Kisspeptin and GPR54 Receptor Expression in Endometrial Cancer Tissue
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Immunohistochemistry
2.3. Statistical Analysis
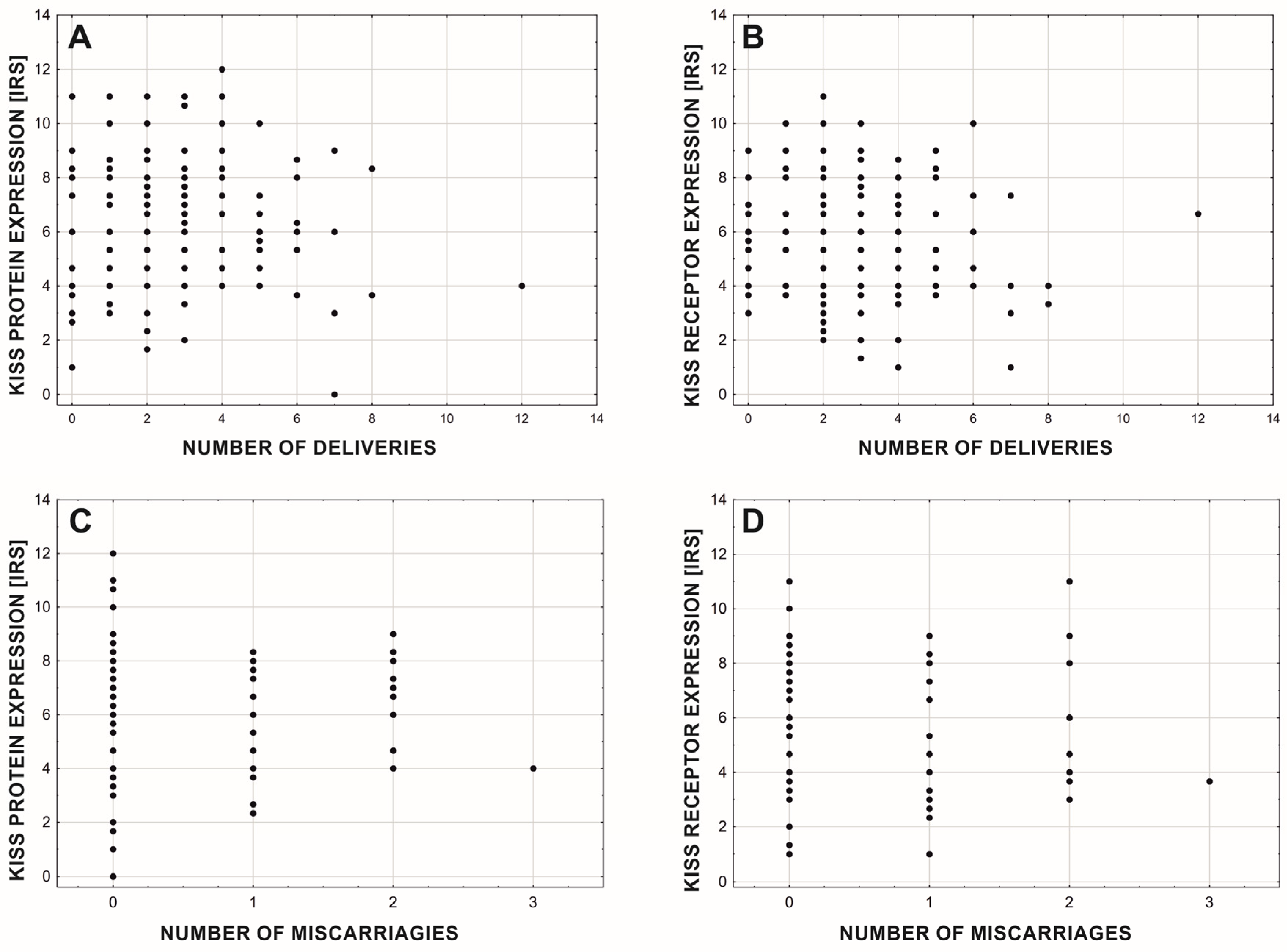
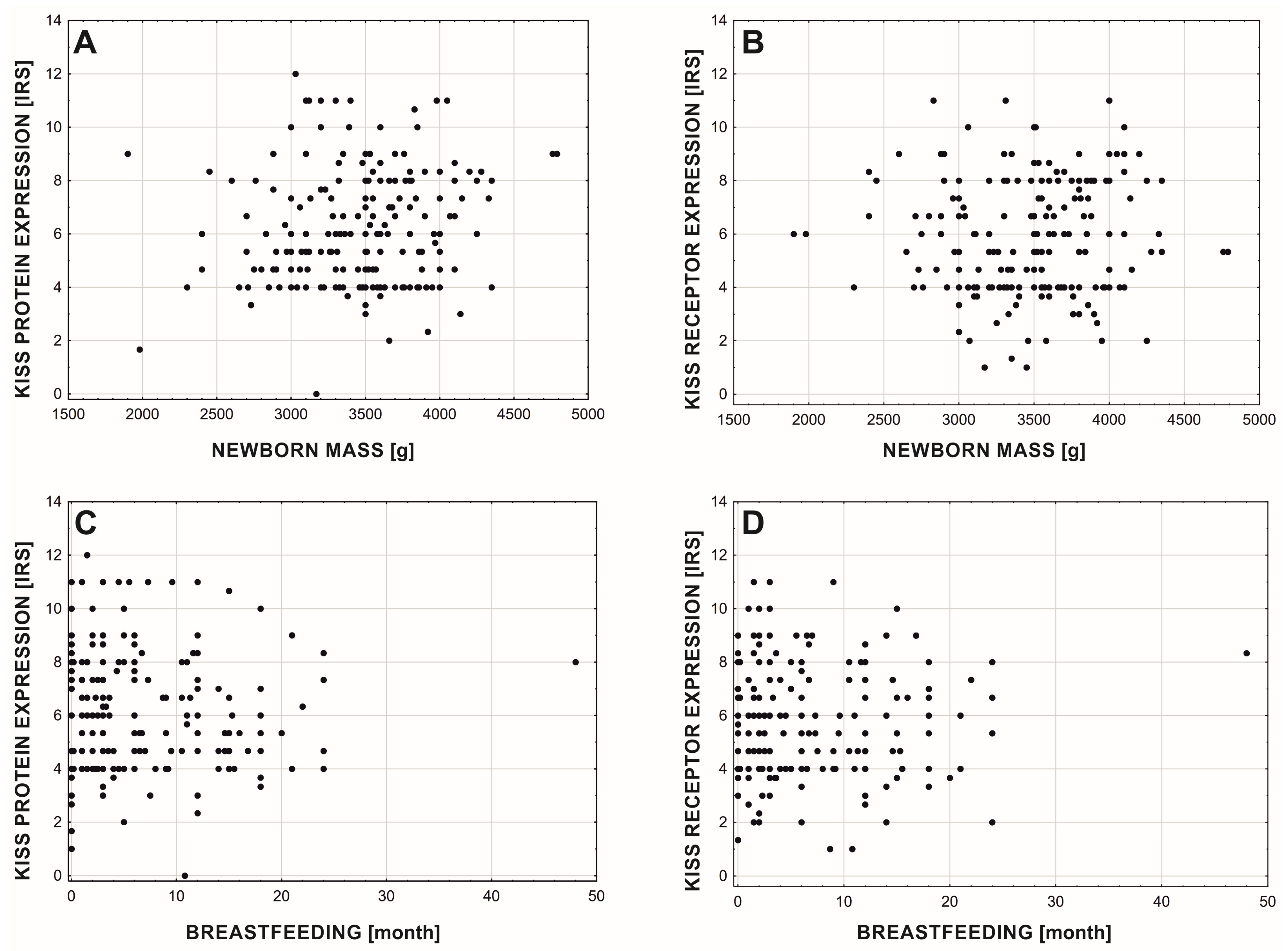
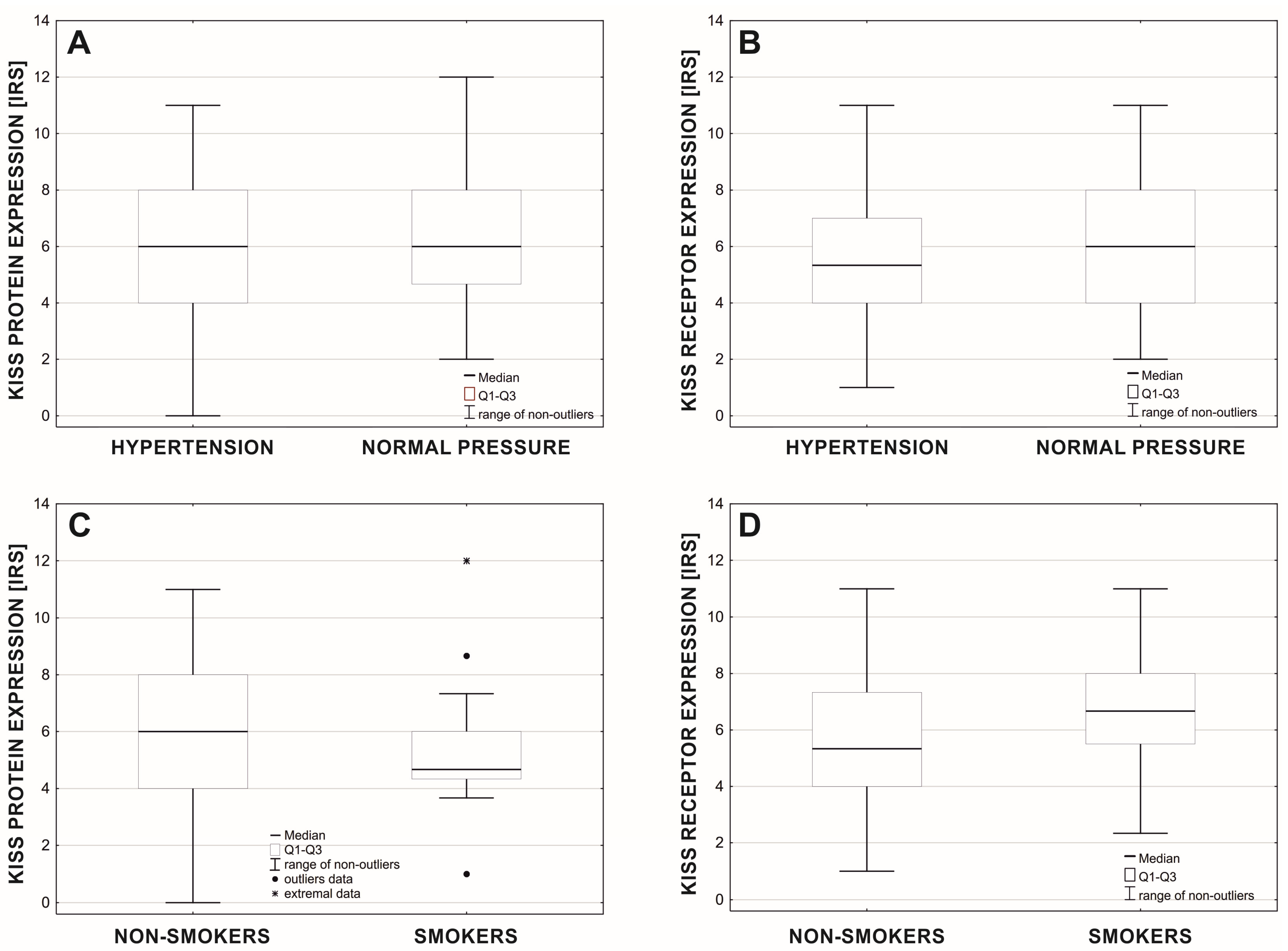
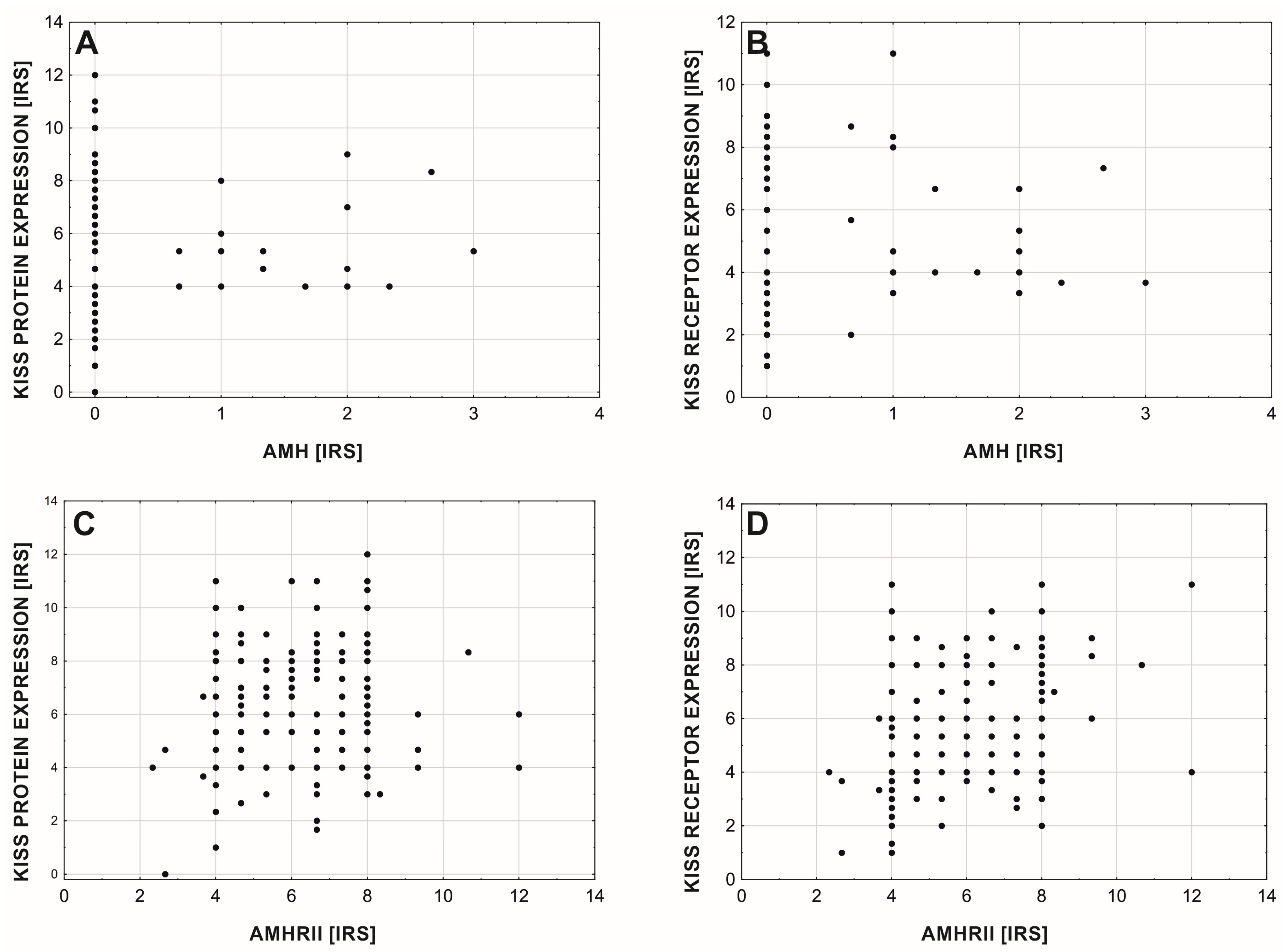
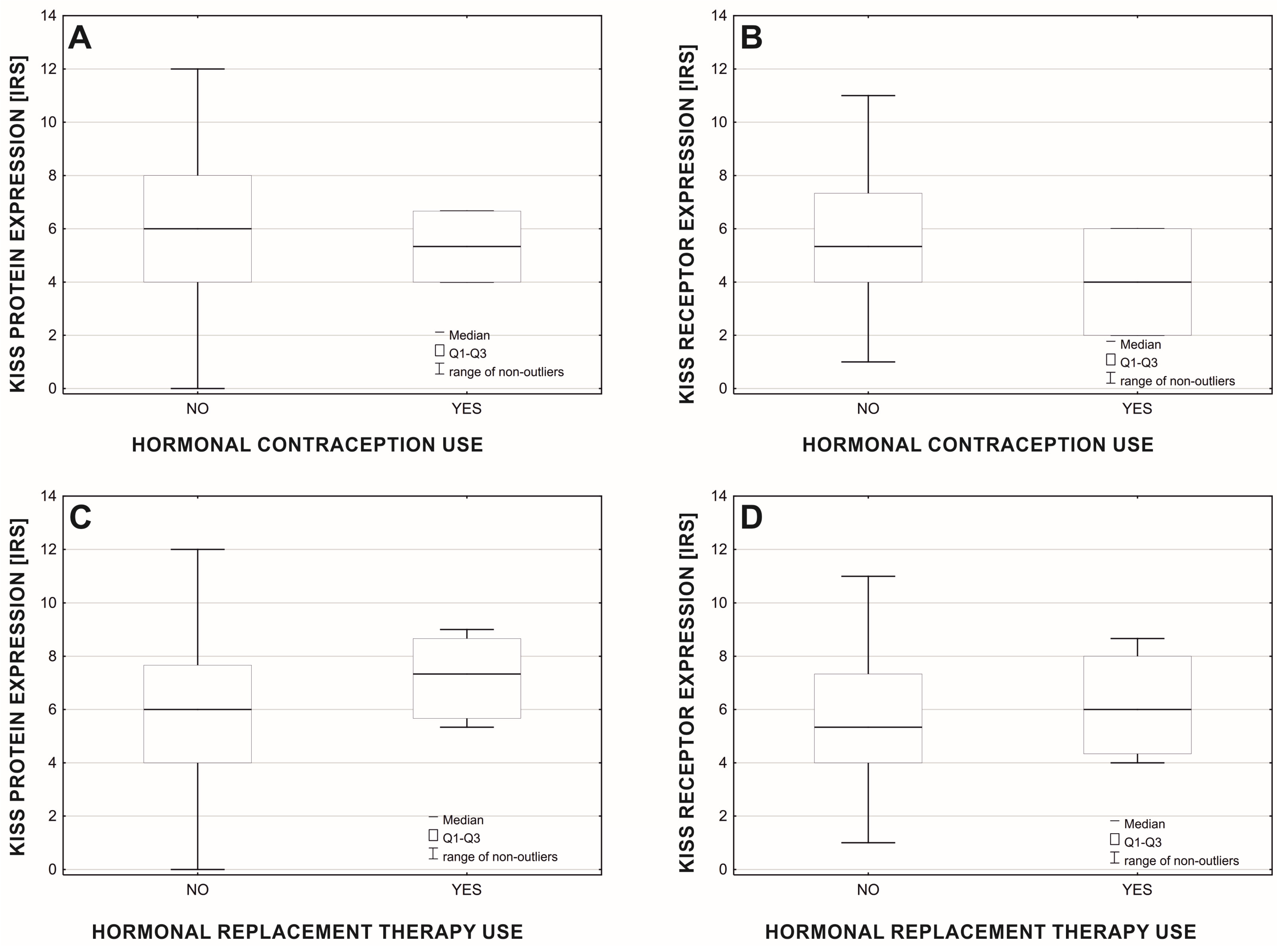
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loosen, S.H.; Luedde, M.; Lurje, G.; Spehlmann, M.; Paffenholz, P.; Ulmer, T.F.; Tacke, F.; Vucur, M.; Trautwein, C.; Neumann, U.P.; et al. Serum Levels of Kisspeptin Are Elevated in Patients with Pancreatic Cancer. Dis. Markers 2019, 2019, 5603474. [Google Scholar] [CrossRef]
- Nejad, S.Z.; Tehrani, F.R.; Zadeh-Vakili, A. The role of Kisspeptin in female reproduction. Int. J. Endocrinol. Metab. 2017, 15, e44337. [Google Scholar] [CrossRef]
- Tsoutsouki, J.; Abbara, A.; Dhillo, W. Novel therapeutic avenues for kisspeptin. Curr. Opin. Pharmacol. 2022, 67, 102319. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Chen, Z.; Li, X.; Cai, E.; Yang, H.; Chen, Y.; Wang, C.; Ju, L.; Deng, W.; Mu, L. Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction. Reprod. Biol. Endocrinol. 2022, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; Izzi-Engbeaya, C.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J. Clin. Investig. 2020, 130, 6739–6753. [Google Scholar] [CrossRef]
- Wang, T.; Cui, X.; Xie, L.; Xing, R.; You, P.; Zhao, Y.; Yang, Y.; Xu, Y.; Zeng, L.; Chen, H.; et al. Kisspeptin Receptor GPR54 Promotes Adipocyte Differentiation and Fat Accumulation in Mice. Front. Physiol. 2018, 9, 209. [Google Scholar] [CrossRef]
- Ziarniak, K.; Dudek, M.; Śliwowska, J.H. Kisspeptyna—Peptyd O Wielu Obliczach. Kosmos 2016, 65, 217–225. [Google Scholar]
- Hu, K.L.; Chang, H.M.; Zhao, H.C.; Yu, Y.; Li, R.; Qiao, J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum. Reprod. Update 2019, 25, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Ye, L.; Mason, M.D.; Jiang, W.G. The Kiss-1/Kiss-1R complex as a negative regulator of cell motility and cancer metastasis (Review). Int. J. Mol. Med. 2013, 32, 747–754. [Google Scholar] [CrossRef]
