microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer
Abstract
Simple Summary
Abstract
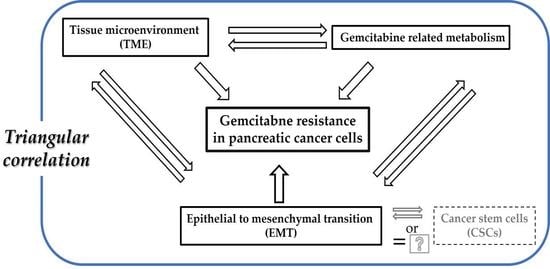
1. Introduction
2. Diverse Functions of miRNAs
3. Gemcitabine Resistance in Pancreatic Cancer
4. EMT-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
4.1. EMT and Gemcitabine Resistance
4.2. miRNAs Related to Gemcitabine and EMT
4.2.1. miRNAs Associated with Gemcitabine Sensitivity
4.2.2. miRNAs Associated with Gemcitabine Resistance
| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Li Y | [74] | let-7 | NA |
| Cioffi M | [62] | miR-17 | NODAL |
| Wang T | [72] | miR-30a | SNAI1 |
| Ji Q | [63] | miR-34 | Bcl-2 |
| Liu G | [75] | miR-125a | Fyn |
| Liu F | [80] | miR-153 | Snail |
| Bai Z | [81] | miR-153 | Snail |
| Wang Z | [82] | miR-183 | ZEB1 |
| Li Y | [74] | miR-200 | NA |
| Funamizu N | [55] | miR-200b | ZEB1 |
| Wang Z | [82] | miR-200b | ZEB1 |
| Ma C | [94] | miR-200c | NA |
| Chaudhary AK | [83] | miR-205 | TUBB3 |
| Yu Q | [93] | miR-497 | NFKB1 |
| Fu X | [76] | miR-506 | NEAT1 |
| Hiramoto H | [78] | miR-509 | NA |
| Yu S | [92] | miR-1206 | ESRP1 |
| Hiramoto H | [78] | miR-1243 | NA |
| Yang RM | [79] | miR-3656 | EMT related genes |
| Resistance | |||
| Xiong G | [86] | miR-10a | TFAP2C |
| Zhang WL | [87] | miR-15b | SMURF2 |
| Yang Z | [84] | miR-210 | Rapamycin |
| Ma J | [91] | miR-223 | FBXW7 |
| Okazaki J | [90] | miR-296 | BOK |
| Zhang KD | [89] | miR-301 | TP63 |
| Funamizu N | [54] | miR-301b | TP63 |
| Yang S | [88] | miR-301b | NR3C2 |
| Hasegawa S | [85] | miR-1246 | CyclinG2 |
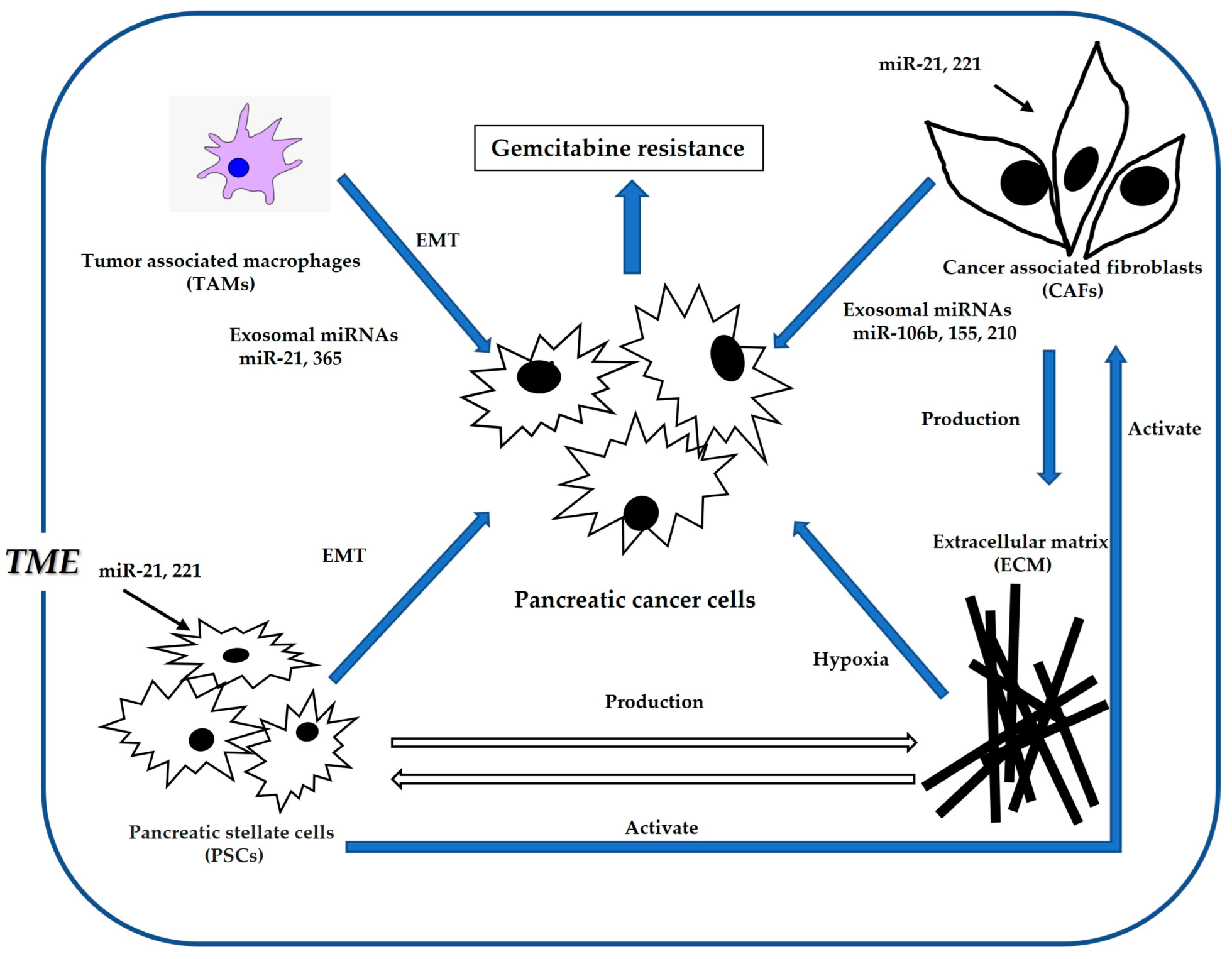
5. TME-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
5.1. TME and Gemcitabine Resistance in Pancreatic Cancer
5.2. Role of ECM in Gemcitabine Resistance
5.3. Role of CAFs in Gemcitabine Resistance
5.4. Role of PSCs in Gemcitabine Resistance
5.5. Role of TAMs in Gemcitabine Resistance
5.6. miRNAs Involved in TME-Mediated Gemcitabine Resistance
| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Liu YF | [132] | miR-143 | HIF-1 |
| Ni J | [137] | miR-210 | HOXA9 |
| Liu A | [136] | miR-3662 | HIF-1 |
| Resistance | |||
| Zhang L | [138] | miR-21 | PDCD4 |
| Kadera BE | [139] | miR-21 | NA |
| Fang Y | [143] | miR-106b | TP53INP1 |
| Masamune A | [144] | miR-221 | NA |
| Guo Y | [122] | miR-222 | TSC1 |
| Luo G | [131] | miR-301a | TP63 |
| Xin X | [133] | miR-519c | HIF-1 |
6. Gemcitabine Metabolism-Mediated Mechanisms of Gemcitabine Resistance in Pancreatic Cancer
6.1. Gemcitabine Metabolism
6.2. Relation of Gemcitabine Metabolism to Gene Expression in Pancreatic Cancer
6.2.1. Transporters Associated with Gemcitabine Resistance
6.2.2. CDA-Induced Gemcitabine Resistance
6.2.3. Relation of dCK and Gemcitabine Resistance
6.2.4. Relation of RR to Gemcitabine Resistance
6.3. Relation of Gemcitabine Metabolism to miRNAs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Bcl-2 | B cell lymphoma-2 |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| TUBB3 | tubulin-beta3 |
| NFKB1 | nuclear factor kappa B subunit 1 |
| NEAT1 | nuclear-enriched abundant transcript 1 |
| ESRP1 | epithelial splicing regulatory protein 1 |
| TFAP2C | transcription factor activating protein 2 gamma |
| SMURF2 | SMAD specific E3 ubiquitin protein ligase 2 |
| FBXW7 | F-Box and WD Repeat Domain Containing 7 |
| BOK | B-cell lymphoma 2 (BCL-2) ovarian killer |
| TP63 | tumor protein p63 |
| NR3C2 | nuclear receptor subfamily 3 group C member 2 |
| SHC1 | src homology domain-containing transforming protein 1 |
| Bax | bcl-2 associated X protein |
| RRM | ribonucleotide reductase regulatory subunit M |
| ABCC | ATP binding cassette C |
| CDA | cytidine deaminase |
| PTEN | phosphatase and tensin homolog |
| dCK | deoxycytidine kinase |
| ST7L | suppression of tumorigenicity 7-like |
| HIF-1 | hypoxia-inducible factor 1 |
| HOXA9 | homeobox A9 |
| PDCD4 | programmed cell death 4 |
| TP53INP1 | tumor protein 53-induced nuclear protein 1 |
| TSC1 | tuberous sclerosis complex 1 |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Paulson, A.S.; Tran Cao, H.S.; Tempero, M.A.; Lowy, A.M. Therapeutic advances in pancreatic cancer. Gastroenterology 2013, 144, 1316–1326. [Google Scholar] [CrossRef]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Louvet, C.; Labianca, R.; Hammel, P.; Lledo, G.; Zampino, M.G.; André, T.; Zaniboni, A.; Ducreux, M.; Aitini, E.; Taïeb, J.; et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005, 23, 3509–3516. [Google Scholar] [CrossRef]
- Barhli, A.; Cros, J.; Bartholin, L.; Neuzillet, C. Prognostic stratification of resected pancreatic ductal adenocarcinoma: Past, present, and future. Dig. Liver Dis. 2018, 50, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Ohuchida, K.; Mizumoto, K.; Kayashima, T.; Fujita, H.; Moriyama, T.; Ohtsuka, T.; Ueda, J.; Nagai, E.; Hashizume, M.; Tanaka, M. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann. Surg. Oncol. 2011, 18, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Dhayat, S.; Mardin, W.A.; Mees, S.T.; Haier, J. Epigenetic markers for chemosensitivity and chemoresistance in pancreatic cancer—A review. Int. J. Cancer 2011, 129, 1031–1041. [Google Scholar] [CrossRef]
- Chiou, S.H.; Dorsch, M.; Kusch, E.; Naranjo, S.; Kozak, M.M.; Koong, A.C.; Winslow, M.M.; Grüner, B.M. Hmga2 is dispensable for pancreatic cancer development, metastasis, and therapy resistance. Sci. Rep. 2018, 8, 14008. [Google Scholar] [CrossRef]
