Simple Summary
We describe the currently known histopathological aspects of the prognostic factors for salivary gland cancers and discuss the genetics or molecules used as diagnostic tools that might serve as treatment targets in the future.
Abstract
Salivary gland cancers (SGCs) are diagnosed using histopathological examination, which significantly contributes to their progression, including lymph node/distant metastasis or local recurrence. In the current World Health Organization (WHO) Classification of Head and Neck Tumors: Salivary Glands (5th edition), malignant and benign epithelial tumors are classified into 21 and 15 tumor types, respectively. All malignant tumors have the potential for lymph node/distant metastasis or local recurrence. In particular, mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (AdCC), salivary duct carcinoma, salivary carcinoma, not otherwise specified (NOS, formerly known as adenocarcinoma, NOS), myoepithelial carcinoma, epithelial–myoepithelial carcinoma, and carcinoma ex pleomorphic adenoma (PA) are relatively prevalent. High-grade transformation is an important aspect of tumor progression in SGCs. MEC, AdCC, salivary carcinoma, and NOS have a distinct grading system; however, a universal histological grading system for SGCs has not yet been recommended. Conversely, PA is considered benign; nonetheless, it should be cautiously treated to avoid the development of metastasizing/recurrent PA. The aim of this review is to describe the current histopathological aspects of the prognostic factors for SGCs and discuss the genes or molecules used as diagnostic tools that might have treatment target potential in the future.
1. Introduction
In the current World Health Organization (WHO) Classification of Head and Neck Tumors: Salivary Glands (5th edition), malignant and benign epithelial tumors are classified into 21 and 15 tumor types, respectively (Table 1) [1].

Table 1.
WHO Classification of Head and Neck Tumors: Salivary Glands (5th edition).
The salivary gland comprises three major salivary glands (parotid, submandibular, and sublingual glands) and several minor salivary glands. Salivary gland cancers (SGCs) can arise from any salivary gland and have a morbidity rate of approximately 10–20% [2,3] (Bishop, J.A. et al. pp. 31–51). SGCs are diagnosed using histopathological examination, and histological findings can be considered the most valuable and distinct prognostic factors [2,3] (Bishop, J.A. et al. pp. 31–51). SGCs show various tumor types because a healthy salivary gland contains inner luminal/epithelial or acinar/mucous cells and outer basal/myoepithelial cells in the duct or the secretory part. Histopathological features correlate with tumor progression, including lymph node/distant metastasis or local recurrence. In 1986, Spiro reported that the significant prognostic factors were the site of origin, histologic subtype, histologic grading, and clinical stage [4]. However, some carcinomas show mild cytological atypia, making the evaluation of tumor invasion challenging. For all malignant tumors, including mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (AdCC), salivary carcinoma, and not otherwise specified (NOS) (formerly known as adenocarcinoma, NOS), validated grading systems exist; however, a universal histological grading system for SGCs has not been recommended [1]. Furthermore, novel genes or genetic components and proteins are being validated as diagnostic tools, and some of them may even serve as targets for new drugs. Herein, we summarize present SGC classifications with a focus on the prognostic factors and discuss new and potential prognostic factors from the histopathological viewpoint.
2. The Past and Present of SGCs
2.1. The General Histopathological Prognostic Factors for SGCs
2.1.1. The Histological Types
The most prevalent malignancies are MEC and AdCC, and most SGCs have a distinct histological grade (Table 2). Histological diagnosis reflects biological behavior in several cases. The current WHO classification (5th edition) describes the grading system for MEC, AdCC, salivary carcinoma, and NOS [1]. Other tumors that show similar classification include carcinoma ex pleomorphic adenoma (CXPA) and intraductal carcinoma (IDC). The WHO classification detaches grades from tumor names since tumors with the same features of a cancer type do not necessarily have the same severity or aggressiveness, allowing for flexibility in describing tumors [1,2,5] (Bishop, J.A. et al. pp. 31–51). Each tumor’s histological features are subsequently discussed.

Table 2.
Native histopathological stratification of salivary gland malignancies.
2.1.2. High-Grade Transformation (Dedifferentiation)
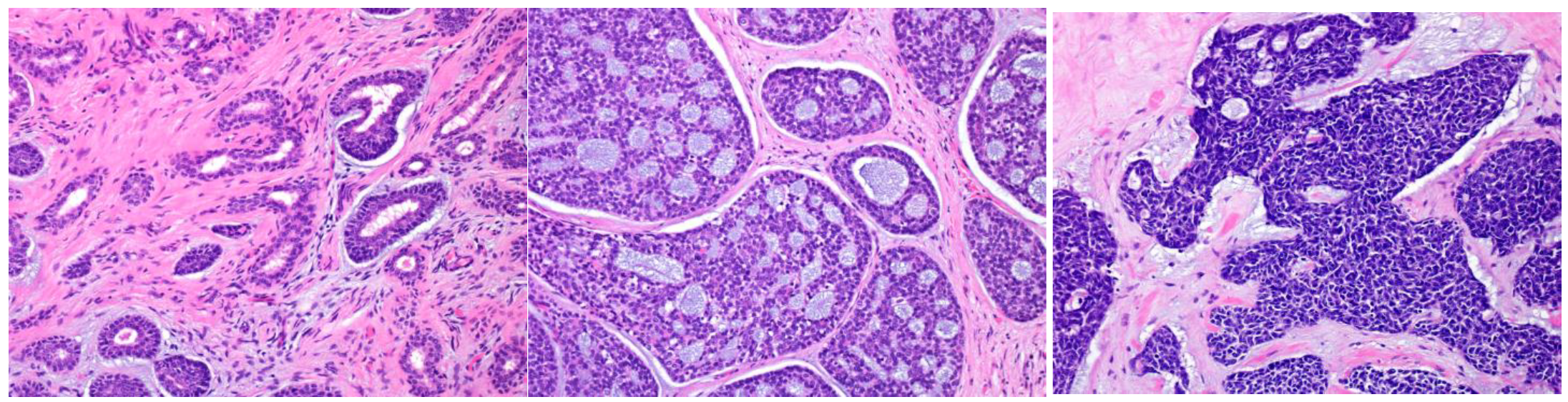
Dedifferentiation is a regression from a more differentiated to a less differentiated state (stem-cell-like). In particular, in a malignant tumor, a differentiated cell loses its specific form or function [6,7]. Histopathologically, dedifferentiation is observed as the abrupt transformation of a well-differentiated tumor into high-grade morphology (poorly differentiated or anaplastic/undifferentiated), lacking the original morphology (Figure 1) [8].
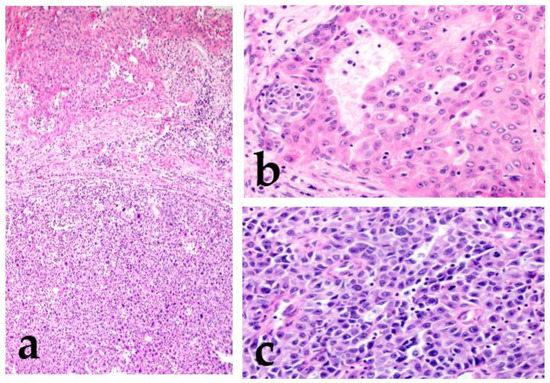
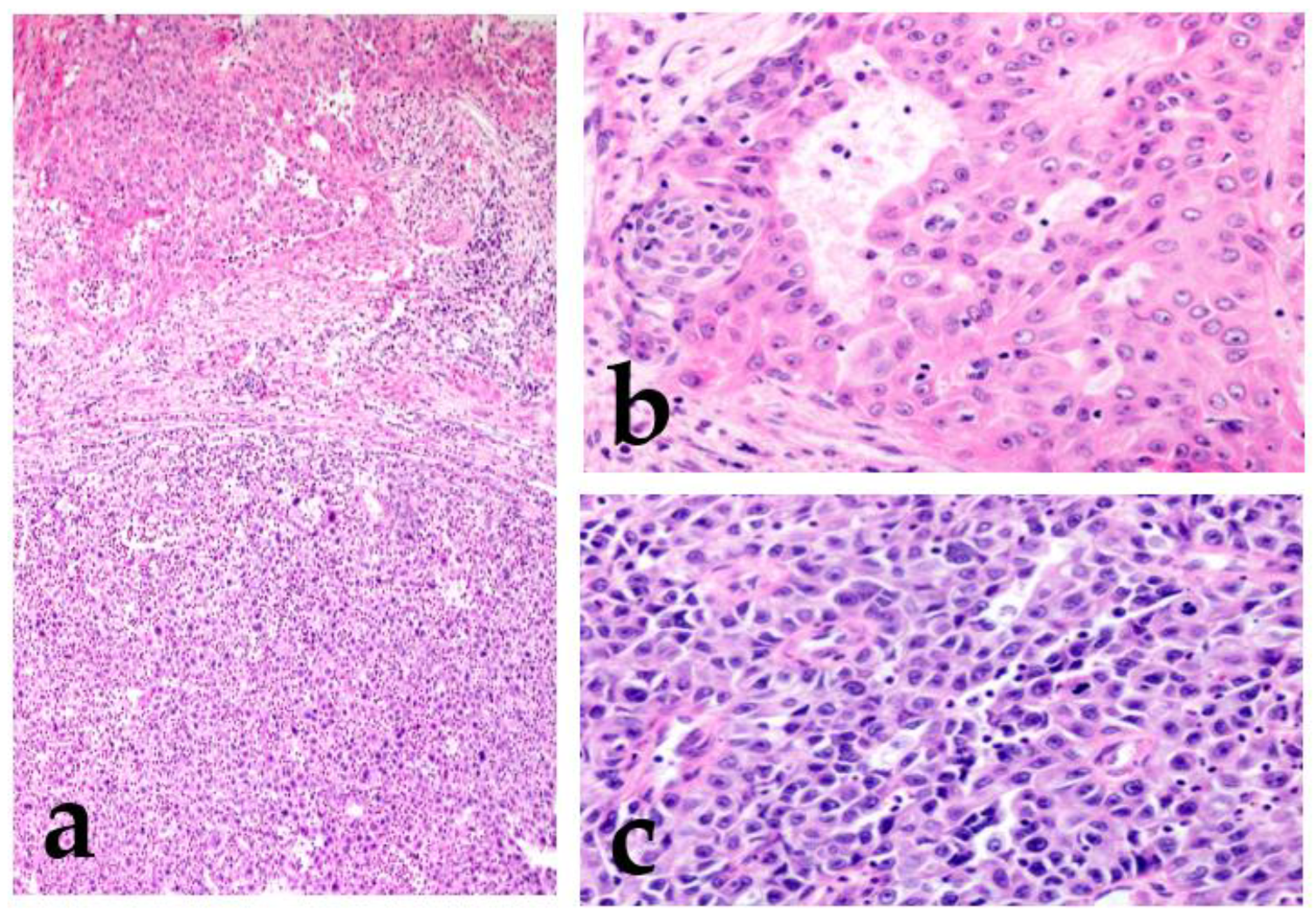
Figure 1.
Mucoepidermoid carcinoma (MEC) with high-grade transformation. There is intermediate-grade MEC in the upper region, and the lower region shows high-grade transformation (a). Intermediate-grade MEC forms glandular or solid structures and consists mainly of intermediate cells with moderate atypia (increased nuclear size with an obvious nucleus) (b). The high-grade region shows undifferentiated features, and the cells lack the original morphology (c).