- Froehlich, K.; Schmidt, A.; Heger, J.I.; Al-Kawlani, B.; Aberl, C.A.; Jeschke, U.; Loibl, S.; Markert, U.R. Breast cancer, placenta and pregnancy. Eur. J. Cancer 2019, 115, 68–78. [Google Scholar] [CrossRef]
- Kim, T.H.; Cho, S.G. Kisspeptin inhibits cancer growth and metastasis via activation of EIF2AK2. Mol. Med. Rep. 2017, 16, 7585–7590. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Law, J.; Cvetkovic, D.D.; Pampillo, M.; McColl, L.; Pape, C.; Guglielmo, G.M.; Postovit, L.M.; Babwah, A.V.; Bhattacharya, M. GPR54 (KISS1R) Transactivates EGFR to Promote Breast Cancer Cell Invasiveness. PLoS ONE 2011, 6, 21599. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Honda, S.; Asada, M.; Ohtaki, T.; Oda, K.; Watanabe, T.; Shintani, Y.; Yamada, T.; Suenaga, M.; Kitada, C.; et al. Metastin Suppresses the Motility and Growth of CHO Cells Transfected with Its Receptor. Biochem. Biophys. Res. Commun. 2001, 286, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef]
- Baba, T.; Kang, H.S.; Hosoe, Y.; Kharma, B.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; et al. Menstrual cyclic change of metastin/GPR54 in endometrium. Med. Mol. Morphol. 2015, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Timologou, A.; Zafrakas, M.; Grimbizis, G.; Miliaras, D.; Kotronis, K.; Stamatopoulos, P.; Tarlatzis, B.C. Immunohistochemical expression pattern of metastasis suppressors KAI1 and KISS1 in endometriosis and normal endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 110–115. [Google Scholar] [CrossRef]
- Guzman, S.; Brackstone, M.; Radovick, S.; Babwah, A.V.; Bhattacharya, M.M. KISS1/KISS1R in cancer: Friend or foe? Front. Endocrinol. (Lausanne) 2018, 9, 437. [Google Scholar] [CrossRef]
- Hata, K.; Dhar, D.K.; Watanabe, Y.; Nakai, H.; Hoshiai, H. Expression of metastin and a G-protein-coupled receptor (AXOR12) in epithelial ovarian cancer. Eur. J. Cancer 2007, 43, 1452–1459. [Google Scholar] [CrossRef]