- Mollah, F.; Varamini, P. Overcoming therapy resistance and relapse in TNBC: Emerging technologies to target breast cancer-associated fibroblasts. Biomedicines 2021, 9, 1921. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Sharma, S.; Obermair, A.; Salomon, C. Extracellular vesicle-associated miRNAs and chemoresistance: A systematic review. Cancers 2021, 13, 4608. [Google Scholar] [CrossRef]
- Santos, P.; Almeida, F. Role of exosomal miRNAs and the tumor microenvironment in drug resistance. Cells 2020, 9, 1450. [Google Scholar] [CrossRef] [PubMed]
- Wellner, U.; Schubert, J.; Burk, U.C.; Schmalhofer, O.; Zhu, F.; Sonntag, A.; Waldvogel, B.; Vannier, C.; Darling, D.; zur Hausen, A.; et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009, 11, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; Karim, S.A.; Morton, J.P.; Del Río Hernández, A. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef]
- Schoepp, M.; Ströse, A.J.; Haier, J. Dysregulation of miRNA expression in cancer associated fibroblasts (CAFs) and its consequences on the tumor microenvironment. Cancers 2017, 9, 54. [Google Scholar] [CrossRef]
- Maftouh, M.; Avan, A.; Funel, N.; Frampton, A.E.; Fiuji, H.; Pelliccioni, S.; Castellano, L.; Galla, V.; Peters, G.J.; Giovannetti, E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 384–393. [Google Scholar] [CrossRef]
- Fazilaty, H.; Rago, L.; Kass Youssef, K.; Ocaña, O.H.; Garcia-Asencio, F.; Arcas, A.; Galceran, J.; Nieto, M.A. A gene regulatory network to control EMT programs in development and disease. Nat. Commun. 2019, 10, 5115. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Lin, H.J.; Lin, J. Seed-in-soil: Pancreatic cancer influenced by tumor microenvironment. Cancers 2017, 9, 93. [Google Scholar] [CrossRef]
- Sunami, Y.; Böker, V.; Kleeff, J. Targeting and reprograming cancer-associated fibroblasts and the tumor microenvironment in pancreatic cancer. Cancers 2021, 13, 697. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in pancreatic cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Jiang, Y.; Pillarisetty, V.G. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine 2016, 95, e5541. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Kamata, Y.; Misawa, T.; Uwagawa, T.; Lacy, C.R.; Yanaga, K.; Manome, Y. Hydroxyurea decreases gemcitabine resistance in pancreatic carcinoma cells with highly expressed ribonucleotide reductase. Pancreas 2012, 41, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Okamoto, A.; Kamata, Y.; Misawa, T.; Uwagawa, T.; Gocho, T.; Yanaga, K.; Manome, Y. Is the resistance of gemcitabine for pancreatic cancer settled only by overexpression of deoxycytidine kinase? Oncol. Rep. 2010, 23, 471–475. [Google Scholar] [CrossRef]
- Funamizu, N.; Lacy, C.R.; Fujita, K.; Furukawa, K.; Misawa, T.; Yanaga, K.; Manome, Y. Tetrahydrouridine inhibits cell proliferation through cell cycle regulation regardless of cytidine deaminase expression levels. PLoS ONE 2012, 7, e37424. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, S.; Nakamori, S.; Tsujie, M.; Takahashi, Y.; Okami, J.; Yoshioka, S.; Yamasaki, M.; Marubashi, S.; Takemasa, I.; Miyamoto, A.; et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int. J. Cancer. 2007, 120, 1355–1363. [Google Scholar] [CrossRef]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic cancer chemoresistance to gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Romano, G.; Di Leva, G.; Nuovo, G.; Jeon, Y.J.; Ngankeu, A.; Sun, J.; Lovat, F.; Alder, H.; Condorelli, G.; et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat. Med. 2011, 18, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.; Komuro, H.; Aminova, S.; Harada, M. A comprehensive review of cancer microRNA therapeutic delivery strategies. Cancers 2020, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ansarullah, A.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Targeting microRNAs in pancreatic cancer: Microplayers in the big game. Can. Res. 2013, 73, 6541–6547. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.H.; Al-Hallak, M.N.; Philip, P.A.; Mohammad, R.M.; Viola, N.; Wagner, K.U.; Azmi, A.S. Exosomal microRNA in pancreatic cancer diagnosis, prognosis, and treatment: From bench to bedside. Cancers 2021, 13, 2777. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Honda, K. Use of biomarkers and imaging for early detection of pancreatic cancer. Cancers 2020, 12, 1965. [Google Scholar] [CrossRef]
- Pavlíková, L.; Šereš, M.; Breier, A.; Sulová, Z. The roles of microRNAs in cancer multidrug resistance. Cancers 2022, 14, 1090. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef]
- Hwang, G.R.; Yuen, J.G.; Ju, J. Roles of microRNAs in gastrointestinal cancer stem cell resistance and therapeutic development. Int. J. Mol. Sci. 2021, 22, 1624. [Google Scholar] [CrossRef]
- Pusceddu, S.; Ghidini, M.; Torchio, M.; Corti, F.; Tomasello, G.; Niger, M.; Prinzi, N.; Nichetti, F.; Coinu, A.; Di Bartolomeo, M.; et al. Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: A systematic review and meta-analysis. Cancers 2019, 11, 484. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.C.; Kim, J.H.; Kim, J.; Hwang, J.H. Pharmacoethnicity of FOLFIRINOX versus gemcitabine plus nab-paclitaxel in metastatic pancreatic cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 20152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Yuan, J.Q.; Di, M.Y.; Zheng, D.Y.; Chen, J.Z.; Ding, H.; Wu, X.Y.; Huang, Y.F.; Mao, C.; Tang, J.L. Gemcitabine plus erlotinib for advanced pancreatic cancer: A systematic review with meta-analysis. PLoS ONE 2013, 8, e57528. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, C.; Zhou, Y.; Chen, R.; Fan, X.; Bi, Z.; Li, Z.; Liu, Y. Gemcitabine compared with gemcitabine and S-1 combination therapy in advanced pancreatic cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1345. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, G.; Yang, J.; Ren, B.; Wang, H.; Chen, G.; Zhao, F.; You, L.; Wang, W.; Zhao, Y. Metabolism of pancreatic cancer: Paving the way to better anticancer strategies. Mol. Cancer 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shi, S.; Meng, Q.; Liang, D.; Ji, S.; Zhang, B.; Qin, Y.; Xu, J.; Ni, Q.; Yu, X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: Where we are and where we are going. Exp. Mol. Med. 2017, 49, e406. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and treatment resistance in pancreatic cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef]