In the high-grade region, the tumor cells show anaplastic cells with large vesicular pleomorphic nuclei, prominent nucleoli, an increased mitoses/Ki67 labeling index, and necrosis [8,9]. The term “dedifferentiation” is occasionally used in malignant soft tissue tumors, such as dedifferentiated liposarcoma or dedifferentiated chondrosarcoma [10,11]. However, in SGCs, a malignant tumor is rarely replaced by a completely different histological morphology, and the original morphological features usually remain. Therefore, the term high-grade transformation is used to describe this phenomenon [8,12]. This transformation is reported not only in variable low-grade malignant tumors (acinic cell carcinoma, MEC, secretory carcinoma, hyalinizing clear cell carcinoma, myoepithelial carcinoma, epithelial–myoepithelial carcinoma, and polymorphous adenocarcinoma) but also in high-grade tumors, including AdCC [12,13,14,15,16,17,18,19,20,21]. Tumors with this finding have an even worse prognosis.
2.1.3. Micropapillary Pattern
Invasive micropapillary carcinoma (IMPC) was first reported in the breast [22]. Neoplastic cell nests are uniformly distributed throughout a reticulated interstitium and exhibit a reverse polarity or “inside-out” growth pattern. This histological finding is observed in other malignant tumors of the urinary bladder, lung, stomach, colon, and bile duct [23,24,25,26,27]. IMPC, a tumor with a micropapillary pattern, frequently shows lymphatic invasion and lymph node metastasis, and its prognosis is very poor. Among SGCs, micropapillary salivary duct carcinoma (SDC) is the most prevalent; nonetheless, micropapillary AdCC and intraductal papillary mucinous neoplasm (IPMN) have also been reported (Figure 2) [21,28,29].
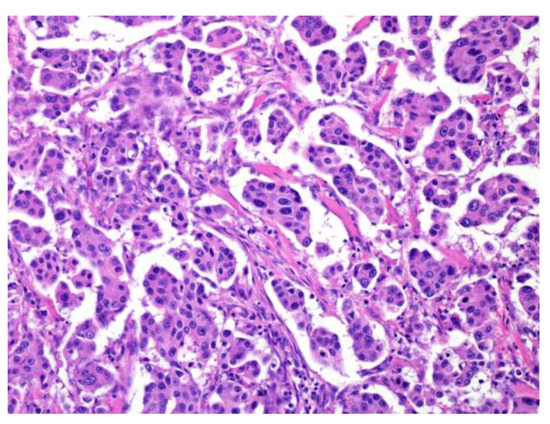
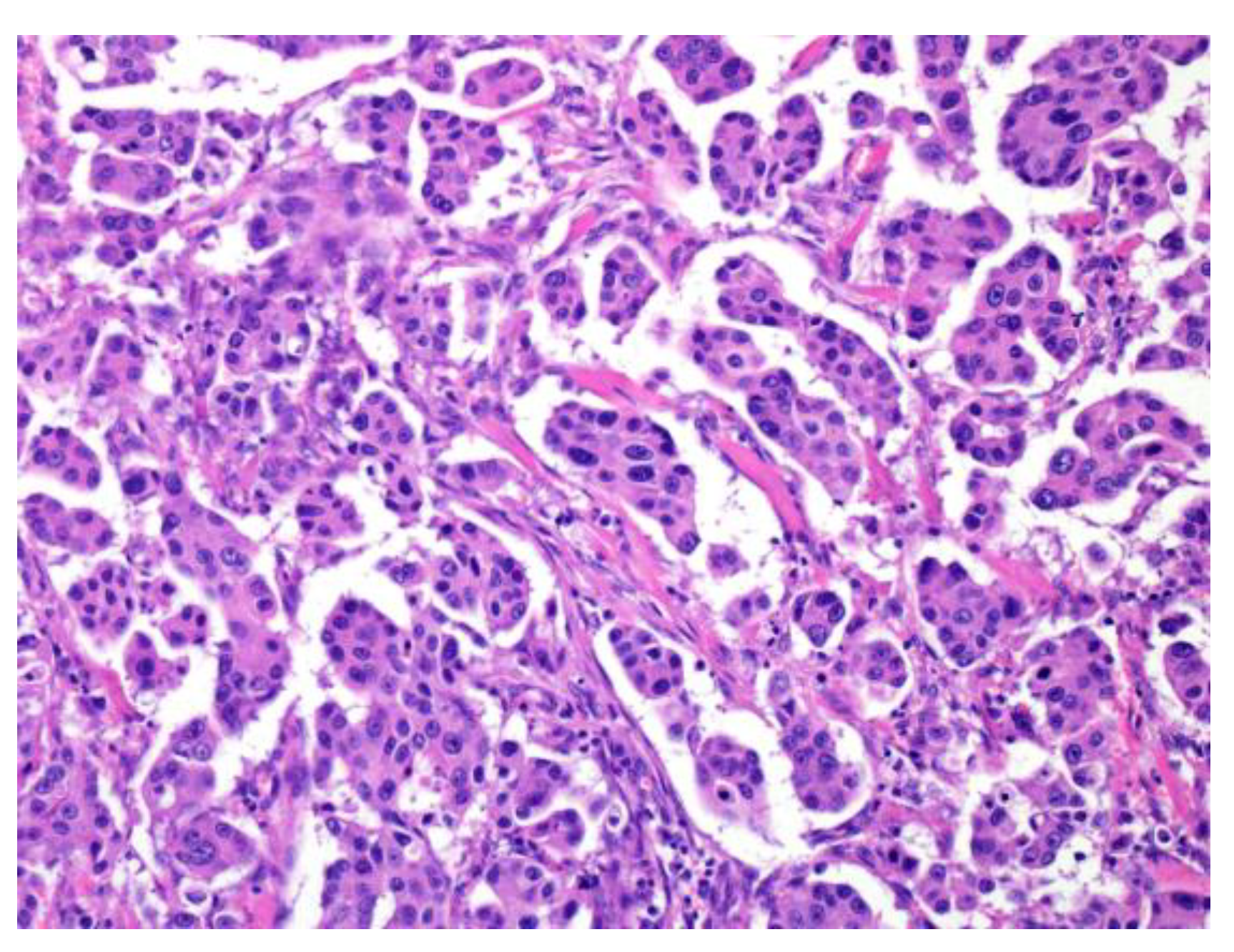
Figure 2.
Micropapillary salivary duct carcinoma (SDC). The tumor forms many micropapillary nests that consist of eosinophilic cytoplasm and irregular nuclear-like SDC.
2.1.4. Other Histologic Findings
Strong prognostic factors prevalent in many tumors include increased cellular atypia, perineural invasion, lymphovascular invasion, an increased mitoses/Ki67 labeling index, necrosis, local recurrence/distant metastasis, and poor surgical margin (Figure 3) [2,3,4,5] (Bishop, J.A. et al. pp. 31–51).
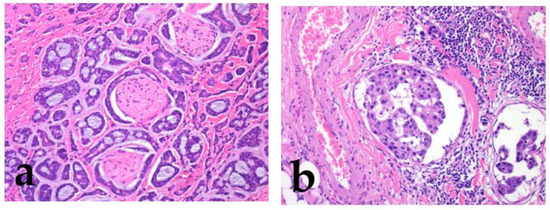
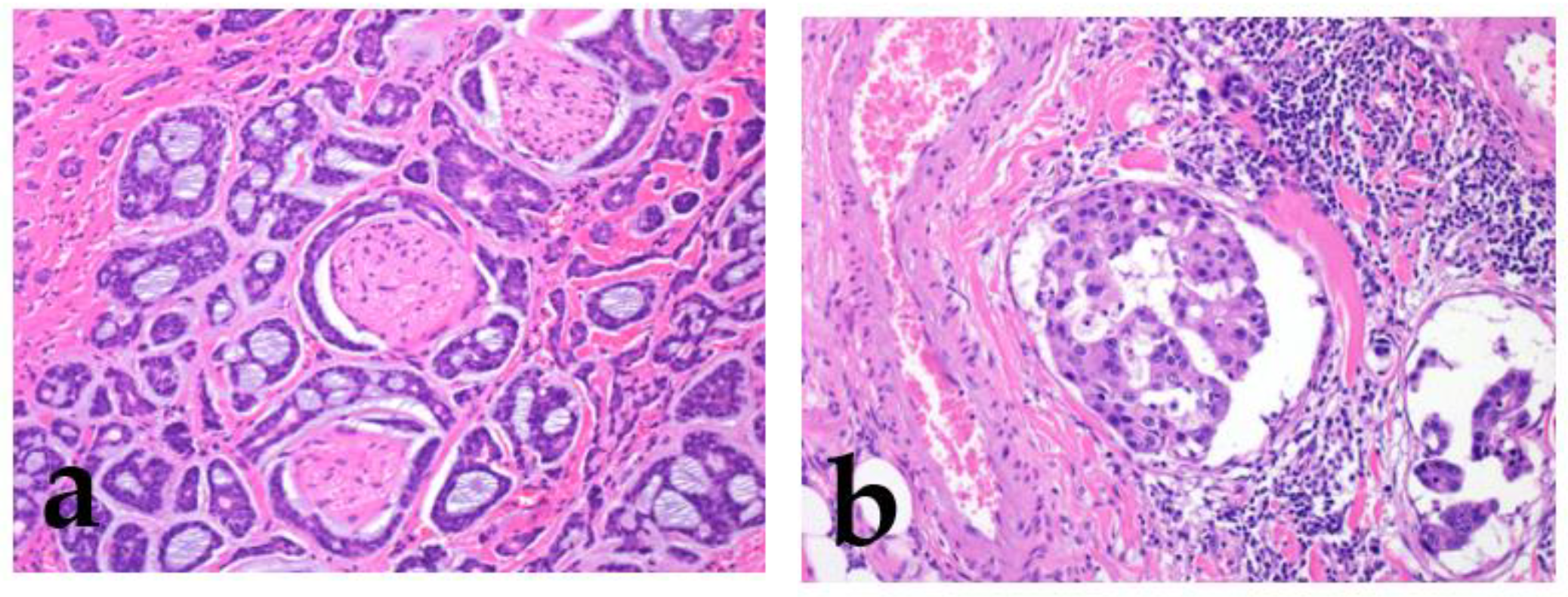
Figure 3.
Perineural invasion and lymphatic invasion. Adenoid cystic carcinoma shows frequent perineural invasion (a), and salivary duct carcinoma shows lymphatic invasion (b).
For lymph node metastasis, important prognostic factors include the number of nodes, foci size, unilateral/bilateral involvement, extranodal extension, and stromal reaction [30,31,32]. Lombardi reported that intraparotid node metastasis implies an increased risk of lateral neck involvement and impact on the survival of patients with SGCs [33]. More specifically, the overall number (0 vs. 1–3 vs. ≥4) and diameter (<20 mm vs. ≥20 mm) of the node metastasis represent major prognostic factors for overall survival [33]. Additionally, for parotid gland carcinoma, facial nerve paralysis and tumor adhesion/immobility could be the predictive factors for high-grade SGCs [34].