- Kang, H.S.; Baba, T.; Mandai, M.; Matsumura, N.; Hamanishi, J.; Kharma, B.; Kondoh, E.; Yoshioka, Y.; Oishi, S.; Fujii, N.; et al. GPR54 is a target for suppression of metastasis in endometrial cancer. Mol. Cancer Ther. 2011, 10, 580–590. [Google Scholar] [CrossRef]
- Gowkielewicz, M.; Lipka, A.; Piotrowska, A.; Szadurska-Noga, M.; Nowakowski, J.; Dzięgiel, P.; Majewski, M.K.; Jozwik, M.; Majewska, M. Anti-Müllerian Hormone Expression in Endometrial Cancer Tissue. Int. J. Mol. Sci. 2019, 20, 1325. [Google Scholar] [CrossRef]
- Gowkielewicz, M.; Lipka, A.; Majewska, M.; Piotrowska, A.; Szadurska-Noga, M.; Nowakowski, J.J.; Wiszpolska, M.; Dzięgiel, P.; Wasniewski, T.; Majewski, M.K.; et al. Anti-Müllerian Hormone Type II Receptor Expression in Endometrial Cancer Tissue. Cells 2020, 9, 2312. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models, 3rd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2008; pp. 1–303. [Google Scholar]
- Akaike, H. Likelohood of a model and Information Criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman: New York, NY, USA, 2012; p. 937. [Google Scholar]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; Agresti, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 978-0-471-22618-5. [Google Scholar]
- Dhillo, W.S.; Chaudhri, O.B.; Patterson, M.; Thompson, E.L.; Murphy, K.G.; Badman, M.K.; McGowan, B.M.; Amber, V.; Patel, S.; Ghatei, M.A.; et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 2005, 90, 6609–6615. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, V.; Della Corte, C.M.; Ciardiello, F.; Morgillo, F. Kisspeptin and Cancer: Molecular Interaction, Biological Functions, and Future Perspectives. Front. Endocrinol. (Lausanne) 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; DiPietro, M.J.; Ramaswamy, S.; Crowley, W.F.; Plant, T.M. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology 2006, 147, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Haase, M.; Ziegler, E.; Emons, G.; Gründker, C. Kisspeptin-10 inhibits stromal-derived factor 1-induced invasion of human endometrial cancer cells. Int. J. Gynecol. Cancer 2014, 24, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.K.; Naora, H.; Kubota, H.; Maruyama, R.; Yoshimura, H.; Tonomoto, Y.; Tachibana, M.; Ono, T.; Otani, H.; Nagasue, N. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int. J. Cancer 2004, 111, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Yamaguchi, K.I.; Kaibara, N. Clinical Significance of the Loss of KiSS-1 and Orphan G-Protein-Coupled Receptor (hOT7T175) Gene Expression in Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2004, 10, 1379–1383. [Google Scholar] [CrossRef]
- Kostadima, L.; Pentheroudakis, G.; Pavlidis, N. The missing kiss of life: Transcriptional activity of the metastasis suppressor gene KiSS1 in early breast cancer. Anticancer Res. 2007, 27, 2499–2504. [Google Scholar]
- Sanchez-Carbayo, M.; Capodieci, P.; Cordon-Cardo, C. Tumor suppressor role of KiSS-1 in bladder cancer: Loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am. J. Pathol. 2003, 162, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-Z.; Zhang, H.-H.; Tang, Q.-F.; Zhang, Q.; Li, J.; Liang, Y.; Huang, L.-J.; Zheng, R.-H.; Deng, J.; Zhang, X.-P. Prognostic value of kisspeptin expression in nasopharyngeal carcinoma. Laryngoscope 2014, 124, E167–E174. [Google Scholar] [CrossRef] [PubMed]
- Matthaiou, S.; Kostakis, I.D.; Kykalos, S.; Machairas, N.; Spartalis, E.; Ntikoudi, E.; Lambropoulou, M.; Karayiannakis, A. KISS1 and KISS1R expression in primary and metastatic colorectal cancer. J. Buon 2018, 23, 598–603. [Google Scholar] [PubMed]
- Wang, X.Q.; Fang, P.F.; Zhang, C.; Xu, Y.Y.; Song, X.B.; Liang, J.; Xia, Q.R. Low KISS1 expression predicts poor prognosis for patients with colorectal cancer: A meta-analysis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef]
- Ahmed, M.L.; Ong, K.K.; Dunger, D.B. Childhood obesity and the timing of puberty. Trends Endocrinol. Metab. 2009, 20, 237–242. [Google Scholar] [CrossRef]
- Li, L.; Tian, J.; Zhou, L.; Wu, S.; Zhang, S.; Qi, L.; Zhang, H. Role of kisspeptin/GPR54 in the first trimester trophoblast of women with a history of recurrent spontaneous abortion. Int. J. Clin. Exp. Pathol. 2017, 10, 8161–8173. [Google Scholar] [PubMed]