- Royam, M.M.; Ramesh, R.; Shanker, R.; Sabarimurugan, S.; Kumarasamy, C.; Gothandam, K.M.; Baxi, S.; Gupta, A.; Krishnan, S.; Jayaraj, R. miRNA predictors of pancreatic cancer chemotherapeutic response: A systematic review and meta-analysis. Cancers 2019, 11, 900. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Kong, D.; Banerjee, S.; Ahmad, A.; Azmi, A.S.; Ali, S.; Abbruzzese, J.L.; Gallick, G.E.; Sarkar, F.H. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009, 69, 2400–2407. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Dhayat, S.A.; Traeger, M.M.; Rehkaemper, J.; Stroese, A.J.; Steinestel, K.; Wardelmann, E.; Kabar, I.; Senninger, N. Clinical impact of epithelial-to-mesenchymal transition regulating microRNAs in pancreatic ductal adenocarcinoma. Cancers 2018, 10, 328. [Google Scholar] [CrossRef]
- Wellner, U.; Brabletz, T.; Keck, T. ZEB1 in Pancreatic Cancer. Cancers 2010, 2, 1617–1628. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Lacy, C.R.; Parpart, S.T.; Takai, A.; Hiyoshi, Y.; Yanaga, K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int. J. Oncol. 2014, 44, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Hu, C.; Lacy, C.; Schetter, A.; Zhang, G.; He, P.; Gaedcke, J.; Ghadimi, M.B.; Ried, T.; Yfantis, H.G.; et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int. J. Cancer 2013, 132, 785–794. [Google Scholar] [CrossRef]
- Shah, A.N.; Summy, J.M.; Zhang, J.; Park, S.I.; Parikh, N.U.; Gallick, G.E. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 2007, 14, 3629–3637. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Liu, J.; Zheng, J.; Liu, Y.; Li, Y.; Su, F.; Ou, W.; Wang, R. Nutlin-3 reverses the epithelial-mesenchymal transition in gemcitabine-resistant hepatocellular carcinoma cells. Oncol. Rep. 2016, 36, 1325–1332. [Google Scholar] [CrossRef]
- Quint, K.; Tonigold, M.; Di Fazio, P.; Montalbano, R.; Lingelbach, S.; Rückert, F.; Alinger, B.; Ocker, M.; Neureiter, D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 2012, 41, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, K.; Tao, X.; Zhao, Y.; Chen, Q.; Li, N.; Liu, J.; Go, V.L.W.; Guo, J.; Gao, G.; et al. MicroRNA-34a alleviates gemcitabine resistance in pancreatic cancer by repression of cancer stem cell renewal. Pancreas 2021, 50, 1260–1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Wang, H.; Xue, X.; An, Y.; Tang, D.; Yuan, Z.; Wang, F.; Wu, J.; Zhang, J.; et al. Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer. Oncol. Rep. 2012, 27, 1599–1605. [Google Scholar] [CrossRef]
- Cioffi, M.; Trabulo, S.M.; Sanchez-Ripoll, Y.; Miranda-Lorenzo, I.; Lonardo, E.; Dorado, J.; Reis Vieira, C.; Ramirez, J.C.; Hidalgo, M.; Aicher, A.; et al. The miR-17-92 cluster counteracts quiescence and chemoresistance in a distinct subpopulation of pancreatic cancer stem cells. Gut 2015, 64, 1936–1948. [Google Scholar] [CrossRef]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; Desano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef]
- Okumura, Y.; Noda, T.; Eguchi, H.; Sakamoto, T.; Iwagami, Y.; Yamada, D.; Asaoka, T.; Wada, H.; Kawamoto, K.; Gotoh, K.; et al. Hypoxia-induced PLOD2 is a key regulator in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Ann. Surg. Oncol. 2018, 25, 3728–3737. [Google Scholar] [CrossRef]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef]
- Carrasco-Garcia, E.; Lopez, L.; Moncho-Amor, V.; Carazo, F.; Aldaz, P.; Collado, M.; Bell, D.; Gaafar, A.; Karamitopoulou, E.; Tzankov, A.; et al. SOX9 triggers different epithelial to mesenchymal transition states to promote pancreatic cancer progression. Cancers 2022, 14, 916. [Google Scholar] [CrossRef]
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94. [Google Scholar] [CrossRef]
- Zhang, G.; Schetter, A.; He, P.; Funamizu, N.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Hassan, R.; Yfantis, H.G.; Lee, D.H.; et al. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS ONE 2012, 7, e31507. [Google Scholar] [CrossRef]
- Lawlor, R.T.; Veronese, N.; Nottegar, A.; Malleo, G.; Smith, L.; Demurtas, J.; Cheng, L.; Wood, L.D.; Silvestris, N.; Salvia, R.; et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: A systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers 2019, 11, 113. [Google Scholar] [CrossRef]
- Krantz, S.B.; Shields, M.A.; Dangi-Garimella, S.; Bentrem, D.J.; Munshi, H.G. Contribution of epithelial-mesenchymal transition to pancreatic cancer progression. Cancers 2010, 2, 2084–2097. [Google Scholar] [CrossRef]
- Maier, H.J.; Wirth, T.; Beug, H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers 2010, 2, 2058–2083. [Google Scholar] [CrossRef]