2.2. Other Related Factors
Although not directly involved histopathologically, the most predictive factors for tumor recurrence are advanced age, male sex, large tumor size, and high clinical stage [2,35,36,37] (Bishop, J.A. et al. pp. 31–51). Concerning the site of tumor origin, small salivary glands have a high frequency of SGC occurrence, and the sublingual salivary gland has the highest frequency of malignancy [2,38] (Bishop, J.A. et al. pp. 31–51).
3. Validated Grading Systems for Individual SGCs and Similar Diseases
This section discusses MEC, AdCC, salivary carcinoma, and NOS, which have validated grading systems in the WHO classification (5th edition), focusing on structural atypia, such as the presence or absence of a tumor nest/solid part and cellular atypia. Furthermore, we describe IDC and CXPA, which are classified by the presence or absence of tumor invasion and atypical cellular morphology. Moreover, metastasizing PA (MPA) is also discussed.
3.1. Grading System in the WHO Classification
3.1.1. Mucoepidermoid Carcinoma (MEC)
MEC is characterized by mucous, as we intermediate and epidermoid/squamoid tumor cells. However, in some tumors, intermediate cells are predominant. MEC forms cystic and solid growth patterns that are usually associated with MAML2 rearrangement [2,39] (Bishop, J.A. et al. pp. 265–290); therefore, some reports describe the histological features for grading (Table 3, Figure 4) [2,39,40,41,42,43], which include structural atypia (cystic/solid component, border invasion pattern, lymphovascular/perineural invasion, and necrosis) and cytological atypia (nuclear anaplasia/pleomorphism and mitoses) [2,36,37,38,39,40,41,42,43]. According to these gradings, MEC should be graded as low, intermediate, or high. The higher the grade, the greater the possibility of metastasis or recurrence [2,39] (Bishop, J.A. et al. pp. 265–290).

Table 3.
Comparison of the mucoepidermoid carcinoma grading systems.
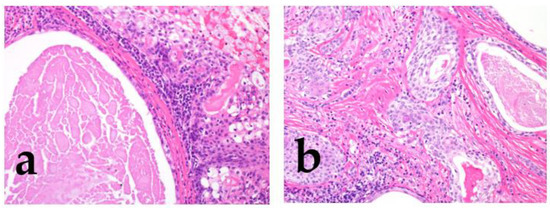
Figure 4.
The morphological features of low/intermediate mucoepidermoid carcinoma (MEC). The tumor forms cystic (a; low grade) and solid growth patterns (b; intermediate). In both cases, tumor cell atypia is mild, but the intermediate category shows slightly different nuclear sizes with mitosis.
The Armed Forces Institute of Pathology (AFIP) grading system was previously used; however, this system may not necessarily indicate the actual degree of some aggressive cases [2,40] (Bishop, J.A. et al. pp. 265–290); hence, the Brandwein system was introduced to classify these cases from the viewpoint of anaplasia [42]. Nevertheless, high-grade MEC is very rare, and there is no difference in outcome between low and intermediate grades using any grading system. Another study reported that mitosis and necrosis may be helpful in the classification of tumor grade [43].
3.1.2. Adenoid Cystic Carcinoma (AdCC)
AdCC consists of two main cell types: ductal cells located in the inner part and myoepithelial cells located in the outer part of the duct. The ductal cells have eosinophilic cytoplasm and uniformly round nuclei, while the myoepithelial cells have a clear cytoplasm and hyperchromatic angular nuclei [2,44] (Bishop, J.A. et al. pp. 337–356). Perineural invasion is an AdCC hallmark, and genetically, it is characterized by MYB or related gene translocations. Typically, AdCC comprises pseudocysts and true glandular lumina. AdCC shows three growth patterns: tubular, cribriform, and solid [2,44] (Bishop, J.A. et al. pp. 337–356). Consequently, the following histological grading is used for its classification: tubular predominant as grade Ⅰ, cribriform predominant as grade Ⅱ, and solid predominant as grade Ⅲ (Figure 5) [2,44,45].
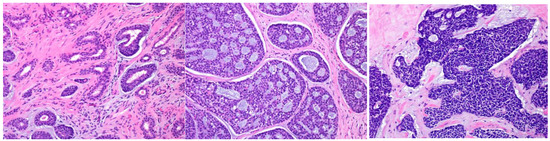
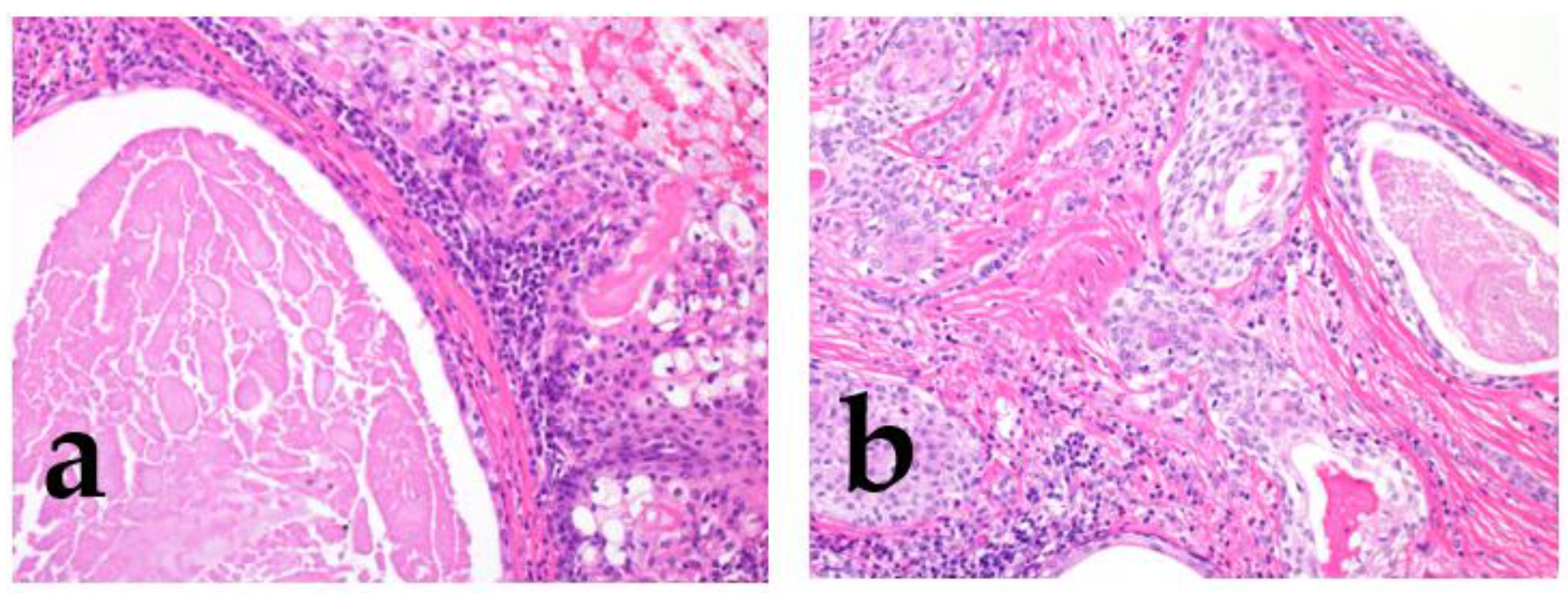
Figure 5.
The histological grade of adenoid cystic carcinoma (AdCC). The tumor shows tubular (left), cribriform (middle), and solid (right) growth patterns.
Although AdCCs with >30% solid components have been shown to be more aggressive, any solid tumor component may be a high-grade tumor, as described in the minAmax system [46,47,48]. Necrosis, marked pleomorphism, or high levels of mitoses are only seen in the solid pattern and are not utilized in the grading system [2] (pp. 337–356).
3.1.3. Salivary Carcinoma, NOS (Adenocarcinoma, Not Otherwise Specified (NOS), Formerly)
In the current WHO classification (5th edition), adenocarcinoma, NOS, is renamed as salivary carcinoma, NOS, and we have used the same term herein [49]. It includes the subtypes of oncocytic and intestinal-type adenocarcinoma. The term salivary carcinoma, NOS, should be used for tumors arising in major/minor salivary glands; however, this category is a heterogeneous spectrum of carcinomas showing ductal and/or glandular differentiation. It represents an exclusive diagnosis of otherwise defined salivary gland carcinoma entities (adenocarcinoma with nonspecific appearance) [2,49,50]. Due to differences in this carcinoma’s interpretation, the percentage or case numbers have varied in previous reports, and the pure entity adenocarcinoma, NOS, now accounts for approximately 10% of SGCs [36,51,52,53,54,55]. Adenocarcinoma, NOS, was considered a heterogeneous group of tumors; however, the strictly selected cases were considered as the pure group [56]. The histological grading system was based on a previous report published in 1982 by Spiro et al., and the tumors were classified as low-, intermediate-, or high-grade [2,49,54,55] (Bishop, J.A. et al. pp. 290–303). They defined anaplastic or high-grade lesions as grade Ⅲ, which are arranged in close clumps and broad bands composed of small glandular tumor cells. The tumor cell nests are separated by collagen connective tissue stroma, similar to that in seen during scirrhous formation. In addition, they are divided into low- (grade Ⅰ) or intermediate-grade (grade II); the low-grade variant shows no stromal invasion, whereas the intermediate grade shows definite stromal infiltration. Grade Ⅱ lesions have prominent sheets or cords of some polymorphic glandular cells with a pale cytoplasm [54]. Although this classification reflected the tumor prognosis at that time, it is still somewhat reasonably useful today; however, it should be revised owing to the differences in current diagnostic criteria.
3.2. Carcinoma ex Pleomorphic Adenoma (CXPA)
CXPA is an epithelial and/or myoepithelial malignancy arising in a primary or recurrent pleomorphic adenoma (PA) [2,57] (Bishop, J.A. et al. pp. 374–400). Reflecting the presence of PA, the typical clinical presentation is a long-term painless mass with recent rapid progression or previous PA diagnosis. PA presents as ductal and myoepithelial cells arranged in bilayered tubular structures, while the stroma is typically mucoid, myxoid, hyalinized, or chondroid. Usually, the transition from PA to the malignant component is distinct; however, some cases may have heavy stromal/hyalinized collagen bundles or chondroid/myxoid stroma only. The common malignancies are SDC, epithelial–myoepithelial carcinoma, salivary carcinoma, NOS, and myoepithelial carcinoma; in contrast, carcinosarcoma is rare [58]. Most carcinosarcomas arise from PA through the intraductal or myoepithelial pathway, the multistep adenoma–carcinoma–sarcoma sequence [59]. The relevant observations are the histological subtype/grade, the proportion of carcinoma (>50%), and the extent of invasion [2,58,59,60] (Bishop, J.A. et al. pp. 374–400). The malignant component progresses from an encapsulated neoplasm to extracapsular invasion. The term “encapsulated” has been rephrased by various alternatives, such as “intracapsular”, “in situ”, “preinvasive”, “intramural”, or “noninvasive”; however, the term intracapsular is preferred. CXPA is subclassified based on the extent of invasion beyond the PA as follows: intracapsular, minimally invasive (the carcinoma invades <4–6 mm beyond the PA borders), and invasive (invasion beyond the PA capsule ≥6 mm) [57]. However, evaluating the fibrous capsule is occasionally challenging because the tumor forms the capsule or the capsule outline is vague, especially in primary minor salivary glands. Several specimen preparations are needed for a precise diagnosis.