- Hu, K.-L.; Zhao, H.; Yu, Y.; Li, R. Kisspeptin as a potential biomarker throughout pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Logie, J.J.; Denison, F.C.; Riley, S.C.; Ramaesh, T.; Forbes, S.; Norman, J.E.; Reynolds, R.M. Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin. Endocrinol. 2012, 76, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Reynolds, R.M.; Leask, R.; Shearing, C.H.; Calder, A.A.; Riley, S.C. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat. Diagn. 2009, 29, 982–985. [Google Scholar] [CrossRef]
- Smets, E.M.L.; Deurloo, K.L.; Go, A.T.J.I.; van Vugt, J.M.G.; Blankenstein, M.A.; Oudejans, C.B.M. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat. Diagn. 2008, 28, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, A.; Miljic, D.; Ljubić, A.; Patterson, M.; Ghatei, M.; Stamenkoví, J.; Nikolic-Djurovic, M.; Pekic, S.; Doknic, M.; Glišić, A.; et al. Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr. Res. 2012, 37, 78–88. [Google Scholar] [CrossRef]
- Jayasena, C.N.; Nijher, G.M.K.; Narayanaswamy, S.; Silva, A.D.; Abbara, A.; Ghatei, M.A.; Bloom, S.R.; Bridges, N.; Dhillo, W.S. Age-dependent elevations in plasma kisspeptin are observed in boys and girls when compared with adults. Ann. Clin. Biochem. Int. J. Lab. Med. 2014, 51, 89–96. [Google Scholar] [CrossRef]
- Park, D.W.; Lee, S.K.; Hong, S.R.; Han, A.R.; Kwak-Kim, J.; Yang, K.M. Expression of Kisspeptin and its Receptor GPR54 in the First Trimester Trophoblast of Women with Recurrent Pregnancy Loss. Am. J. Reprod. Immunol. 2012, 67, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Aikawa, S.; Iijima, N.; Ozawa, H. Rapid modulation of hypothalamic Kiss1 levels by the suckling stimulus in the lactating rat. J. Endocrinol. 2015, 227, 105–115. [Google Scholar] [CrossRef] [PubMed]
- True, C.; Kirigiti, M.; Ciofi, P.; Grove, K.L.; Smith, M.S. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J. Neuroendocrinol. 2011, 23, 52–64. [Google Scholar] [CrossRef]