- Wang, T.; Chen, G.; Ma, X.; Yang, Y.; Chen, Y.; Peng, Y.; Bai, Z.; Zhang, Z.; Pei, H.; Guo, W. Mir-30a regulates cancer cell response to chemotherapy through snai1/irs1/akt pathway. Cell Death Dis. 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Lacy, C.R.; Kamada, M.; Yanaga, K.; Manome, Y. MicroRNA-200b and −301 are associated with gemcitabine response as biomarkers in pancreatic carcinoma cells. Int. J. Oncol. 2019, 54, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vandenboom, T.G.; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009, 69, 6704–6712. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ji, L.; Ke, M.; Ou, Z.; Tang, N.; Li, Y. miR-125a-3p is responsible for chemosensitivity in PDAC by inhibiting epithelial-mesenchymal transition via Fyn. Biomed. Pharmacother. 2018, 106, 523–531. [Google Scholar] [CrossRef]
- Fu, X.; Deng, X.; Xiao, W.; Huang, B.; Yi, X.; Zou, Y. Downregulation of NEAT1 sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine through modulation of the miR-506-3p/ZEB2/EMT axis. Am. J. Cancer Res. 2021, 11, 3841–3856. [Google Scholar]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef]
- Hiramoto, H.; Muramatsu, T.; Ichikawa, D.; Tanimoto, K.; Yasukawa, S.; Otsuji, E.; Inazawa, J. miR-509-5p and miR-1243 increase the sensitivity to gemcitabine by inhibiting epithelial-mesenchymal transition in pancreatic cancer. Sci. Rep. 2017, 7, 4002. [Google Scholar] [CrossRef]
- Yang, R.M.; Zhan, M.; Xu, S.W.; Long, M.M.; Yang, L.H.; Chen, W.; Huang, S.; Liu, Q.; Zhou, J.; Zhu, J.; et al. miR-3656 expression enhances the chemosensitivity of pancreatic cancer to gemcitabine through modulation of the RHOF/EMT axis. Cell Death Dis. 2017, 8, e3129. [Google Scholar] [CrossRef]
- Liu, F.; Liu, B.; Qian, J.; Wu, G.; Li, J.; Ma, Z. miR-153 enhances the therapeutic effect of gemcitabine by targeting Snail in pancreatic cancer. Acta Biochim. Biophys. Sin. 2017, 49, 520–529. [Google Scholar] [CrossRef]
- Bai, Z.; Sun, J.; Wang, X.; Wang, H.; Pei, H.; Zhang, Z. MicroRNA-153 is a prognostic marker and inhibits cell migration and invasion by targeting SNAI1 in human pancreatic ductal adenocarcinoma. Oncol. Rep. 2015, 34, 595–602. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Lin, Y.; Wang, X.; Cui, X.; Zhang, Z.; Xian, G.; Qin, C. Novel crosstalk between KLF4 and ZEB1 regulates gemcitabine resistance in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2017, 51, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Mondal, G.; Kumar, V.; Kattel, K.; Mahato, R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017, 402, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell. Oncol. 2020, 43, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Eguchi, H.; Nagano, H.; Konno, M.; Tomimaru, Y.; Wada, H.; Hama, N.; Kawamoto, K.; Kobayashi, S.; Nishida, N.; et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer 2014, 111, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Huang, H.; Feng, M.; Yang, G.; Zheng, S.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; Zhao, Y. Mir-10a-5p targets tfap2c to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhang, J.H.; Wu, X.Z.; Yan, T.; Lv, W. miR-15b promotes epithelial-mesenchymal transition by inhibiting SMURF2 in pancreatic cancer. Int. J. Oncol. 2015, 47, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, P.; Wang, J.; Schetter, A.; Tang, W.; Funamizu, N.; Yanaga, K.; Uwagawa, T.; Satoskar, A.R.; Gaedcke, J.; et al. A novel MIF signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res. 2016, 76, 3838–3850. [Google Scholar] [CrossRef]
- Zhang, K.D.; Hu, B.; Cen, G.; Yang, Y.H.; Chen, W.W.; Guo, Z.Y.; Wang, X.F.; Zhao, Q.; Qiu, Z.J. Mir-301a transcriptionally activated by hif-2alpha promotes hypoxia-induced epithelial-mesenchymal transition by targeting tp63 in pancreatic cancer. World J. Gastroenterol. 2020, 26, 2349–2373. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanahashi, T.; Sato, Y.; Miyoshi, J.; Nakagawa, T.; Kimura, T.; Miyamoto, H.; Fujino, Y.; Nakamura, F.; Takehara, M.; et al. MicroRNA-296-5p promotes cell invasion and drug resistance by targeting Bcl2-related ovarian killer, leading to a poor prognosis in pancreatic cancer. Digestion 2020, 101, 794–806. [Google Scholar] [CrossRef]
- Ma, J.; Fang, B.; Zeng, F.; Ma, C.; Pang, H.; Cheng, L.; Shi, Y.; Wang, H.; Yin, B.; Xia, J.; et al. Down-regulation of mir-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget 2015, 6, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, M.; Zhang, H.; Guo, X.; Qin, R. Circ_0092367 inhibits EMT and gemcitabine resistance in pancreatic cancer via regulating the miR-1206/ESRP1 axis. Genes 2021, 12, 1701. [Google Scholar] [CrossRef]
- Yu, Q.; Xiu, Z.; Jian, Y.; Zhou, J.; Chen, X.; Chen, X.; Chen, C.; Chen, H.; Yang, S.; Yin, L.; et al. microRNA-497 prevents pancreatic cancer stem cell gemcitabine resistance, migration, and invasion by directly targeting nuclear factor kappa B 1. Aging 2022, 14, 5908–5924. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Huang, T.; Ding, Y.C.; Yu, W.; Wang, Q.; Meng, B.; Luo, S.X. Microrna-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6533–6539. [Google Scholar]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Patel, R.; Chiang, C.Y.; Hou, P. Role of the tumor microenvironment in regulating pancreatic cancer therapy resistance. Cells 2022, 11, 2952. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Michl, P.; Frese, K.K.; Feig, C.; Cook, N.; Jacobetz, M.A.; Lolkema, M.P.; Buchholz, M.; Olive, K.P.; Gress, T.M.; et al. Stromal biology and therapy in pancreatic cancer. Gut 2011, 60, 861–868. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Tyagi, N.; Khan, M.A.; Srivastava, S.K.; Al-Ghadhban, A.; Dugger, K.; Carter, J.E.; Singh, S.; Singh, A.P. Gemcitabine treatment promotes immunosuppressive microenvironment in pancreatic tumors by supporting the infiltration, growth, and polarization of macrophages. Sci. Rep. 2018, 8, 12000. [Google Scholar] [CrossRef]