3.3. Intraductal Carcinoma (IDC)
IDC is a salivary gland malignancy located entirely or predominantly intraductally [2,61] (Bishop, J.A. et al. pp. 461–475). It has papillary, cribriform, and solid structures mimicking atypical ductal hyperplasia or ductal carcinoma in situ of the breast [2,61] (Bishop, J.A. et al. pp. 461–475). It shows four subtypes based on the tumor cells: intercalated duct, apocrine, oncocytic, and mixed IDC; hence, tumors in this category are heterogeneous. Usually, intercalated duct and oncocytic IDC are low-grade, whereas apocrine and mixed IDC are low- or high-grade [2,61] (Bishop, J.A. et al. pp. 461–475). Independent of tumor cell subtype, pure IDC behaves indolently, although invasive carcinomas ex-IDC (arising from IDC) can behave aggressively [62,63]. Therefore, these tumors may be reassessed as “IDC, noninvasive (low-grade IDC)”, “IDC, noninvasive (high-grade IDC)”, or “IDC, invasive”, similar to intraductal papillary mucinous neoplasm (IPMN) of the pancreas or CXPA of the salivary gland.
3.4. Metastasizing Pleomorphic Adenoma (MPA)
PA is a benign tumor composed of benign ductal and myoepithelial cells with a chondromyxoid or sclerosing fibrous component in the background [2,64] (Bishop, J.A. et al. pp. 536–543). In peculiar cases, benign-looking PA metastasizes to the bone, lung, and neck lymph nodes with some local recurrence [65,66]. Knight et al. reported that 41 patients (80.4%) with MPA were alive at the time of 1-year follow-up. However, survival was poor, and 17.6% (9/51 cases) died from MPA [66]. Although the term includes “PA”, considering the clinical course, it was managed at least as a low-grade malignancy. There are no histological or molecular features to predict metastasis, and it is not distinguishable from a benign tumor at the primary site [66,67]. Thus, first-time surgery for PA must be performed cautiously if the patient is young, the PA is multinodular, or there is a tumor rupture. Furthermore, a postoperative status of incomplete tumor excision, incomplete pseudocapsule, extracapsular extension (microscopic pseudopods or skip/satellite lesion beyond the pseudocapsule), or poor margin should also be considered due to the possibility of recurrent PA [68,69,70,71].
4. Future Perspectives on SGCs
This section discusses SGC-specific genes or characteristic proteins, including their immunohistochemistry. These have emerged as diagnostic tools in recent years and could be clinicopathological predictors of SGCs. Although many drugs are not included in the usual regimens, drug-targetable proteins and genes, such as hormone receptors in breast cancers, have been shown to alter SGCs prognosis. Although various methods are used to investigate SGCs or target genes, in several cases, immunohistochemistry (IHC), reverse transcription polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH), and Sanger sequencing/next-generation sequencing (NGS) are commonly used. IHC, RT-PCR, and FISH detect protein expression, fusion genes and gene translocation, and gene translocation and amplification, respectively. Furthermore, Sanger sequencing detects point mutations or minor genetic alterations, and NGS can be used for whole-genome sequencing; nevertheless, IHC is the most commonly performed investigation, as it is economic and convenient. Recently, targeted therapies have been developed to target the signaling pathways involving the molecular signatures detected by these techniques. For example, if the detected oncogene signature is VEGF/ANG2, VEGFR/FGFR/PDGFR, HER2, EGFR, or TrkB/BDNF, the targeted therapy for MEC is sorafenib, nintedanib, trastuzumab, lapatinib, or ANA-12, respectively [72].
4.1. Genetics as a Diagnostic Tool
SGCs are heterogeneous tumors, and genetics aids in understanding the molecular biology of each tumor. All malignant tumors listed in the WHO classification (5th edition) and related genes are summarized in Table 4 [1,2,73,74,75,76,77,78] (Bishop, J.A. et al. pp. 31–51).

Table 4.
Comparison of histological diagnoses and gene alterations.
These gene alterations are used as diagnostic tools and represent the tumor’s specific characteristics. Some of these genes are druggable, and HER2 or tropomyosin receptor kinase (TRK) inhibitors have been used to treat salivary duct or secretory carcinoma, respectively [76,77,78]. Some of these drugs significantly change patients’ prognoses, and they are discussed in the following section.
4.2. Druggable Genes and Proteins (Including Drug Repositioning/Drug Repurposing)
4.2.1. Human Epidermal Growth Factor Receptor 2 (HER2)
HER2 is a proto-oncogene that is expressed or overexpressed in a variety of epithelial malignancies, including, breast, stomach, colon, rectum, biliary tract, and lung cancer [79,80,81,82,83,84,85,86,87]. Its overexpression is associated with HER2 gene amplification or mutation. That is, the HER2 gene is amplified in 20% to 25% of primary breast cancers. Accordingly, HER2 inhibitors are used to treat HER2-positive breast and gastric cancers [80]. In SGCs, the overall frequency of HER2 overexpression is 17% and is predominantly seen in SDCs [88]. Other HER2-high expression tumors are CXPA, adenocarcinoma, NOS, squamous cell carcinoma, and MEC [89]. HER2-positive tumors, for example, breast carcinoma, have been treated with trastuzumab (Herceptin), the use of which is expanding to gastric or colon cancers [81,82]. Recently, its use has been explored for the management of SDC, urothelial carcinoma, bile duct adenocarcinoma, etc. [83,84,85,86,87]. Trastuzumab treatment for HER2-positive patients is correlated with good response and long-term survival [84]. Notably, an advanced anti-HER2 antibody, trastuzumab deruxtecan (T-Dxd, Enhertu) was developed, which is used for HER2-low breast cancers (HER2 IHC score of 1+ or 2+ without gene amplification) [90]. T-Dxd is an antibody–drug conjugate combination of trastuzumab and topoisomerase I inhibitor, which implies that its use could be expanded in the future to treat a variety of tumors, including HER2-positive SDCs [91,92].
4.2.2. Androgen Receptor (AR)/NK3 Homeobox 1 (NKX3.1)
AR expression is mainly characteristic of SDC among all SGCs [2,87] (Bishop, J.A. et al. pp. 475–497). Owing to AR copy number gain, ligand-independent splice variants, and mutations, AR is overexpressed in typical SDCs [2] (pp. 475–497). Some SDCs are also positive for NKX3.1, α-methylacyl-CoA racemase (AMACR), and prostatic acid phosphatase (PAP), and the positivity of these androgen hormone-related proteins was previously reported in prostatic cancer [93,94]. Hence, androgen deprivation therapy is part of the standard of care for advanced and metastatic prostate cancer [95]. Similar to that for prostatic cancer, androgen deprivation therapy has been performed, and some reports have stated that it is effective for SDC patients [96]. However, the prognosis of patients with AR-, AMACR-, or PAP-negative SDC remains poor [87,93]. In particular, AR negativity is associated with significantly worse overall survival, as splice variants and increased gene copy number may reduce the drug response and increase therapeutic resistance [97,98].
4.2.3. Protein Receptor Kinase/Protein Kinase
Various genetic alterations have been reported for SGCs, including some oncogenic driver alterations (for example, EGFR mutation and ALK translocation; Table 4) [1,2,99] (Bishop, J.A. et al. pp. 31–51). These alterations accelerate tumorigenesis. For example, RET or MET acts on tumors with activating alterations as proto-oncogenes, such as point mutations or fusions. Therefore, these alterations are an easy therapeutic target. Alterations in MET and RET have been reported in 1.2% and 0.8% of non-small cell lung cancers, respectively [100,101,102]. Meanwhile, MET and RET inhibitors exhibit high efficacy rates and good tolerability [100,101,102,103]. Particularly for SGCs, the use of tyrosine kinase inhibitor (TKI)/TRK inhibitor is expected to be beneficial because these protein-coding gene alterations are detectable. Representative TKIs include those against vascular endothelial growth factor receptors (VEGFRs), fibroblast growth factor receptors (FGFRs), platelet-derived growth factor receptors (PDGFRs), etc. [104]. These TKIs are related to cell regulation and survival, as they influence angiogenesis and lymphangiogenesis. VEGFR inhibitors have been successfully used for the treatment of lung, stomach, liver, and kidney cancer [105]. Additionally, PDGFR inhibitors target gastrointestinal tumors, glioblastomas, sarcomas, leukemias, and dermatofibrosarcoma protuberans [106]. TRK is encoded by the NTRK gene family (NTRK1, NTRK2, and NTRK3) [107]. This proto-oncogene is responsible for cancer cell transformation, tumor cell proliferation, migration, and invasion. NTRK expression is detectable in approximately 90% of certain cancer types, including secretory breast carcinoma, secretory carcinoma in the salivary gland, and congenital infantile fibrosarcoma, while it is reported in less than 1% of common cancers such as non-small cell lung, colorectal, thyroid, and salivary gland cancers [108]. Currently, numerous protein inhibitors are being developed for various tumors and are expected to be effective against SGCs, as many carry genetic alterations associated with tumorigenesis [59,77,81,109,110,111]. Among SGCs, the overall response rates to protein inhibitors and disease control rates (0–46.2% and 59.7–100.0%, respectively) are similar to those reported in chemotherapy trials [112].
4.2.4. Tumor-Infiltrating Lymphocytes (TILs)/Immunotherapy-Related Proteins
SGCs have shown lymphocytic infiltrations with or without lymphoid follicles, and certain cases are called tumor-associated lymphoid proliferation (TALP) [113]. This may be misdiagnosed as lymph node metastasis by pathologists if they are not aware; nonetheless, its relationship with the prognosis remains unclear [113]. In addition, the special SGC type, lymphoepithelial carcinoma, presents non-keratinizing, poorly differentiated squamous-cell-like carcinoma with a predominant lymphoid stroma [114]. Most cases show Epstein–Barr virus (EBV) infection, and this type of tumor has a relatively good prognosis [115]. Moreover, in a tumor-immune environment, TILs are related to the prognosis of breast cancer [116]. Although reports are conflicting, the number of TILs is associated with a better prognosis. Moreover, the therapeutic effect is frequently correlated with the number of TILs and tumor mutation burden (TMB) in many tumors, such as malignant melanoma, colon cancer, pancreatic cancer, or biliary tract cancer [117,118,119,120]. TMB is the total number of DNA alterations in cancer cells and is measurable using NGS. Moreover, mismatch repair deficiency (dMMR) or microsatellite instability (MSI-H) causes high TMB; colorectal cancer includes two subtypes (Lynch syndrome and sporadic MSI-H cancer) [121]. Approximately 20% of colon cancer patients are MSI-H, and approximately 3% of patients with MSI-H colon cancer are diagnosed with Lynch syndrome [122]. The US Food and Drug Administration approved immune checkpoint inhibitors for metastatic MSI-H colon cancer and solid tumors with dMMR/MSI-H [123]. In addition, anticytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) agents and anti-PD-1 agent combination therapy have exhibited acceptable antitumor efficacy in MSI-H/dMMR metastatic colorectal cancers [123]. However, research on TILs related to global SGCs showed no significant difference in CD8+ TIL density between disease-free survival and overall survival [124]. This may be owing to the limitation of not treating the tumors individually or the low case numbers. Hence, further examination is required.