- Matjila, M.; Millar, R.; van der Spuy, Z.; Katz, A. Elevated placental expression at the maternal–fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens 2016, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shirai, R.; Hontani, M.; Shinooka, R.; Hasegawa, A.; Kichise, T.; Yamashita, T.; Yoshizawa, H.; Watanabe, R.; Matsuyama, T.; et al. Potent Vasoconstrictor Kisspeptin-10 Induces Atherosclerotic Plaque Progression and Instability: Reversal by its Receptor GPR54 Antagonist. J. Am. Heart Assoc. 2017, 6, e005790. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sato, K. Roles of the kisspeptin/GPR54 system in pathomechanisms of atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, I.; Smillie, S.-J.; Bodkin, J.V.; Fernandes, E.; O’Byrne, K.T.; Brain, S.D. The Vasoactive Potential of Kisspeptin-10 in the Peripheral Vasculature. PLoS ONE 2011, 6, e14671. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Lehman, M.N.; Smith, J.T.; Coolen, L.M.; de Oliveira, C.V.R.; Jafarzadehshirazi, M.R.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin Neurons in the Arcuate Nucleus of the Ewe Express Both Dynorphin A and Neurokinin B. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Coolen, L.M.; Lehman, M.N. A Role for Neurokinin B in Pulsatile GnRH Secretion in the Ewe. Neuroendocrinology 2014, 99, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; Han, S.Y.; Piet, R.; McLennan, T.; Kane, G.M.; Ng, J.; Porteous, R.W.; Kim, J.S.; Colledge, W.H.; Iremonger, K.J.; et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10216–E10223. [Google Scholar] [CrossRef] [PubMed]
- Akkoyun, D.C.; Akyuz, A.; Alpsoy, S.; Aydin, M.; Erselcan, K. Serum neurokinin B levels in newly diagnosed nondipper hypertensive patients. Blood Press. Monit. 2015, 20, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.A.; Dhillo, W.S. Kisspeptin across the human lifespan: Evidence from animal studies and beyond. J. Endocrinol. 2016, 229, R83–R98. [Google Scholar] [CrossRef] [PubMed]
- Colledge, W.H.; d’Anglemont de Tassigny, X. The role of kisspeptin signalling in the regulation of the GnRH-gonadotrophin ovarian axis in mice. Ann. Endocrinol. 2010, 71, 198–200. [Google Scholar] [CrossRef]