- Ahmad, I.M.; Dafferner, A.J.; O’Connell, K.A.; Mehla, K.; Britigan, B.E.; Hollingsworth, M.A.; Abdalla, M.Y. Heme Oxygenase-1 inhibition potentiates the effects of nab-paclitaxel-gemcitabine and modulates the tumor microenvironment in pancreatic ductal adenocarcinoma. Cancers 2021, 13, 2264. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018, 9, 1065. [Google Scholar] [CrossRef]
- Gu, Z.; Du, Y.; Zhao, X.; Wang, C. Tumor microenvironment and metabolic remodeling in gemcitabine-based chemoresistance of pancreatic cancer. Cancer Lett. 2021, 521, 98–108. [Google Scholar] [CrossRef]
- Park, J.; Schwarzbauer, J.E. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene 2014, 33, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Murakami, T.; Tsuchida, K.; Sugino, H.; Miyake, H.; Tashiro, S. Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas 2004, 28, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Aasrum, M.; Verbeke, C.S.; Gladhaug, I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 2019, 19, 596. [Google Scholar] [CrossRef]
- Topalovski, M.; Brekken, R.A. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2016, 381, 252–258. [Google Scholar] [CrossRef]
- Dangi-Garimella, S.; Krantz, S.B.; Barron, M.R.; Shields, M.A.; Heiferman, M.J.; Grippo, P.J.; Bentrem, D.J.; Munshi, H.G. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011, 71, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.Y.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III trial of Pegvorhyaluronidase Alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. 2020, 38, 3185–3194. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Richards, K.E.; Xiao, W.; Hill, R.; On Behalf Of The Usc Pancreas Research Team. Cancer-associated fibroblasts confer gemcitabine resistance to pancreatic cancer cells through PTEN-targeting miRNAs in exosomes. Cancers 2022, 14, 2812. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Head, R.; Liu, J.; Ruzinova, M.B.; Lim, K.H. Tumor-stroma IL1beta-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef]
- Hu, C.; Xia, R.; Zhang, X.; Li, T.; Ye, Y.; Li, G.; He, R.; Li, Z.; Lin, Q.; Zheng, S.; et al. circFARP1 enables cancer-associated fibroblasts to promote gemcitabine resistance in pancreatic cancer via the LIF/STAT3 axis. Mol. Cancer 2022, 21, 24. [Google Scholar] [CrossRef]
- Wei, L.; Lin, Q.; Lu, Y.; Li, G.; Huang, L.; Fu, Z.; Chen, R.; Zhou, Q. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-β1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 2021, 12, 334. [Google Scholar] [CrossRef]
- Ikenaga, N.; Ohuchida, K.; Mizumoto, K.; Akagawa, S.; Fujiwara, K.; Eguchi, D.; Kozono, S.; Ohtsuka, T.; Takahata, S.; Tanaka, M. Pancreatic cancer cells enhance the ability of collagen internalization during epithelial-mesenchymal transition. PLoS ONE 2012, 7, e40434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, H.; Sun, L.; Brigstock, D.R.; Gao, R. Interleukin-6 participates in human pancreatic stellate cell activation and collagen I production via TGF-β1/Smad pathway. Cytokine 2021, 143, 155536. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Li, J.; Sun, H.; Liu, S.; Cui, Y.; Li, F. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol. Rep. 2015, 33, 1883–1889. [Google Scholar] [CrossRef]
- Lee, E.; Ryu, G.R.; Ko, S.H.; Ahn, Y.B.; Song, K.H. A role of pancreatic stellate cells in islet fibrosis and β-cell dysfunction in type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2017, 485, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, K.; Masamune, A.; Watanabe, T.; Ariga, H.; Itoh, H.; Hamada, S.; Satoh, K.; Egawa, S.; Unno, M.; Shimosegawa, T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2010, 403, 380–384. [Google Scholar] [CrossRef] [PubMed]
- LaRue, M.M.; Parker, S.; Puccini, J.; Cammer, M.; Kimmelman, A.C.; Bar-Sagi, D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2119168119. [Google Scholar] [CrossRef]
- Helm, O.; Held-Feindt, J.; Grage-Griebenow, E.; Reiling, N.; Ungefroren, H.; Vogel, I.; Krüger, U.; Becker, T.; Ebsen, M.; Röcken, C.; et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int. J. Cancer 2014, 135, 843–861. [Google Scholar] [CrossRef]
- Weizman, N.; Krelin, Y.; Shabtay-Orbach, A.; Amit, M.; Binenbaum, Y.; Wong, R.J.; Gil, Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 2014, 33, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, H.; Xiong, J.; Gou, S.; Cui, J.; Peng, T. miR-222-3p-containing macrophage-derived extracellular vesicles confer gemcitabine resistance via TSC1-mediated mTOR/AKT/PI3K pathway in pancreatic cancer. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Castellanos, J.A.; Shi, C.; Beesetty, Y.; Reyzer, M.L.; Caprioli, R.; Chen, X.; Walsh, A.J.; Skala, M.C.; Moses, H.L.; et al. Signal transducer and activator of transcription 3, mediated remodeling of the tumor microenvironment results in enhanced tumor drug delivery in a mouse model of pancreatic cancer. Gastroenterology 2015, 149, 1932–1943.e9. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Sato, N.; Adachi, Y.; Amaike, T.; Kudo, Y.; Koga, A.; Kohi, S.; Hirata, K. Hypoxia increases KIAA1199/CEMIP expression and enhances cell migration in pancreatic cancer. Sci. Rep. 2021, 11, 18193. [Google Scholar] [CrossRef]