Programmed death (PD)-1/programmed death-ligand 1 (PD-L1) exists in the tumor microenvironment. Immunotherapy can kill tumor cells by activating antitumor immunity against tumor antigens. In particular, PD-1 is the most important receptor responsible for activating T cells and mediating immunosuppression. However, PD-L1 is also involved in PD-1-related pathways, leading to the induction of T-cell apoptosis or anergy [125]. The PD-1/PD-L1 pathway is the most notable checkpoint inhibitor pathway. Moreover, CTLA-4 is also the immune checkpoint target in clinical practice for many tumors [125,126]. When CTLA-4 translocates to the cell surface, CTLA-4 mediates inhibitory signaling into the T cell and arrests cell proliferation and activation [127]. Hence, anti-CTLA-4 agents are expected to exhibit beneficial therapeutic effects. In particular, a monoclonal antibody against CTLA-4 effectively amplifies immune stimulation and boosts tumor annihilation [128]. This antibody has been applied for the treatment of non-small or small cell lung cancer, renal cell carcinoma, urothelial carcinoma, pancreatic cancer, gastric cancer, and malignant melanoma [128]. However, limited data are available regarding the therapeutic potential of immune checkpoint inhibitors for SGCs, especially MEC and AdCC [129]. In particular, PD-L2 may be an important biomarker in SGCs (for example, MEC, AdCC, and SDC) [126,130].
4.2.5. Other Targetable Genes and Proteins
In addition, although rare, the following tumors have specific proteins or gene alterations involving tumorigenesis: (i) nuclear protein in testis (NUT); NUT carcinoma, (ii) subfamily of ATP-dependent chromatin remodeling complexes SWI/SNF (switch/sucrose non-fermentable); SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily B, member 1 (SMARCB1)-deficient high-grade transformed/dedifferentiated acinic cell carcinoma, (iii) BRAF; IPMN, (iv) neuroendocrine granules; small cell neuroendocrine carcinoma “Merkel type” (SNECM), large cell neuroendocrine carcinoma, and (v) salivary gland carcinoma with viral infection (human papillomavirus, EBV, polyomaviruses, and so on) [131,132,133,134,135,136,137,138,139,140,141,142]. These unique proteins or gene alterations might be related to tumorigenesis and may therefore represent novel therapeutic targets.
5. Conclusions
Herein, we discussed prognostic factors, focusing on the histopathological findings for SGCs, and described the current scenario and future perspectives. The interaction between clinicians and pathologists is essential since the pathological report includes many prognostic factors for patients and should be read carefully. Furthermore, genetics and molecular pathology are continuously advancing. Thus, novel information is continuously emerging, requiring further exploration.
Author Contributions
Conceptualization, H.N.; methodology, H.N.; investigation, T.K., K.K. and Y.O.; writing—original draft preparation, H.N.; writing—review and editing, T.K., K.K. and Y.O.; supervision, T.D.; project administration, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hyrcza, M.D.; Skalova, A.; Thompson, L.D.R.; Bishop, J.A.; Mehrotra, R. Introduction. WHO Classification of Tumours Edited by the WHO Classification of Tumours Editorial Board. Head and Neck Tumours; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/53 (accessed on 20 November 2022).
- Bishop, J.A.; Thompson, L.D.R.; Wakely, P.E., Jr.; Weinreb, I. AFIP Atlases of tumor and non-tumor pathology. In Tumors of the Salivary Glands; 5th Series Fascicle 5; American Registry of Pathology: Arlington, VA, USA, 2021. [Google Scholar]
- Seethala, R.R.; Altemani, A.; Ferris, R.L.; Fonseca, I.; Gnepp, D.R.; Ha, P.; Nagao, T.; Skalova, A.; Stenman, G.; Thompson, L.D.R. Data Set for the Reporting of Carcinomas of the Major Salivary Glands: Explanations and Recommendations of the Guidelines from the International Collaboration on Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H. Salivary Neoplasms: Overview of a 35-Year Experience with 2,807 Patients. Head Neck Surg. 1986, 8, 177–184. [Google Scholar] [CrossRef]
- Seethala, R.R. Histologic Grading and Prognostic Biomarkers in Salivary Gland Carcinomas. Adv. Anat. Pathol. 2011, 18, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Fu, X.; Sheng, Z. Dedifferentiation: A New Approach in Stem Cell Research. Bioscience 2007, 57, 655–662. [Google Scholar] [CrossRef]
- Mills, J.C.; Stanger, B.Z.; Sander, M. Nomenclature for Cellular Plasticity: Are the Terms as Plastic as the Cells Themselves? EMBO J. 2019, 38, e103148. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T. ‘Dedifferentiation’ and High-Grade Transformation in Salivary Gland Carcinomas. Head Neck Pathol. 2013, 7 (Suppl. 1), S37–S47. [Google Scholar] [CrossRef]
- Skalova, A.; Leivo, I.; Hellquist, H.; Agaimy, A.; Simpson, R.H.W.; Stenman, G.; Vander Poorten, V.; Bishop, J.A.; Franchi, A.; Hernandez-Prera, J.C.; et al. High-Grade Transformation/Dedifferentiation in Salivary Gland Carcinomas: Occurrence Across Subtypes and Clinical Significance. Adv. Anat. Pathol. 2021, 28, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Dei Tos, A.P.; Pedeutour, F.; Marino-Enriquez, A. Dedifferentiated Liposarcoma. In WHO Classification of Tumours; Soft Tissue and Bone Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/33/14 (accessed on 20 November 2022).
- Bovée, J.V.; Inwards, C.Y.; Hogendoorn, P.C.; Bloem, J.L. Dedifferentiated Chondrosarcoma. In WHO Classification of Tumours; Soft Tissue and Bone Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/33/144 (accessed on 20 November 2022).
- Thompson, L.D.; Aslam, M.N.; Stall, J.N.; Udager, A.M.; Chiosea, S.; McHugh, J.B. Clinicopathologic and Immunophenotypic Characterization of 25 Cases of Acinic Cell Carcinoma with High-Grade Transformation. Head Neck Pathol. 2016, 10, 152–160. [Google Scholar] [CrossRef]
- Van Weert, S.; Valstar, M.; Lissenberg-Witte, B.; Bloemena, E.; Smit, L.; van der Wal, J.; Vergeer, M.; Smeele, L.; Leemans, C.R. Prognostic Factors in Acinic Cell Carcinoma of the Head and Neck: The Amsterdam Experience. Oral Oncol. 2022, 125, 105698. [Google Scholar] [CrossRef]
- Lee, H.; Roh, J.L.; Choi, Y.J.; Choi, J.; Cho, K.J. High Grade Transformation in Mucoepidermoid Carcinoma of the Minor Salivary Gland with Polyploidy of the Rearranged MAML2 Gene. Head Neck Pathol. 2020, 14, 822–827. [Google Scholar] [CrossRef]
- Asai, S.; Sumiyoshi, S.; Yamada, Y.; Tateya, I.; Nagao, T.; Minamiguchi, S.; Haga, H. High-Grade Salivary Gland Carcinoma with the ETV6-NTRK3 Gene Fusion: A Case Report and Literature Review of Secretory Carcinoma with High-Grade Transformation. Pathol. Int. 2021, 71, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Wang, S.; Wei, J.; Yuan, J.; Liu, H. Clinicopathological and Molecular Study of 10 Salivary Gland Clear Cell Carcinomas, with Emphasis on Rare Cases with High Grade Transformation and Occurring in Uncommon Sites. Diagn. Pathol. 2022, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R.; Ohanessian, S.E.; Adil, E.; Crist, H.S.; Goldenberg, D.; Mani, H. Dedifferentiated Epithelial-Myoepithelial Carcinoma: Analysis of a Rare Entity Based on a Case Report and Literature Review. Int. J. Surg. Pathol. 2013, 21, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, I.; Nishida, T.; Miyauchi, M.; Sato, S.; Takata, T. Dedifferentiated Malignant Myoepithelioma of the Parotid Gland. Pathol. Int. 2003, 53, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Nagao, T.; Ide, F.; Takizawa, S.; Sakashita, H.; Tsujino, I.; Li, T.J.; Kusama, K. Palatal Polymorphous Adenocarcinoma with High-Grade Transformation: A Case Report and Literature Review. Head Neck Pathol. 2019, 13, 131–139. [Google Scholar] [CrossRef]
- Dutta, A.; Arun, P.; Arun, I. Adenoid Cystic Carcinoma with Transformation to High Grade Carcinomatous and Sarcomatoid Components: A Rare Case Report with Review of Literature. Head Neck Pathol. 2020, 14, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Hunt, J.L.; Baloch, Z.W.; Livolsi, V.A.; Leon Barnes, E. Adenoid Cystic Carcinoma with High-Grade Transformation: A Report of 11 Cases and a Review of the Literature. Am. J. Surg. Pathol. 2007, 31, 1683–1694. [Google Scholar] [CrossRef]
- Siriaunkgul, S.; Tavassoli, F.A. Invasive Micropapillary Carcinoma of the Breast. Mod. Pathol. 1993, 6, 660. [Google Scholar]
- Sangoi, A.R.; Beck, A.H.; Amin, M.B.; Cheng, L.; Epstein, J.I.; Hansel, D.E.; Iczkowski, K.A.; Lopez-Beltran, A.; Oliva, E.; Paner, G.P.; et al. Interobserver Reproducibility in the Diagnosis of Invasive Micropapillary Carcinoma of the Urinary Tract Among Urologic Pathologists. Am. J. Surg. Pathol. 2010, 34, 1367–1376. [Google Scholar] [CrossRef]
- Ohe, M.; Yokose, T.; Sakuma, Y.; Miyagi, Y.; Okamoto, N.; Osanai, S.; Hasegawa, C.; Nakayama, H.; Kameda, Y.; Yamada, K.; et al. Stromal Micropapillary Component as a Novel Unfavorable Prognostic Factor of Lung Adenocarcinoma. Diagn. Pathol. 2012, 7, 3. [Google Scholar] [CrossRef]
- Shimoda, M.; Okada, Y.; Hayashi, Y.; Hatano, S.; Kawakubo, H.; Omori, T.; Ishii, S.; Sugiura, H. Primary Invasive Micropapillary Carcinoma of the Stomach. Pathol. Int. 2008, 58, 513–517. [Google Scholar] [CrossRef]
- Kondo, T. Colon Invasive Micropapillary Carcinoma Arising in Tubulovillous Adenoma. Pol. J. Pathol. 2008, 59, 183–185. [Google Scholar]
- Kondo, T. Bile Duct Adenocarcinoma with Minor Micropapillary Component: A Case Report. Cases J. 2009, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Gaffey, T.A.; Visscher, D.W.; Kay, P.A.; Minato, H.; Serizawa, H.; Lewis, J.E. Invasive Micropapillary Salivary Duct Carcinoma: A Distinct Histologic Variant with Biologic Significance. Am. J. Surg. Pathol. 2004, 28, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zeng, M.; Chen, X. Intraductal Papillary Mucinous Neoplasm of the Minor Salivary Gland with Associated Invasive Micropapillary Carcinoma. Am. J. Surg. Pathol. 2019, 43, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. Major Salivary Glands. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; p. 95. [Google Scholar]
- Erovic, B.M.; Shah, M.D.; Bruch, G.; Johnston, M.; Kim, J.; O’Sullivan, B.; Perez-Ordonez, B.; Weinreb, I.; Atenafu, E.G.; de Almeida, J.R.; et al. Outcome Analysis of 215 Patients with Parotid Gland Tumors: A Retrospective Cohort Analysis. J. Otolaryngol. Head Neck Surg. 2015, 44, 43. [Google Scholar] [CrossRef]
- Hosni, A.; Huang, S.H.; Goldstein, D.; Xu, W.; Chan, B.; Hansen, A.; Weinreb, I.; Bratman, S.V.; Cho, J.; Giuliani, M.; et al. Outcomes and Prognostic Factors for Major Salivary Gland Carcinoma Following Postoperative Radiotherapy. Oral Oncol. 2016, 54, 75–80. [Google Scholar] [CrossRef]
- Lombardi, D.; Tomasoni, M.; Paderno, A.; Mattavelli, D.; Ferrari, M.; Battocchio, S.; Missale, F.; Mazzola, F.; Peretti, G.; Mocellin, D.; et al. The Impact of Nodal Status in Major Salivary Gland Carcinoma: A Multicenter Experience and Proposal of a Novel N-Classification. Oral Oncol. 2021, 112, 105076. [Google Scholar] [CrossRef]
- Mikoshiba, T.; Ozawa, H.; Watanabe, Y.; Kawaida, M.; Sekimizu, M.; Saito, S.; Yoshihama, K.; Nakamura, S.; Nagai, R.; Imanishi, Y.; et al. Pretherapeutic Predictive Factors for Histological High-Grade Parotid Gland Carcinoma. Laryngoscope 2022, 132, 96–102. [Google Scholar] [CrossRef]
- Ali, S.; Palmer, F.L.; Yu, C.; DiLorenzo, M.; Shah, J.P.; Kattan, M.W.; Patel, S.G.; Ganly, I. A Predictive Nomogram for Recurrence of Carcinoma of the Major Salivary Glands. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 698–705. [Google Scholar] [CrossRef]
- Bjørndal, K.; Krogdahl, A.; Therkildsen, M.H.; Overgaard, J.; Johansen, J.; Kristensen, C.A.; Homøe, P.; Sørensen, C.H.; Andersen, E.; Bundgaard, T.; et al. Salivary Gland Carcinoma in Denmark 1990–2005: Outcome and Prognostic Factors. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. 2012, 48, 179–185. [Google Scholar] [CrossRef]
- Therkildsen, M.H.; Christensen, M.; Andersen, L.J.; Schiødt, T.; Hansen, H.S. Salivary Gland Carcinomas--Prognostic Factors. Acta Oncol. 1998, 37, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.J. Frequency and Histopathology by Site, Major Pathologies, Symptoms and Signs of Salivary Gland Neoplasms. Adv. Otorhinolaryngol. 2016, 78, 9–16. [Google Scholar] [CrossRef]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Leivo, I.; Bishop, J.A.; Vielh, P.; Inagaki, H.; Cipriani, N.A.; Costes-Martineau, V. Mucoepidermoid Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/77 (accessed on 20 November 2022).