- Lippincott, M.F.; Chan, Y.M.; Morales, D.R.; Seminara, S.B. Continuous kisspeptin administration in postmenopausal women: Impact of estradiol on luteinizing hormone secretion. J. Clin. Endocrinol. Metab. 2017, 102, 2091–2099. [Google Scholar] [CrossRef]
- George, J.T.; Anderson, R.A.; Millar, R.P. Kisspeptin-10 stimulation of gonadotrophin secretion in women is modulated by sex steroid feedback. Hum. Reprod. 2012, 27, 3552–3559. [Google Scholar] [CrossRef]
- Chan, Y.M.; Butler, J.P.; Sidhoum, V.F.; Pinnell, N.E.; Seminara, S.B. Kisspeptin administration to women: A window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J. Clin. Endocrinol. Metab. 2012, 97, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- George, J.T.; Seminara, S.B. Kisspeptin and the hypothalamic control of reproduction: Lessons from the human. Endocrinology 2012, 153, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Z.; Jiang, W.; Ling, Y.; Kuang, H. Reproductive functions of Kisspeptin/KISS1R Systems in the Periphery. Reprod. Biol. Endocrinol. 2019, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Mattam, U.; Talari, N.; Thiriveedi, V.; Fareed, M.; Velmurugan, S.; Mahadev, K.; Sepuri, N.B. Aging reduces kisspeptin receptor (GPR54) expression levels in the hypothalamus and extra-hypothalamic brain regions. Exp. Ther. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Thornton, K.; Bonney, E.; Cipolla, M.J.; Charron, M.J.; Buyuk, E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J. Assist. Reprod. Genet. 2016, 33, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Shah, M.; Habib, S.H.; Shah, F.A.; Malik, M.O. Correlation of plasma kisspeptin with total testosterone levels in smokeless tobacco and smoking tobacco users in a healthy cohort: A cross-sectional study. Andrologia 2019, 51, e13409. [Google Scholar] [CrossRef] [PubMed]
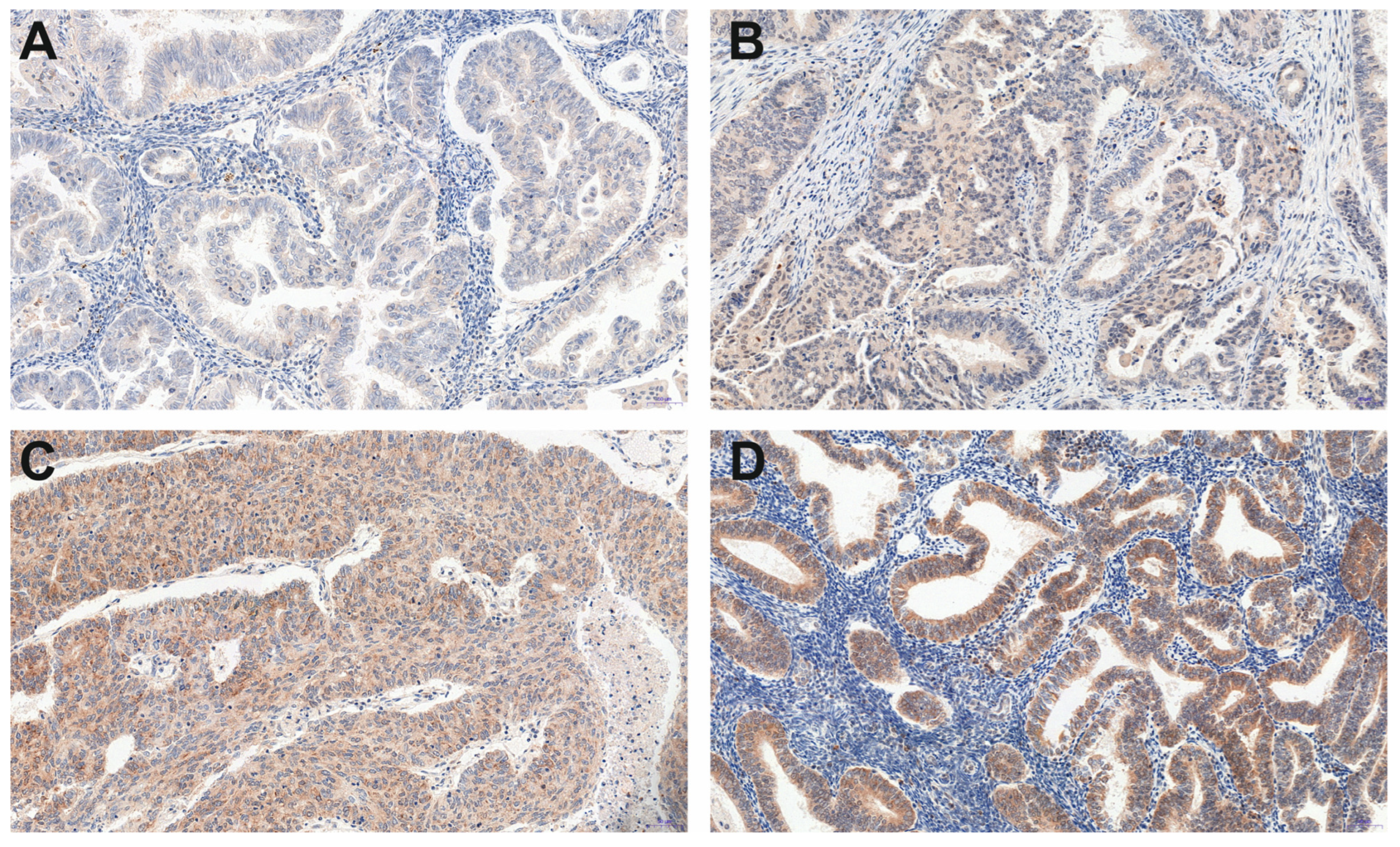
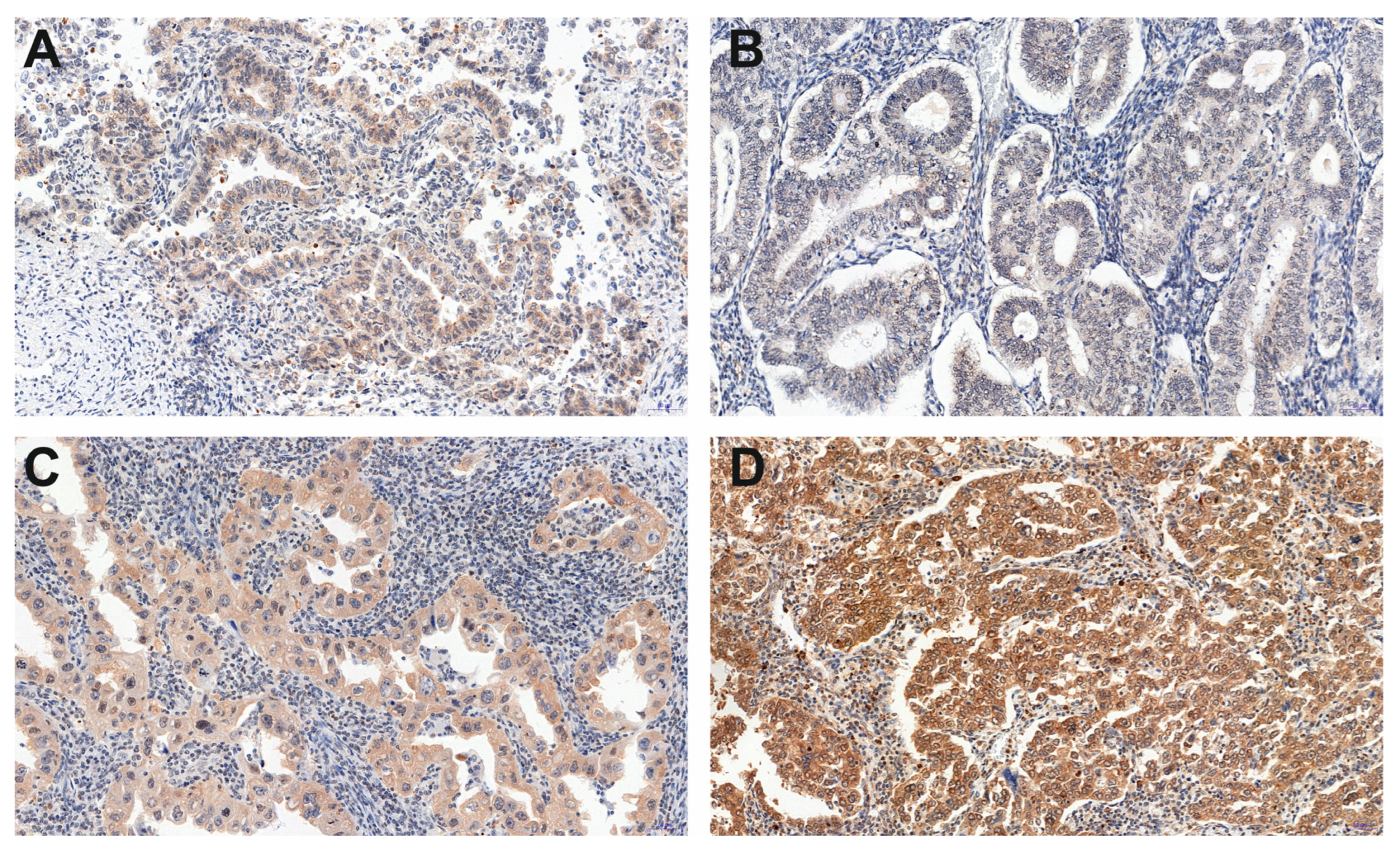
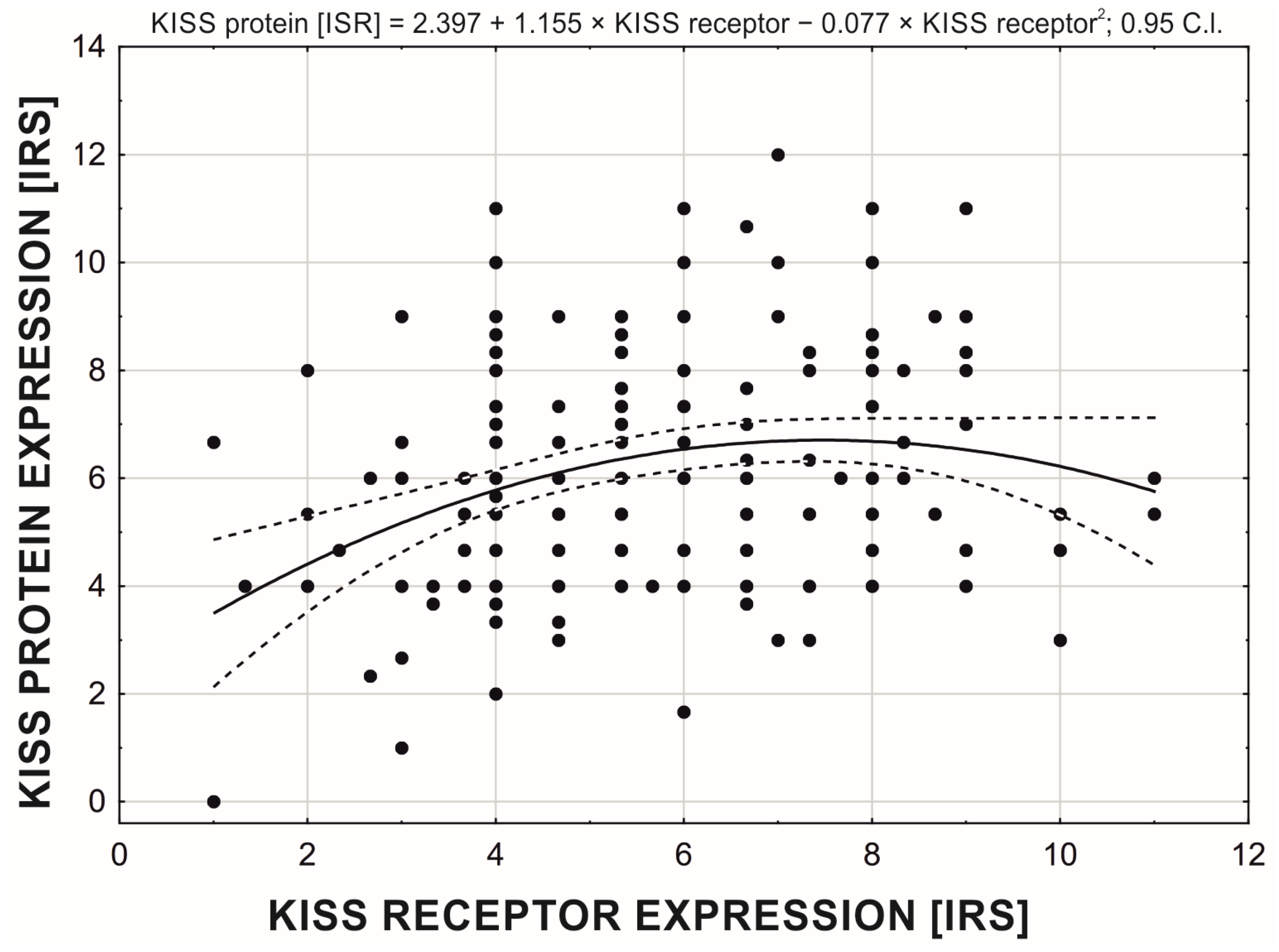
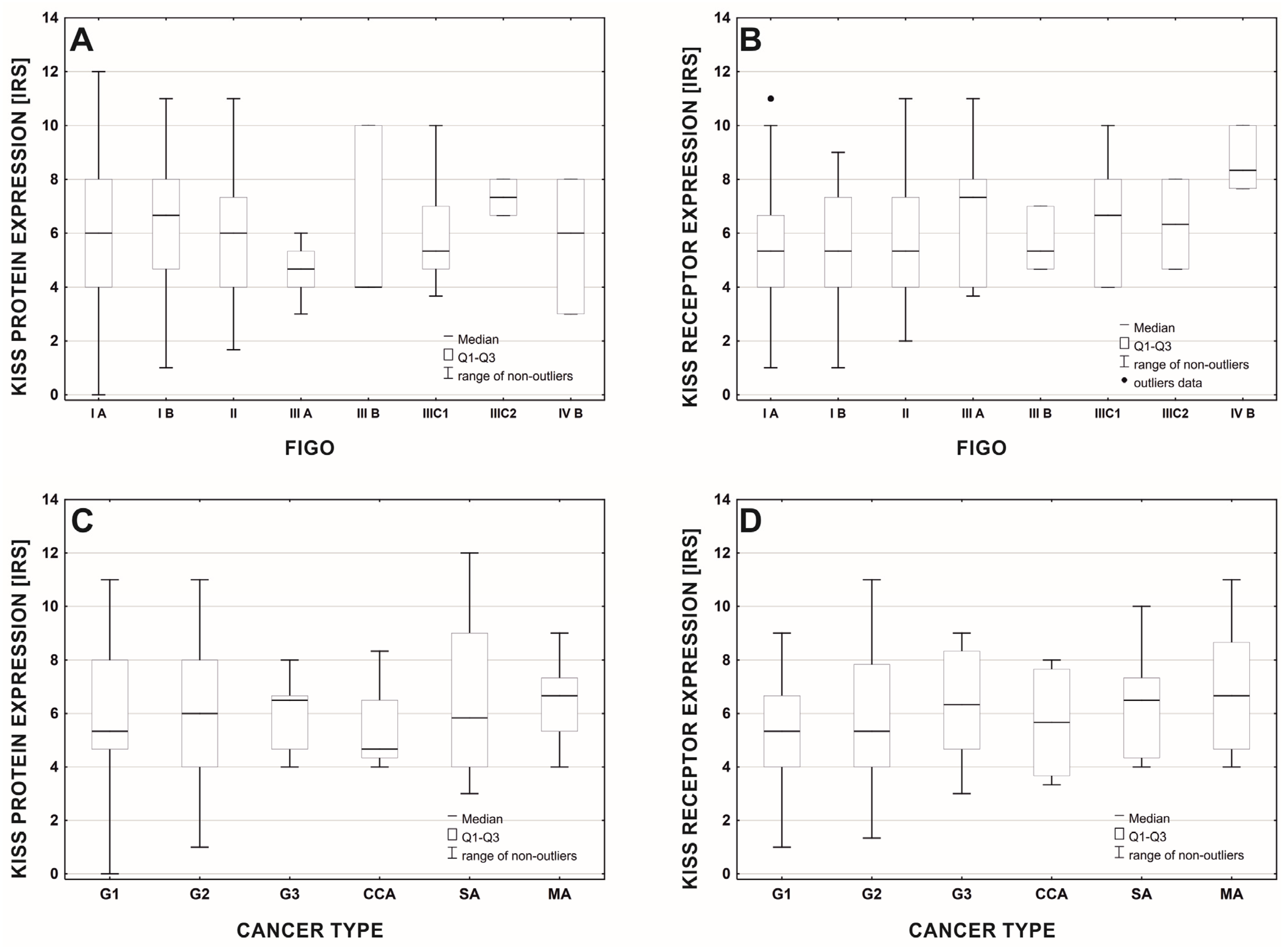

| Histopathological Type of Endometrial Lesion | Number of Patients | Variable | Mean ± SD | Minimal Expression | Maximal Expression |