- Keleg, S.; Kayed, H.; Jiang, X.; Penzel, R.; Giese, T.; Büchler, M.W.; Friess, H.; Kleeff, J. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int. J. Cancer. 2007, 121, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chee, N.T.; Carriere, C.H.; Miller, Z.; Welford, S.; Brothers, S.P. Activating transcription factor 4 regulates hypoxia inducible factor 1α in chronic hypoxia in pancreatic cancer cells. Oncol. Rep. 2023, 49, 14. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, L.L.; Shi, C.Y.; Wei, W.; Wang, D.S.; Cai, D.F. MicroRNA-454 regulates stromal cell derived factor-1 in the control of the growth of pancreatic ductal adenocarcinoma. Sci. Rep. 2016, 6, 22793. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Ahmad, A.; Azmi, A.S.; Li, Y.; Banerjee, S.; Kong, D.; Sethi, S.; Aboukameel, A.; Padhye, S.B.; et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE 2012, 7, e50165. [Google Scholar] [CrossRef]
- Nong, K.; Zhang, D.; Chen, C.; Yang, Y.; Yang, Y.; Liu, S.; Cai, H. MicroRNA-519 inhibits hypoxia-induced tumorigenesis of pancreatic cancer by regulating immune checkpoint PD-L1. Oncol. Lett. 2020, 19, 1427–1433. [Google Scholar] [CrossRef]
- Luo, G.; Xia, X.; Wang, X.; Zhang, K.; Cao, J.; Jiang, T.; Zhao, Q.; Qiu, Z. miR-301a plays a pivotal role in hypoxia-induced gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2018, 369, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Luo, D.; Li, X.; Li, Z.Q.; Yu, X.; Zhu, H.W. PVT1 knockdown inhibits autophagy and improves gemcitabine sensitivity by regulating the MiR-143/HIF-1α/VMP1 axis in pancreatic cancer. Pancreas 2021, 50, 227–234. [Google Scholar] [CrossRef]
- Xin, X.; Kumar, V.; Lin, F.; Kumar, V.; Bhattarai, R.; Bhatt, V.R.; Tan, C.; Mahato, R.I. Redox-responsive nanoplatform for codelivery of miR-519c and gemcitabine for pancreatic cancer therapy. Sci. Adv. 2020, 6, eabd6764. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, K.; Tsuchida, A.; Nagakawa, Y.; Suzuki, M.; Abe, Y.; Itoi, T.; Serizawa, H.; Nagao, T.; Shimazu, M.; Aoki, T. Hypoxia-inducible factor-1α expression and gemcitabine chemotherapy for pancreatic cancer. Oncol. Rep. 2011, 26, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, H.; Zhang, Y.; Li, X.; Tong, H.; Zeng, Y.; Wang, Q.; He, W. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1α activation. Int. J. Oncol. 2018, 53, 215–224. [Google Scholar] [CrossRef]
- Liu, A.; Zhou, Y.; Zhao, T.; Tang, X.; Zhou, B.; Xu, J. MiRNA-3662 reverses the gemcitabine resistance in pancreatic cancer through regulating the tumor metabolism. Cancer Chemother. Pharmacol. 2021, 88, 343–357. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, S.; Yuan, W.; Cen, F.; Yan, Q. Mechanism of miR-210 involved in epithelial-mesenchymal transition of pancreatic cancer cells under hypoxia. J. Recept. Signal Transduct. 2019, 39, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, J.; Li, W.; Zhang, C. Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol. Res. 2018, 26, 827–835. [Google Scholar] [CrossRef]
- Kadera, B.E.; Li, L.; Toste, P.A.; Wu, N.; Adams, C.; Dawson, D.W.; Donahue, T.R. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS ONE 2013, 8, e71978. [Google Scholar] [CrossRef]
- Mace, T.A.; Collins, A.L.; Wojcik, S.E.; Croce, C.M.; Lesinski, G.B.; Bloomston, M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J. Surg. Res. 2013, 184, 855–860. [Google Scholar] [CrossRef]
- Wang, P.; Zhuang, L.; Zhang, J.; Fan, J.; Luo, J.; Chen, H.; Wang, K.; Liu, L.; Chen, Z.; Meng, Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FASL. Mol. Oncol. 2013, 7, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Lee, E.J.; Esau, C.; Schmittgen, T.D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 2009, 38, e190–e199. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, W.; Rong, Y.; Kuang, T.; Xu, X.; Wu, W.; Wang, D.; Lou, W. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2019, 383, 111543. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Nakano, E.; Hamada, S.; Takikawa, T.; Yoshida, N.; Shimosegawa, T. Alteration of the microRNA expression profile during the activation of pancreatic stellate cells. Scand. J. Gastroenterol. 2014, 49, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yang, J.; Cui, X.; Zhou, Z.; Zhan, H.; Ding, K.; Tian, X.; Yang, Z.; Fung, K.A.; et al. ZIP4 Increases Expression of transcription factor ZEB1 to Promote integrin α3β1 Signaling and Inhibit Expression of the gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology 2020, 158, 679–692.e1. [Google Scholar] [CrossRef]
- Hu, Q.; Qin, Y.; Zhang, B.; Liang, C.; Ji, S.; Shi, S.; Xu, W.; Xiang, J.; Liang, D.; Ni, Q.; et al. FBW7 increases the chemosensitivity of pancreatic cancer cells to gemcitabine through upregulation of ENT1. Oncol. Rep. 2017, 38, 2069–2077. [Google Scholar] [CrossRef]
- Michalski, C.W.; Erkan, M.; Sauliunaite, D.; Giese, T.; Stratmann, R.; Sartori, C.; Giese, N.A.; Friess, H.; Kleeff, J. Ex vivo chemosensitivity testing and gene expression profiling predict response towards adjuvant gemcitabine treatment in pancreatic cancer. Br. J. Cancer 2008, 99, 760–767. [Google Scholar] [CrossRef]
- Saiki, Y.; Hirota, S.; Horii, A. Attempts to remodel the pathways of gemcitabine metabolism: Recent approaches to overcoming tumours with acquired chemoresistance. Cancer Drug Resist. 2020, 3, 819–831. [Google Scholar] [CrossRef]
- Hagmann, W.; Faissner, R.; Schnölzer, M.; Löhr, M.; Jesnowski, R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers 2010, 3, 106–125. [Google Scholar] [CrossRef]