- Auclair, P.L.; Goode, R.K.; Ellis, G.L. Mucoepidermoid Carcinoma of Intraoral Salivary Glands. Evaluation and Application of Grading Criteria in 143 Cases. Cancer. 1992, 69, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.K.; Auclair, P.L.; Ellis, G.L. Mucoepidermoid Carcinoma of the Major Salivary Glands: Clinical and Histopathologic Analysis of 234 Cases with Evaluation of Grading Criteria. Cancer 1998, 82, 1217–1224. [Google Scholar] [CrossRef]
- Brandwein, M.S.; Ivanov, K.; Wallace, D.I.; Hille, J.J.; Wang, B.; Fahmy, A.; Bodian, C.; Urken, M.L.; Gnepp, D.R.; Huvos, A.; et al. Mucoepidermoid Carcinoma: A Clinicopathologic Study of 80 Patients with Special Reference to Histological Grading. Am. J. Surg. Pathol. 2001, 25, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Ghossein, R.; Ali, S.; Dogan, S.; Klimstra, D.; Ganly, I. Prognostic Features in Mucoepidermoid Carcinoma of Major Salivary Glands with Emphasis on Tumour Histologic Grading. Histopathology 2014, 65, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Inagaki, H.; Faquin, W.C.; Stenman, G.; Urano, M. Adenoid Cystic Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/78 (accessed on 24 November 2022).
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic Grading of Adenoid Cystic Carcinoma of the Salivary Glands. Cancer. 1984, 54, 1062–1069. [Google Scholar] [CrossRef]
- Seethala, R.R. An Update on Grading of Salivary Gland Carcinomas. Head Neck Pathol. 2009, 3, 69–77. [Google Scholar] [CrossRef]
- van Weert, S.; van der Waal, I.; Witte, B.I.; Leemans, C.R.; Bloemena, E. Histopathological Grading of Adenoid Cystic Carcinoma of the Head and Neck: Analysis of Currently Used Grading Systems and Proposal for a Simplified Grading Scheme. Oral Oncol. 2015, 51, 71–76. [Google Scholar] [CrossRef]
- Morita, N.; Murase, T.; Ueda, K.; Nagao, T.; Kusafuka, K.; Nakaguro, M.; Urano, M.; Taguchi, K.I.; Yamamoto, H.; Kano, S.; et al. Pathological Evaluation of Tumor Grade for Salivary Adenoid Cystic Carcinoma: A Proposal of an Objective Grading System. Cancer Sci. 2021, 112, 1184–1195. [Google Scholar] [CrossRef]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Ihrler, S.; Bishop, J.A. Salivary Carcinoma, NOS and Emerging Entities. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/84 (accessed on 20 November 2022).
- Batsakis, J.G.; El-Naggar, A.K.; Luna, M.A. ‘Adenocarcinoma, not otherwise specified’: A Diminishing Group of Salivary Carcinomas. Ann. Otol. Rhinol. Laryngol. 1992, 101, 102–104. [Google Scholar] [CrossRef]
- Nagao, K.; Matsuzaki, O.; Saiga, H.; Sugano, I.; Kaneko, T.; Katoh, T.; Kitamura, T.; Shigematsu, H.; Maruyama, N. Histopathologic Studies on Adenocarcinoma of the Parotid Gland. Acta Pathol. Jpn. 1986, 36, 337–347. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Huang, A.T.; Khaja, S.F.; Bell, D.; Day, T.A. Predictors of Survival in Parotid Adenocarcinoma Not Otherwise Specified: A National Cancer Database Study of 3155 Patients. Head Neck. 2016, 38, 1208–1212. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.Y.; Nelson, M.; Li, L.; Hu, Y.; Urken, M.L.; Brandwein-Gensler, M. Salivary Adenocarcinoma, Not Otherwise Specified: A Collection of Orphans. Arch. Pathol. Lab. Med. 2004, 128, 1385–1394. [Google Scholar] [CrossRef]
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenocarcinoma of Salivary Origin. Clinicopathologic Study of 204 Patients. Am. J. Surg. 1982, 144, 423–431. [Google Scholar] [CrossRef]
- Blanck, C.; Eneroth, C.M.; Jakobsson, P.A. Mucus-Producing Adenopapillary (Non-epidermoid) Carcinoma of the Parotid Gland. Cancer. 1971, 28, 676–685. [Google Scholar] [CrossRef]
- Wang, K.; Russell, J.S.; McDermott, J.D.; Elvin, J.A.; Khaira, D.; Johnson, A.; Jennings, T.A.; Ali, S.M.; Murray, M.; Marshall, C.; et al. Profiling of 149 Salivary Duct Carcinomas, Carcinoma Ex Pleomorphic Adenomas, and Adenocarcinomas, Not Otherwise Specified Reveals Actionable Genomic Alterations. Clin. Cancer Res. 2016, 22, 6061–6068. [Google Scholar] [CrossRef]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Katabi, N.; Chiosea, S.; Fonseca, I.; Ihrler, S.; Klijanienko, J.; Altemani, A. Carcinoma Ex Pleomorphic Adenoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/88 (accessed on 25 November 2022).
- Kwon, M.Y.; Gu, M. True Malignant Mixed Tumor (Carcinosarcoma) of Parotid Gland with Unusual Mesenchymal Component: A Case Report and Review of the Literature. Arch. Pathol. Lab. Med. 2001, 125, 812–815. [Google Scholar] [CrossRef]
- Ihrler, S.; Stiefel, D.; Jurmeister, P.; Sandison, A.; Chaston, N.; Laco, J.; Zidar, N.; Brcic, L.; Stoehr, R.; Agaimy, A. Salivary Carcinosarcoma: Insight into Multistep Pathogenesis Indicates Uniform Origin as Sarcomatoid Variant of Carcinoma Ex Pleomorphic Adenoma with Frequent Heterologous Elements. Histopathology 2023, 82, 576–586. [Google Scholar] [CrossRef]
- Lewis, J.E.; Olsen, K.D.; Sebo, T.J. Carcinoma Ex Pleomorphic Adenoma: Pathologic Analysis of 73 Cases. Hum. Pathol. 2001, 32, 596–604. [Google Scholar] [CrossRef]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Bishop, J.A.; Thompson, L.D.R.; Agaimy, A.; Nagao, T.; Weinreb, I. Intraductal Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/83 (accessed on 20 November 2022).
- Cheuk, W.; Miliauskas, J.R.; Chan, J.K. Intraductal Carcinoma of the Oral Cavity: A Case Report and a Reappraisal of the Concept of Pure Ductal Carcinoma in Situ in Salivary Duct Carcinoma. Am. J. Surg. Pathol. 2004, 28, 266–270. [Google Scholar] [CrossRef]
- Skalova, A.; Ptáková, N.; Santana, T.; Agaimy, A.; Ihrler, S.; Uro-Coste, E.; Thompson, L.D.R.; Bishop, J.A.; Baněčkova, M.; Rupp, N.J.; et al. NCOA4-RET and TRIM27-RET Are Characteristic Gene Fusions in Salivary Intraductal Carcinoma, Including Invasive and Metastatic Tumors: Is “Intraductal” Correct? Am. J. Surg. Pathol. 2019, 43, 1303–1313. [Google Scholar] [CrossRef]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Hernandez-Prera, J.C.; Faquin, W.C.; Ihrler, S.; Katabi, N.; Weinreb, I.; Altemani, A.; Machado de Sousa, S.O.; et al. Pleomorphic Adenoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/63 (accessed on 25 November 2022).