|---|---|---|---|---|---|
| Endometrioid adenocarcinoma G1 | 47 | KISS | 5.96 ± 2.28 | 0.00 | 11.00 |
| GPR54 | 5.35 ± 1.97 | 1.00 | 9.00 | ||
| Endometrioid adenocarcinoma G2 | 144 | KISS | 6.20 ± 2.17 | 1.00 | 11.00 |
| GPR54 | 5.76 ± 2.08 | 1.33 | 11.00 | ||
| Endometrioid adenocarcinoma G3 | 6 | KISS | 6.06 ± 1.47 | 4.00 | 8.00 |
| GPR54 | 6.28 ± 2.32 | 3.00 | 9.00 | ||
| Serous adenocarcinoma (SA) | 8 | KISS | 6.58 ± 3.31 | 3.00 | 12.00 |
| GPR54 | 6.29 ± 2.06 | 4.00 | 10.00 | ||
| Clear cell adenocarcinoma (CCA) | 4 | KISS | 5.42 ± 1.97 | 4.00 | 8.00 |
| GPR54 | 5.67 ± 2.34 | 3.33 | 8.00 | ||
| Mixed adenocarcinoma (MA) | 5 | KISS | 6.47 ± 1.91 | 4.00 | 9.00 |
| GPR54 | 7.00 ± 2.89 | 4.00 | 11.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowkielewicz, M.; Lipka, A.; Piotrowska, A.; Szadurska-Noga, M.; Nowakowski, J.J.; Lepiarczyk, E.; Wiszpolska, M.; Waśniewski, T.; Dzięgiel, P.; Kaleczyc, J.; et al. Kisspeptin and GPR54 Receptor Expression in Endometrial Cancer Tissue. Cancers 2023, 15, 1228. https://doi.org/10.3390/cancers15041228
Gowkielewicz M, Lipka A, Piotrowska A, Szadurska-Noga M, Nowakowski JJ, Lepiarczyk E, Wiszpolska M, Waśniewski T, Dzięgiel P, Kaleczyc J, et al. Kisspeptin and GPR54 Receptor Expression in Endometrial Cancer Tissue. Cancers. 2023; 15(4):1228. https://doi.org/10.3390/cancers15041228
Chicago/Turabian StyleGowkielewicz, Marek, Aleksandra Lipka, Aleksandra Piotrowska, Marta Szadurska-Noga, Jacek J. Nowakowski, Ewa Lepiarczyk, Marta Wiszpolska, Tomasz Waśniewski, Piotr Dzięgiel, Jerzy Kaleczyc, and et al. 2023. "Kisspeptin and GPR54 Receptor Expression in Endometrial Cancer Tissue" Cancers 15, no. 4: 1228. https://doi.org/10.3390/cancers15041228
APA StyleGowkielewicz, M., Lipka, A., Piotrowska, A., Szadurska-Noga, M., Nowakowski, J. J., Lepiarczyk, E., Wiszpolska, M., Waśniewski, T., Dzięgiel, P., Kaleczyc, J., Majewski, M. K., & Majewska, M. (2023). Kisspeptin and GPR54 Receptor Expression in Endometrial Cancer Tissue. Cancers, 15(4), 1228. https://doi.org/10.3390/cancers15041228







