, R.; Shigesawa, T.; Suzuki, K.; et al. Characteristics and lenvatinib treatment response of unresectable hepatocellular carcinoma with iso-high intensity in the hepatobiliary phase of EOB-MRI. Cancers 2021, 13, 3633. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Ding, H.; Zhou, X.; Zeng, Z.; Long, L. Gd-EOB-DTPA-enhanced magnetic resonance imaging may help identify patients with hepatocellular carcinoma eligible for treatment targeted at RAF1. Abdom. Radiol. 2022, 47, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Huang, K.; Liao, B.; Cai, H.; Dong, Y.; Huang, M.; Zhou, X.; Jia, Y.; Xu, L.; Luo, Y.; et al. Prediction of sorafenib treatment-related gene expression for hepatocellular carcinoma: Preoperative MRI and histopathological correlation. Eur. Radiol. 2019, 29, 2272–2282. [Google Scholar] [CrossRef]
- Chen, S.; Feng, S.; Wei, J.; Liu, F.; Li, B.; Li, X.; Hou, Y.; Gu, D.; Tang, M.; Xiao, H.; et al. Pretreatment prediction of immunoscore in hepatocellular cancer: A radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur. Radiol. 2019, 29, 4177–4187. [Google Scholar] [CrossRef] [PubMed]
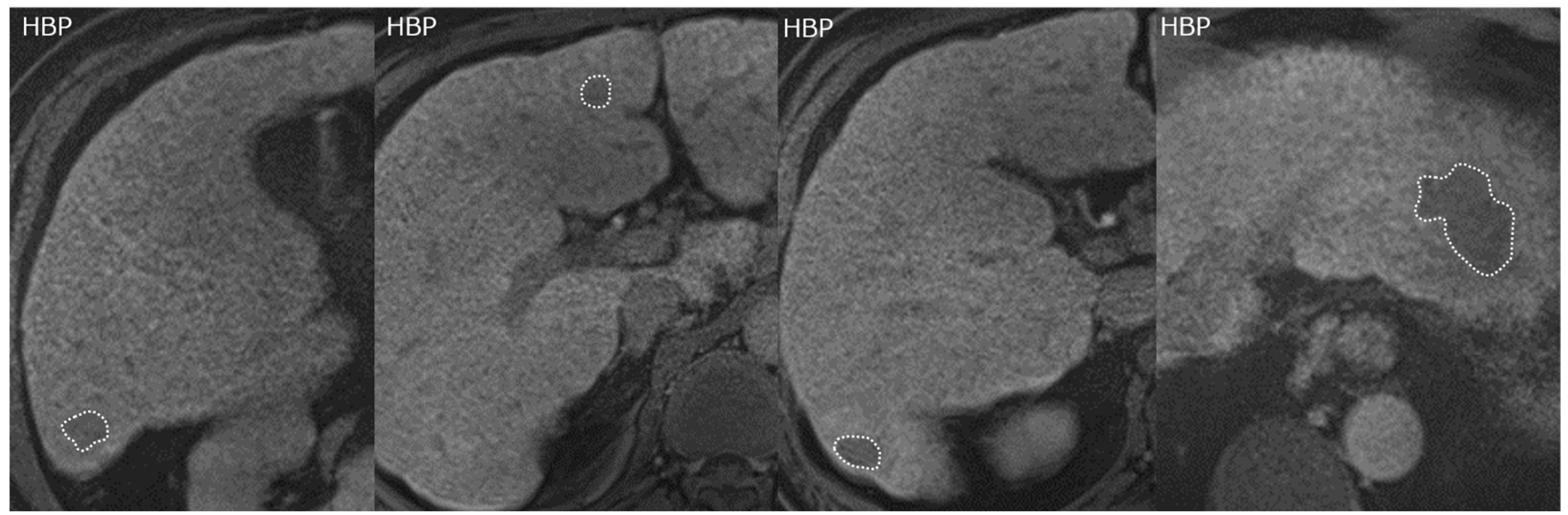
| Characteristic | All Patients (n = 64) | % |
|---|---|---|
| Age (year) | ||
| <80 | 58 | 90.6 |
| ≥80 | 6 | 9.4 |
| Sex | ||
| Male | 47 | 73.4 |
| Female | 17 | 26.6 |
| Child-Pugh score | ||
| 5.6 | 59 | 92.2 |
| 7 | 5 | 7.8 |
| PT (%) | ||
| <70 | 3 | 4.7 |
| ≥70 | 61 | 95.3 |
| Total bilirubin(mg/dL) | ||
| <2 | 63 | 98.4 |
| ≥2 | 1 | 1.6 |
| Albumin(g/dL) | ||
| <3.5 | 3 | 4.7 |
| ≥3.5 | 61 | 95.3 |
| AFP (ng/mL) | ||
| <200 | 54 | 84.4 |
| ≥200 | 10 | 15.6 |
| Up-to-7 | ||
| in | 39 | 60.9 |
| out | 25 | 39.1 |
| Up-to-11 | ||
| in | 57 | 89.1 |
| out | 7 | 10.9 |
| Etiology of liver disease | ||
| No | 6 | 9.4 |
| Yes | 58 | 90.6 |
| ALBI grade | ||
| 1 | 23 | 35.9 |
| 2 | 41 | 64.1 |
| MTA after refractory to TACE | ||
| No | 52 | 81.2 |
| Yes | 12 | 18.8 |
| MRI scanner | ||
| 1.5 T | ||
| Avanto | 26 | 40.6 |
| Sonata | 7 | 10.9 |
| Other institutions | 8 | 12.5 |
| 3.0 T | ||
| Skyra | 1 | 1.6 |
| Verio | 22 | 34.4 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Risk Factor | p-Value | HR (95% CI) | p-Value | HR (95% CI) |
| Age ≥ 80 years | 0.339 | 2.049 (0.472–8.903) | ||
| Etiology of liver disease | 0.364 | 0.575 (0.174–1.901) | ||
| Coefficient of variation ≥0.16 | 0.001 | 3.211 (1.615–6.384) | 0.038 | 2.354 (1.049–5.281) |
| AFP ≥ 200 ng/mL | 0.005 | 3.208 (1.428–7.207) | 0.673 | 1.244 (0.452–3.429) |
| Up-to-7 out | 0.002 | 2.745 (1.440–5.236) | 0.157 | 1.777 (0.801–3.943) |