- Wenig, B.M.; Hitchcock, C.L.; Ellis, G.L.; Gnepp, D.R. Metastasizing Mixed Tumor of Salivary Glands. A Clinicopathologic and Flow Cytometric Analysis. Am. J. Surg. Pathol. 1992, 16, 845–858. [Google Scholar] [CrossRef]
- Knight, J.; Ratnasingham, K. Metastasising Pleomorphic Adenoma: Systematic Review. Int. J. Surg. 2015, 19, 137–145. [Google Scholar] [CrossRef]
- Wasserman, J.K.; Dickson, B.C.; Smith, A.; Swanson, D.; Purgina, B.M.; Weinreb, I. Metastasizing Pleomorphic Adenoma: Recurrent PLAG1/HMGA2 Rearrangements and Identification of a Novel HMGA2-TMTC2 Fusion. Am. J. Surg. Pathol. 2019, 43, 1145–1151. [Google Scholar] [CrossRef]
- Kanatas, A.; Ho, M.W.S.; Mücke, T. Current Thinking About the Management of Recurrent Pleomorphic Adenoma of the Parotid: A Structured Review. Br. J. Oral Maxillofac. Surg. 2018, 56, 243–248. [Google Scholar] [CrossRef]
- Malard, O.; Thariat, J.; Cartier, C.; Chevalier, D.; Courtade-Saidi, M.; Uro-Coste, E.; Garrel, R.; Kennel, T.; Mogultay, P.; Tronche, S.; et al. Guidelines of the French Society of Otorhinolaryngology-Head and Neck Surgery (SFORL), part II: Management of Recurrent Pleomorphic Adenoma of the Parotid Gland. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 45–49. [Google Scholar] [CrossRef]
- Witt, R.L.; Eisele, D.W.; Morton, R.P.; Nicolai, P.; Poorten, V.V.; Zbären, P. Etiology and Management of Recurrent Parotid Pleomorphic Adenoma. Laryngoscope 2015, 125, 888–893. [Google Scholar] [CrossRef]
- Guerra, G.; Testa, D.; Montagnani, S.; Tafuri, D.; Salzano, F.A.; Rocca, A.; Amato, B.; Salzano, G.; Dell’Aversana Orabona, G.; Piombino, P.; et al. Surgical Management of Pleomorphic Adenoma of Parotid Gland in Elderly Patients: Role of Morphological Features. Int. J. Surg. 2014, 12 (Suppl. 2), S12–S16. [Google Scholar] [CrossRef]
- Sama, S.; Komiya, T.; Guddati, A.K. Advances in the Treatment of Mucoepidermoid Carcinoma. World J. Oncol. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Yin, L.X.; Ha, P.K. Genetic Alterations in Salivary Gland Cancers. Cancer 2016, 122, 1822–1831. [Google Scholar] [CrossRef]
- Andersson, M.K.; Stenman, G. The Landscape of Gene Fusions and Somatic Mutations in Salivary Gland Neoplasms—Implications for Diagnosis and Therapy. Oral Oncol. 2016, 57, 63–69. [Google Scholar] [CrossRef]
- Yousaf, A.; Sulong, S.; Abdullah, B.; Lazim, N.M. Heterogeneity of Genetic Landscapes in Salivary Gland Tumors and Their Critical Roles in Current Management. Medeni. Med. J. 2022, 37, 194–202. [Google Scholar] [CrossRef]
- Gargano, S.M.; Senarathne, W.; Feldman, R.; Florento, E.; Stafford, P.; Swensen, J.; Vranic, S.; Gatalica, Z. Novel Therapeutic Targets in Salivary Duct Carcinoma Uncovered by Comprehensive Molecular Profiling. Cancer Med. 2019, 8, 7322–7329. [Google Scholar] [CrossRef]
- Lassche, G.; van Helvert, S.; Eijkelenboom, A.; Tjan, M.J.H.; Jansen, E.A.M.; van Cleef, P.H.J.; Verhaegh, G.W.; Kamping, E.J.; Grünberg, K.; van Engen-van Grunsven, A.C.H.; et al. Identification of Fusion Genes and Targets for Genetically Matched Therapies in a Large Cohort of Salivary Gland Cancer Patients. Cancers 2022, 14, 4156. [Google Scholar] [CrossRef]
- Moore, A.; Bar, Y.; Maurice-Dror, C.; Ospovat, I.; Sarfaty, M.; Korzets, Y.; Goldvaser, H.; Gordon, N.; Billan, S.; Gutfeld, O.; et al. Next-Generation Sequencing in Salivary Gland Carcinoma: Targetable Alterations Lead to a Therapeutic Advantage-Multicenter Experience. Head Neck 2020, 42, 599–607. [Google Scholar] [CrossRef]
- Glisson, B.; Colevas, A.D.; Haddad, R.; Krane, J.; El-Naggar, A.; Kies, M.; Costello, R.; Summey, C.; Arquette, M.; Langer, C.; et al. HER2 Expression in Salivary Gland Carcinomas: Dependence on Histological Subtype. Clin. Cancer Res. 2004, 10, 944–946. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, S.; Zhou, L.; Yin, W.; Lin, Y.; Du, Y.; Wang, Y.; Jiang, Y.; Yin, K.; Zhang, J.; et al. Clinical Significance of Quantitative HER2 Gene Amplification as Related to Its Predictive Value in Breast Cancer Patients in Neoadjuvant Setting. Onco Targets Ther. 2018, 11, 801–808. [Google Scholar] [CrossRef]
- Coutzac, C.; Funk-Debleds, P.; Cattey-Javouhey, A.; Desseigne, F.; Guibert, P.; Marolleau, P.; Rochefort, P.; de la Fouchardière, C. Targeting HER2 in Metastatic Gastroesophageal Adenocarcinomas: What Is New? Bull. Cancer 2022, in press. [Google Scholar] [CrossRef]
- Strickler, J.H.; Yoshino, T.; Graham, R.P.; Siena, S.; Bekaii-Saab, T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol. 2022, 8, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Quan, R.; Han, L. Trastuzumab-Based Therapy Is Effective for Salivary Duct Carcinoma: Case Report and Review of the Literature. Oral Oncol. 2019, 91, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2-Positive Salivary Duct Carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef]
- Giridhar, P.; Venkatesulu, B.P.; Yoo, R.; Pragathee, V.; Rath, G.K.; Mallick, S.; Upadhyay, A.; Chan, D.P. Demography, Patterns of Care, and Survival Outcomes in Patients with Salivary Duct Carcinoma: An Individual Patient Data Analysis of 857 Patients. Future Sci. OA 2022, 8, FSO791. [Google Scholar] [CrossRef]
- Patelli, G.; Zeppellini, A.; Spina, F.; Righetti, E.; Stabile, S.; Amatu, A.; Tosi, F.; Ghezzi, S.; Siena, S.; Sartore-Bianchi, A. The Evolving Panorama of HER2-Targeted Treatments in Metastatic Urothelial Cancer: A Systematic Review and Future Perspectives. Cancer Treat. Rev. 2022, 104, 102351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, H.; Wang, Y.; Zhang, Z.; Cao, G.; Song, T.; Zhang, T.; Li, Q. Systemic Treatment of Advanced or Recurrent Biliary Tract Cancer. BioSci. Trends. 2020, 14, 328–341. [Google Scholar] [CrossRef]
- Mueller, S.K.; Haderlein, M.; Lettmaier, S.; Agaimy, A.; Haller, F.; Hecht, M.; Fietkau, R.; Iro, H.; Mantsopoulos, K. Targeted Therapy, Chemotherapy, Immunotherapy and Novel Treatment Options for Different Subtypes of Salivary Gland Cancer. J. Clin. Med. 2022, 11, 720. [Google Scholar] [CrossRef]
- Javaheripour, A.; Saatloo, M.V.; Vahed, N.; Gavgani, L.F.; Kouhsoltani, M. Evaluation of HER2/neu Expression in Different Types of Salivary Gland Tumors: A Systematic Review and Meta-analysis. J. Med. Life. 2022, 15, 595–600. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Kalmuk, J.; Rinder, D.; Heltzel, C.; Lockhart, A.C. An Overview of the Preclinical Discovery and Development of Trastuzumab Deruxtecan: A Novel Gastric Cancer Therapeutic. Expert Opin. Drug Discov. 2022, 17, 427–436. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter Phase II Trial of Trastuzumab Deruxtecan for HER2-Positive Unresectable or Recurrent Biliary Tract Cancer: HERB Trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Nishida, H.; Oyama, Y.; Kusaba, T.; Kadowaki, H.; Arakane, M.; Wada, J.; Urabe, S.; Daa, T. Immunohistochemical Reactivity of Prostate-Specific Markers for Salivary Duct Carcinoma. Pathobiology 2020, 87, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, I.; Stephan, C.; Jung, K.; Dietel, M.; Rieger, A.; Tolkach, Y.; Kristiansen, G. Sensitivity of HOXB13 as a Diagnostic Immunohistochemical Marker of Prostatic Origin in Prostate Cancer Metastases: Comparison to PSA, Prostein, Androgen Receptor, ERG, NKX3.1, PSAP, and PSMA. Int. J. Mol. Sci. 2017, 18, 1151. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen Deprivation Therapy for Prostate Cancer. JAMA. 2005, 294, 238–244. [Google Scholar] [CrossRef]
- Kawakita, D.; Nagao, T.; Takahashi, H.; Kano, S.; Honma, Y.; Hirai, H.; Saigusa, N.; Akazawa, K.; Tani, K.; Ojiri, H.; et al. Survival Benefit of HER2-Targeted or Androgen Deprivation Therapy in Salivary Duct Carcinoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221119538. [Google Scholar] [CrossRef]
- Mitani, Y.; Rao, P.H.; Maity, S.N.; Lee, Y.C.; Ferrarotto, R.; Post, J.C.; Licitra, L.; Lippman, S.M.; Kies, M.S.; Weber, R.S.; et al. Alterations Associated with Androgen Receptor Gene Activation in Salivary Duct Carcinoma of Both Sexes: Potential Therapeutic Ramifications. Clin. Cancer Res. 2014, 20, 6570–6581. [Google Scholar] [CrossRef]
- Otsuka, K.; Imanishi, Y.; Tada, Y.; Kawakita, D.; Kano, S.; Tsukahara, K.; Shimizu, A.; Ozawa, H.; Okami, K.; Sakai, A.; et al. Clinical Outcomes and Prognostic Factors for Salivary Duct Carcinoma: A Multi-institutional Analysis of 141 Patients. Ann. Surg. Oncol. 2016, 23, 2038–2045. [Google Scholar] [CrossRef]
- Martínez-Jiménez, F.; Muiños, F.; Sentís, I.; Deu-Pons, J.; Reyes-Salazar, I.; Arnedo-Pac, C.; Mularoni, L.; Pich, O.; Bonet, J.; Kranas, H.; et al. A Compendium of Mutational Cancer Driver Genes. Nat. Rev. Cancer. 2020, 20, 555–572. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Chen, S.; Eldredge, J.B.; Schokrpur, S.; Li, D.; Quan, Z.; Chan, J.W.; Cummings, A.L.; Daly, M.E.; Goldman, J.W.; et al. The Role of Chemotherapy Plus Immune Checkpoint Inhibitors in Oncogenic-Driven NSCLC: A University of California Lung Cancer Consortium Retrospective Study. JTO Clin. Res. Rep. 2022, 3, 100427. [Google Scholar] [CrossRef]
- Addeo, A.; Miranda, E.; den Hollander, P.; Friedlaender, A.; Sintim, H.; Wu, J.; Mani, S.A.; Subbiah, V. RET Aberrant Cancers and RET Inhibitor Therapies: Current State-of-the-Art and Future Perspectives. Pharmacol. Ther. 2023, 242, 108344. [Google Scholar] [CrossRef]
- Olmedo, M.E.; Cervera, R.; Cabezon-Gutierrez, L.; Lage, Y.; Corral de la Fuente, E.; Gómez Rueda, A.; Mielgo-Rubio, X.; Trujillo, J.C.; Couñago, F. New Horizons for Uncommon Mutations in Non-small Cell Lung Cancer: BRAF, KRAS, RET, MET, NTRK, HER2. World J. Clin. Oncol. 2022, 13, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Girard, N. New Strategies and Novel Combinations in EGFR TKI-Resistant Non-small Cell Lung Cancer. Curr. Treat. Options Oncol. 2022, 23, 1626–1644. [Google Scholar] [CrossRef]
- Lee, R.H.; Wai, K.C.; Chan, J.W.; Ha, P.K.; Kang, H. Approaches to the Management of Metastatic Adenoid Cystic Carcinoma. Cancers 2022, 14, 5698. [Google Scholar] [CrossRef] [PubMed]
- Elebiyo, T.C.; Rotimi, D.; Evbuomwan, I.O.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.M.; Adeyemi, O.S. Reassessing Vascular Endothelial Growth Factor (VEGF) in Anti-angiogenic Cancer Therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Lennartsson, J. The PDGF/PDGFR Pathway as a Drug Target. Mol. Aspects Med. 2018, 62, 75–88. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Riondino, S.; Sganga, S.; Rosenfeld, R.; Guerriero, S.; Carlucci, M.; Capotondi, B.; Torino, F.; Roselli, M. NTRK Gene Fusions in Solid Tumors and TRK Inhibitors: A Systematic Review of Case Reports and Case Series. J. Pers. Med. 2022, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Csanyi-Bastien, M.; Lanic, M.D.; Beaussire, L.; Ferric, S.; François, A.; Meseure, D.; Jardin, F.; Wassef, M.; Ruminy, P.; Laé, M. Pan-TRK Immunohistochemistry Is Highly Correlated with NTRK3 Gene Rearrangements in Salivary Gland Tumors. Am J Surg Pathol. 2021, 45, 1487–1498. [Google Scholar] [CrossRef]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Corrigendum: Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef]
- Ernst, M.S.; Lysack, J.T.; Hyrcza, M.D.; Chandarana, S.P.; Hao, D. TRK Inhibition with Entrectinib in Metastatic Salivary Secretory Carcinoma (SC): A Case Report. Curr. Oncol. 2022, 29, 3933–3939. [Google Scholar] [CrossRef]
- Kacew, A.J.; Hanna, G.J. Systemic and Targeted Therapies in Adenoid Cystic Carcinoma. Curr. Treat. Options Oncol. 2023, 24, 45–60. [Google Scholar] [CrossRef]
- Kurian, E.M.; Miller, R.; Mclean-Holden, A.L.; Oliai, B.R.; Bishop, J.A. Low Molecular Weight Cytokeratin Immunostaining for Extrafollicular Reticulum Cells Is an Effective Means of Separating Salivary Gland Tumor-Associated Lymphoid Proliferation from True Lymph Node Involvement. Head Neck Pathol. 2020, 14, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Seethala, R.; Thompson, L.D.R.; Wenig, B.M.; Nagao, T.; Whaley, R.D. Lymphoepithelial Carcinoma. In WHO Classification of Tumours; Head and Neck Tumours; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2022; Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/93 (accessed on 29 November 2022).