- Hung, S.W.; Marrache, S.; Cummins, S.; Bhutia, Y.D.; Mody, H.; Hooks, S.B.; Dhar, S.; Govindarajan, R. Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015, 359, 233–240. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Hung, S.W.; Patel, B.; Lovin, D.; Govindarajan, R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 2011, 71, 1825–1835. [Google Scholar] [CrossRef]
- Maréchal, R.; Mackey, J.R.; Lai, R.; Demetter, P.; Peeters, M.; Polus, M.; Cass, C.E.; Young, J.; Salmon, I.; Devière, J.; et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin. Cancer Res. 2009, 15, 2913–2919. [Google Scholar] [CrossRef]
- García-Manteiga, J.; Molina-Arcas, M.; Casado, F.J.; Mazo, A.; Pastor-Anglada, M. Nucleoside transporter profiles in human pancreatic cancer cells: Role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clin. Cancer Res. 2003, 9, 5000–5008. [Google Scholar] [PubMed]
- Rauchwerger, D.R.; Firby, P.S.; Hedley, D.W.; Moore, M.J. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000, 60, 6075–6079. [Google Scholar]
- Tsesmetzis, N.; Paulin, C.B.J.; Rudd, S.G.; Herold, N. Nucleobase and nucleoside analogues: Resistance and re-sensitisation at the level of pharmacokinetics, pharmacodynamics and metabolism. Cancers 2018, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; Del Tacca, M.; Mey, V.; Funel, N.; Nannizzi, S.; Ricci, S.; Orlandini, C.; Boggi, U.; Campani, D.; Del Chiaro, M.; et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006, 66, 3928–3935. [Google Scholar] [CrossRef]
- Mackey, J.R.; Mani, R.S.; Selner, M.; Mowles, D.; Young, J.D.; Belt, J.A.; Crawford, C.R.; Cass, C.E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998, 58, 4349–4357. [Google Scholar]
- Aughton, K.; Elander, N.O.; Evans, A.; Jackson, R.; Campbell, F.; Costello, E.; Halloran, C.M.; Mackey, J.R.; Scarfe, A.G.; Valle, J.W.; et al. hENT1 predicts benefit from gemcitabine in pancreatic cancer but only with low CDA mRNA. Cancers 2021, 13, 5758. [Google Scholar] [CrossRef]
- Farrell, J.J.; Elsaleh, H.; Garcia, M.; Lai, R.; Ammar, A.; Regine, W.F.; Abrams, R.; Benson, A.B.; Macdonald, J.; Cass, C.E.; et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 2009, 136, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Skrypek, N.; Duchêne, B.; Hebbar, M.; Leteurtre, E.; van Seuningen, I.; Jonckheere, N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative nucleoside Transporter family. Oncogene 2013, 32, 1714–1723. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, J.; Cao, J.; Diao, F.; Huang, P. Low-intensity low-frequency ultrasound enhances the chemosensitivity of gemcitabine-resistant ASPC-1 cells via PI3K/AKT/NF-κB pathway-mediated ABC transporters. Oncol. Rep. 2020, 44, 1158–1168. [Google Scholar] [CrossRef]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. S5), v7–v12. [Google Scholar] [CrossRef] [PubMed]
- Neff, T.; Blau, C.A. Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp. Hematol. 1996, 24, 1340–1346. [Google Scholar] [PubMed]
- Eliopoulos, N.; Cournoyer, D.; Momparler, R.L. Drug resistance to 5-aza-2′-deoxycytidine, 2′,2′-difluorodeoxycytidine, and cytosine arabinoside conferred by retroviral-mediated transfer of human cytidine deaminase cDNA into murine cells. Cancer Chemother. Pharmacol. 1998, 42, 373–378. [Google Scholar] [CrossRef]
- Kanno, S.; Hiura, T.; Ohtake, T.; Koiwai, K.; Suzuki, H.; Ujibe, M.; Ishikawa, M. Characterization of resistance to cytosine arabinoside (ara-C) in NALM-6 human B leukemia cells. Clin. Chim. Acta 2007, 377, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Hori, H.; Ogawa, M.; Miyahara, M.; Kawasaki, H.; Taniguchi, N.; Komada, Y. Impact of cytidine deaminase activity on intrinsic resistance to cytarabine in carcinoma cells. Oncol. Rep. 2004, 12, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ludovini, V.; Floriani, I.; Pistola, L.; Minotti, V.; Meacci, M.; Chiari, R.; Garavaglia, D.; Tofanetti, F.R.; Flacco, A.; Siggillino, A.; et al. Association of cytidine deaminase and xeroderma pigmentosum group D polymorphisms with response, toxicity, and survival in cisplatin/gemcitabine-treated advanced non-small cell lung cancer patients. J. Thorac. Oncol. 2011, 6, 2018–2026. [Google Scholar] [CrossRef]
- Blackstock, A.W.; Lightfoot, H.; Case, L.D.; Tepper, J.E.; Mukherji, S.K.; Mitchell, B.S.; Swarts, S.G.; Hess, S.M. Tumor uptake and elimination of 2′, 2. Clin. Cancer Res. 2001, 7, 3263–3268. [Google Scholar]
- Manome, Y.; Wen, P.Y.; Dong, Y.; Tanaka, T.; Mitchell, B.S.; Kufe, D.W.; Fine, H.A. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat. Med. 1996, 2, 567–573. [Google Scholar] [CrossRef]
- Ohhashi, S.; Ohuchida, K.; Mizumoto, K.; Fujita, H.; Egami, T.; Yu, J.; Toma, H.; Sadatomi, S.; Nagai, E.; Tanaka, M. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008, 28, 2205–2212. [Google Scholar]
- Ohmine, K.; Kawaguchi, K.; Ohtsuki, S.; Motoi, F.; Ohtsuka, H.; Kamiie, J.; Abe, T.; Unno, M.; Terasaki, T. Quantitative targeted proteomics of pancreatic cancer: Deoxycytidine kinase protein level correlates to progression-free survival of patients receiving gemcitabine treatment. Mol. Pharm. 2015, 12, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.; Akiyoshi, K.; Akiyama, N.; Funamizu, N.; Watanabe, M.; Fujioka, K.; Ikeda, K.; Manome, Y. Cholangiocarcinoma cell line TK may be useful for the pharmacokinetic study of the chemotherapeutic agent gemcitabine. Oncol. Rep. 2014, 32, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.J.; Zhou, Z.; Allen, J.B. Ribonucleotide reductase: Regulation, regulation, regulation. Trends Biochem. Sci. 1992, 17, 119–123. [Google Scholar] [CrossRef]