| Up-to-11 out | 0.042 | 3.810 (1.048–13.852) | ||
| Child–Pugh score 7 | 0.016 | 3.688 (1.270–10.704) | 0.190 | 2.145 (0.685–6.719) |
| ALBI grade 2 | 0.02 | 2.663 (1.163–6.096) | ||
| PT < 70% | 0.262 | 1.986 (0.599–6.588) | ||
| Totalbilirubin ≥ 2.0 mg/dL | 0.528 | 0.047 (0.000–621.057) | ||
| Albumin < 3.5 g/dL | 0.072 | 2.031 (0.938–4.397) | ||
| Post-TACE MTA | 0.071 | 1.886 (0.947–3.755) | ||
| Non-CR after first TACE | 0.072 | 0.525 (0.260–1.060) | ||
| Within Up-To-7 (n = 39) | Beyond Up-To-7 (n = 25) | |||
|---|---|---|---|---|
| High CV (n = 8) | Low CV (n=31) | High CV (n = 13) | Low CV (n = 12) | |
| MST (months) | 37.7 | 82.9 | 18 | 38.3 |
| Within Up-To-7 | Beyond Up-To-7 | |
|---|---|---|
| Low CV | TACE | TACE + systemic therapy |
| High CV | TACE + systemic therapy | Systemic therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minamiguchi, K.; Nishiofuku, H.; Saito, N.; Sato, T.; Taiji, R.; Matsumoto, T.; Maeda, S.; Chanoki, Y.; Tachiiri, T.; Kunichika, H.; et al. Quantitative Analysis of Signal Heterogeneity in the Hepatobiliary Phase of Pretreatment Gadoxetic Acid-Enhanced MRI as a Prognostic Imaging Biomarker in Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma. Cancers 2023, 15, 1238. https://doi.org/10.3390/cancers15041238
Minamiguchi K, Nishiofuku H, Saito N, Sato T, Taiji R, Matsumoto T, Maeda S, Chanoki Y, Tachiiri T, Kunichika H, et al. Quantitative Analysis of Signal Heterogeneity in the Hepatobiliary Phase of Pretreatment Gadoxetic Acid-Enhanced MRI as a Prognostic Imaging Biomarker in Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma. Cancers. 2023; 15(4):1238. https://doi.org/10.3390/cancers15041238
Chicago/Turabian StyleMinamiguchi, Kiyoyuki, Hideyuki Nishiofuku, Natsuhiko Saito, Takeshi Sato, Ryosuke Taiji, Takeshi Matsumoto, Shinsaku Maeda, Yuto Chanoki, Tetsuya Tachiiri, Hideki Kunichika, and et al. 2023. "Quantitative Analysis of Signal Heterogeneity in the Hepatobiliary Phase of Pretreatment Gadoxetic Acid-Enhanced MRI as a Prognostic Imaging Biomarker in Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma" Cancers 15, no. 4: 1238. https://doi.org/10.3390/cancers15041238
APA StyleMinamiguchi, K., Nishiofuku, H., Saito, N., Sato, T., Taiji, R., Matsumoto, T., Maeda, S., Chanoki, Y., Tachiiri, T., Kunichika, H., Inoue, T., Marugami, N., & Tanaka, T. (2023). Quantitative Analysis of Signal Heterogeneity in the Hepatobiliary Phase of Pretreatment Gadoxetic Acid-Enhanced MRI as a Prognostic Imaging Biomarker in Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma. Cancers, 15(4), 1238. https://doi.org/10.3390/cancers15041238