- Wenig, B.M. Lymphoepithelial-Like Carcinomas of the Head and Neck. Semin. Diagn. Pathol. 2015, 32, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Caro, C.A.; Ramirez, M.A.; Gonzalez-Torres, H.J.; Sanabria-Salas, M.C.; Serrano-Gómez, S.J. Immune Lymphocyte Infiltrate and Its Prognostic Value in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 910976. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, Y.; Huang, Z.; Li, X. Identification of Prognostic Biomarkers of Cutaneous Melanoma Based on Analysis of Tumor Mutation Burden. Comput. Math. Methods Med. 2020, 2020, 8836493. [Google Scholar] [CrossRef] [PubMed]
- Keshinro, A.; Vanderbilt, C.; Kim, J.K.; Firat, C.; Chen, C.T.; Yaeger, R.; Ganesh, K.; Segal, N.H.; Gonen, M.; Shia, J.; et al. Tumor-Infiltrating Lymphocytes, Tumor Mutational Burden, and Genetic Alterations in Microsatellite Unstable, Microsatellite Stable, or Mutant POLE/POLD1 Colon Cancer. JCO Precis. Oncol. 2021, 5, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Karamitopoulou, E.; Andreou, A.; Wenning, A.S.; Gloor, B.; Perren, A. High Tumor Mutational Burden (TMB) Identifies a Microsatellite Stable Pancreatic Cancer Subset with Prolonged Survival and Strong Anti-tumor Immunity. Eur. J. Cancer. 2022, 169, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef]
- Yamaura, T.; Miyoshi, H.; Maekawa, H.; Morimoto, T.; Yamamoto, T.; Kakizaki, F.; Higasa, K.; Kawada, K.; Matsuda, F.; Sakai, Y.; et al. Accurate Diagnosis of Mismatch Repair Deficiency in Colorectal Cancer Using High-Quality DNA Samples from Cultured Stem Cells. Oncotarget 2018, 9, 37534–37548. [Google Scholar] [CrossRef]
- Li, S.K.H.; Martin, A. Mismatch Repair and Colon Cancer: Mechanisms and Therapies Explored. Trends Mol. Med. 2016, 22, 274–289. [Google Scholar] [CrossRef]
- Hou, W.; Yi, C.; Zhu, H. Predictive Biomarkers of Colon Cancer Immunotherapy: Present and Future. Front. Immunol. 2022, 13, 1032314. [Google Scholar] [CrossRef]
- Sato, F.; Ono, T.; Kawahara, A.; Matsuo, K.; Kondo, R.; Sato, K.; Akiba, J.; Kawaguchi, T.; Kakuma, T.; Chitose, S.I.; et al. Prognostic Value of Tumor Proportion Score in Salivary Gland Carcinoma. Laryngoscope 2021, 131, E1481–E1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, G.; Zhang, X.; Gao, J.; Meng, C.; Liu, Y.; Wei, Q.; Sun, L.; Wei, P.; Bai, Z.; et al. Current Progress and Future Perspectives of Neoadjuvant Anti-PD-1/PD-L1 Therapy for Colorectal Cancer. Front. Immunol. 2022, 13, 1001444. [Google Scholar] [CrossRef]
- Mosconi, C.; de Arruda, J.A.A.; de Farias, A.C.R.; Oliveira, G.A.Q.; de Paula, H.M.; Fonseca, F.P.; Mesquita, R.A.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immune Microenvironment and Evasion Mechanisms in Adenoid Cystic Carcinomas of Salivary Glands. Oral Oncol. 2019, 88, 95–101. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Karimi, A.; Alilou, S.; Mirzaei, H.R. Adverse Events Following Administration of Anti-CTLA4 Antibody Ipilimumab. Front. Oncol. 2021, 11, 624780. [Google Scholar] [CrossRef]
- Gerdabi, S.; Asadian, F.; Kiani, R.; Khademi, B.; Haghshenas, M.R.; Erfani, N. Simultaneous Expression of PD-1 and PD-L1 in Peripheral and Central Immune Cells and Tumor Cells in the Benign and Malignant Salivary Gland Tumors Microenvironment. Head Neck Pathol. 2022, 1–15. [Google Scholar] [CrossRef]
- Chang, H.; Kim, J.S.; Choi, Y.J.; Cho, J.G.; Woo, J.S.; Kim, A.; Kim, J.S.; Kang, E.J. Overexpression of PD-L2 Is Associated with Shorter Relapse-Free Survival in Patients with Malignant Salivary Gland Tumors. Onco Targets Ther. 2017, 10, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Fonseca, I.; Martins, C.; Thway, K.; Barrette, R.; Harrington, K.J.; Hartmann, A.; French, C.A.; Fisher, C. NUT Carcinoma of the Salivary Glands: Clinicopathologic and Molecular Analysis of 3 Cases and a Survey of NUT Expression in Salivary Gland Carcinomas. Am. J. Surg. Pathol. 2018, 42, 877–884. [Google Scholar] [CrossRef]
- Maghami, E.; Afkhami, M.; Villaflor, V.; Bell, D. Heterotopic SMARCB1-Deficient High-Grade Transformed/Dedifferentiated Acinic Cell Carcinoma and Sine-Qua-Non Radiology- Pathology with TNM Challenge. Ann. Diagn. Pathol. 2022, 57, 151900. [Google Scholar] [CrossRef]
- Lam-Ubol, A.; Phattarataratip, E. Distinct Histone H3 Modification Profiles Correlate with Aggressive Characteristics of Salivary Gland Neoplasms. Sci. Rep. 2022, 12, 15063. [Google Scholar] [CrossRef]
- Nakaguro, M.; Urano, M.; Ogawa, I.; Hirai, H.; Yamamoto, Y.; Yamaguchi, H.; Tanigawa, M.; Matsubayashi, J.; Hirano, H.; Shibahara, J.; et al. Histopathological Evaluation of Minor Salivary Gland Papillary-Cystic Tumours: Focus on Genetic Alterations in Sialadenoma Papilliferum and Intraductal Papillary Mucinous Neoplasm. Histopathology 2020, 76, 411–422. [Google Scholar] [CrossRef]
- Mete, O.; Wenig, B.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head Neck Pathol. 2022, 16, 123–142. [Google Scholar] [CrossRef]
- Chernock, R.D.; Duncavage, E.J. Proceedings of the NASHNP Companion Meeting, March 18th, 2018, Vancouver, BC, Canada: Salivary Neuroendocrine Carcinoma-an Overview of a Rare Disease with an Emphasis on Determining Tumor Origin. Head Neck Pathol. 2018, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, D.; Accorona, R.; Ungari, M.; Melocchi, L.; Bell, D.; Nicolai, P. Primary Merkel Cell Carcinoma of the Submandibular Gland: When CK20 Status Complicates the Diagnosis. Head Neck Pathol. 2015, 9, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Oh, J.; Bukhari, H.; Ng, T.; Chau, N.; Tran, E. Primary Parotid Merkel Type Small Cell Neuroendocrine Carcinoma with Oligometastasis to the Brain and Adrenal Gland: Case Report and Review of Literature. Head Neck Pathol. 2021, 15, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Gaffey, T.A.; Olsen, K.D.; Serizawa, H.; Lewis, J.E. Small Cell Carcinoma of the Major Salivary Glands: Clinicopathologic Study with Emphasis on Cytokeratin 20 Immunoreactivity and Clinical Outcome. Am. J. Surg. Pathol. 2004, 28, 762–770. [Google Scholar] [CrossRef]
- Mascitti, M.; Luconi, E.; Togni, L.; Rubini, C. Large Cell Neuroendocrine Carcinoma of the Submandibular Gland: A Case Report and Literature Review. Pathologica 2019, 111, 70–75. [Google Scholar] [CrossRef]
- Ramqvist, T.; Ursu, R.G.; Haeggblom, L.; Mirzaie, L.; Gahm, C.; Hammarstedt-Nordenvall, L.; Dalianis, T.; Näsman, A. Human Polyomaviruses Are Not Frequently Present in Cancer of the Salivary Glands. Anticancer Res. 2018, 38, 2871–2874. [Google Scholar] [CrossRef]
- Zupancic, M.; Holzhauser, S.; Cheng, L.; Ramqvist, T.; Du, J.; Friesland, S.; Näsman, A.; Dalianis, T. Analysis of Human Papillomavirus (HPV) and Polyomaviruses (HPyVs) in Adenoid Cystic Carcinoma (AdCC) of the Head and Neck Region Reveals Three HPV-Positive Cases with Adenoid Cystic-Like Features. Viruses 2022, 14, 1040. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).