- Duxbury, M.S.; Ito, H.; Benoit, E.; Zinner, M.J.; Ashley, S.W.; Whang, E.E. Retrovirally mediated RNA interference targeting the M2 subunit of ribonucleotide reductase: A novel therapeutic strategy in pancreatic cancer. Surgery 2004, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Vena, F.; Li Causi, E.; Rodriguez-Justo, M.; Goodstal, S.; Hagemann, T.; Hartley, J.A.; Hochhauser, D. The MEK1/2 inhibitor Pimasertib enhances gemcitabine efficacy in pancreatic cancer models by altering ribonucleotide reductase subunit-1 (RRM1). Clin. Cancer Res. 2015, 21, 5563–5577. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Kohya, N.; Kai, K.; Ohtaka, K.; Hashiguchi, K.; Hiraki, M.; Kitajima, Y.; Tokunaga, O.; Noshiro, H.; Miyazaki, K. Ribonucleotide reductase subunit M1 assessed by quantitative double-fluorescence immunohistochemistry predicts the efficacy of gemcitabine in biliary tract carcinoma. Int. J. Oncol. 2010, 37, 845–852. [Google Scholar]
- Sierzega, M.; Pach, R.; Kulig, P.; Legutko, J.; Kulig, J. Prognostic implications of expression profiling for gemcitabine-related genes (hENT1, dCK, RRM1, RRM2) in patients with resectable pancreatic adenocarcinoma receiving adjuvant chemotherapy. Pancreas 2017, 46, 684–689. [Google Scholar] [CrossRef]
- Amponsah, P.S.; Fan, P.; Bauer, N.; Zhao, Z.; Gladkich, J.; Fellenberg, J.; Herr, I. MicroRNA, 210 overexpression inhibits tumor growth and potentially reverses gemcitabine resistance in pancreatic cancer. Cancer Lett. 2017, 388, 107–117. [Google Scholar] [CrossRef]
- Gu, J.; Huang, W.; Wang, X.; Zhang, J.; Tao, T.; Zheng, Y.; Liu, S.; Yang, J.; Chen, Z.S.; Cai, C.Y.; et al. Hsa-miR-3178/RhoB/PI3K/Akt, a novel signaling pathway regulates ABC transporters to reverse gemcitabine resistance in pancreatic cancer. Mol. Cancer 2022, 21, 112. [Google Scholar] [CrossRef]
- Wang, F.; Xue, X.; Wei, J.; An, Y.; Yao, J.; Cai, H.; Wu, J.; Dai, C.; Qian, Z.; Xu, Z.; et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br. J. Cancer 2010, 103, 567–574. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Yang, Y.; Zhang, Z. miRNA-93-5p promotes gemcitabine resistance in pancreatic cancer cells by targeting the PTEN-mediated PI3K/Akt signaling pathway. Ann. Clin. Lab. Sci. 2021, 51, 310–320. [Google Scholar] [PubMed]
- Zhan, T.; Chen, X.; Tian, X.; Han, Z.; Liu, M.; Zou, Y.; Huang, S.; Chen, A.; Cheng, X.; Deng, J.; et al. MiR-331-3p links to drug resistance of pancreatic cancer cells by activating WNT/β-catenin signal via ST7L. Technol. Cancer Res. Treat. 2020, 19, 1533033820945801. [Google Scholar] [CrossRef]
- Fan, P.; Liu, L.; Yin, Y.; Zhao, Z.; Zhang, Y.; Amponsah, P.S.; Xiao, X.; Bauer, N.; Abukiwan, A.; Nwaeburu, C.C.; et al. MicroRNA-101-3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Lett. 2016, 373, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Hung, S.W.; Krentz, M.; Patel, D.; Lovin, D.; Manoharan, R.; Thomson, J.M.; Govindarajan, R. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: Role of LIN-28 and SET oncoprotein. PLoS ONE 2013, 8, e53436. [Google Scholar] [CrossRef]
- Rajabpour, A.; Afgar, A.; Mahmoodzadeh, H.; Radfar, J.E.; Rajaei, F.; Teimoori-Toolabi, L. MiR-608 regulating the expression of ribonucleotide reductase M1 and cytidine deaminase is repressed through induced gemcitabine chemoresistance in pancreatic cancer cells. Cancer Chemother. Pharmacol. 2017, 80, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lu, S.; Yang, D.; Zhang, L.; Ye, J.; Li, M.; Hu, W. MiR-20a-5p regulates gemcitabine chemosensitivity by targeting RRM2 in pancreatic cancer cells and serves as a predictor for gemcitabine-based chemotherapy. Biosci. Rep. 2019, 39, BSR20181374. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.E.; Zeleniak, K.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 30, 1770–1778. [Google Scholar] [CrossRef]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 controls exosome synthess and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef]
- Comanedatore, A.; Immordino, B.; Balsano, M.; Capula, M.; Garajova, I.; Ciccolini, J.; Giovannetti, E.; Morelli, L. Potenital role of exosomes in the chemoresistance to gemcitabine and nab-paclitaxel in pancreatic cancer. Diagnostics 2022, 12, 286. [Google Scholar] [CrossRef] [PubMed]


| Author | Ref. Number | miRNA | Target Gene |
|---|---|---|---|
| Sensitivity | |||
| Bhutia YD | [184] | let-7a | RRM2 |
| Lu H | [186] | miR-20a | RRM2 |
| Fan P | [183] | miR-101 | RRM1 |
| Amponsah PS | [178] | miR-210 | ABCC5 |
| Maftouh M | [17] | miR-211 | RRM2 |
| Rajabpour A | [185] | miR-608 | CDA, RRM1 |
| Gu J | [179] | miR-3178 | ABC transporter |
| Resistance | |||
| Wu Y | [181] | miR-93 | PTEN |
| Patel GK | [187] | miR-155 | dCK |
| Zhan T | [182] | miR-331 | ST7L |
| Xin X | [133] | miR-519c | ABCG2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funamizu, N.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer. Cancers 2023, 15, 1230. https://doi.org/10.3390/cancers15041230
Funamizu N, Honjo M, Tamura K, Sakamoto K, Ogawa K, Takada Y. microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer. Cancers. 2023; 15(4):1230. https://doi.org/10.3390/cancers15041230
Chicago/Turabian StyleFunamizu, Naotake, Masahiko Honjo, Kei Tamura, Katsunori Sakamoto, Kohei Ogawa, and Yasutsugu Takada. 2023. "microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer" Cancers 15, no. 4: 1230. https://doi.org/10.3390/cancers15041230
APA StyleFunamizu, N., Honjo, M., Tamura, K., Sakamoto, K., Ogawa, K., & Takada, Y. (2023). microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer. Cancers, 15(4), 1230. https://doi.org/10.3390/cancers15041230