Targeted Degradation of Androgen Receptor by VNPP433-3β in Castration-Resistant Prostate Cancer Cells Implicates Interaction with E3 Ligase MDM2 Resulting in Ubiquitin-Proteasomal Degradation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Immunoblotting
2.3. Preparation of Nuclear and Cytoplasmic Fractions
2.4. Molecular Docking
2.5. Co-Immunoprecipitation (Co-IP) Assay
2.6. Real Time Quantitative Studies of AR Degradation in CRISPR Knock-In HiBiT Reporter Cell Line
2.7. RNA-Sequencing and Transcriptome Analyses
2.8. Statistical Analysis
3. Results
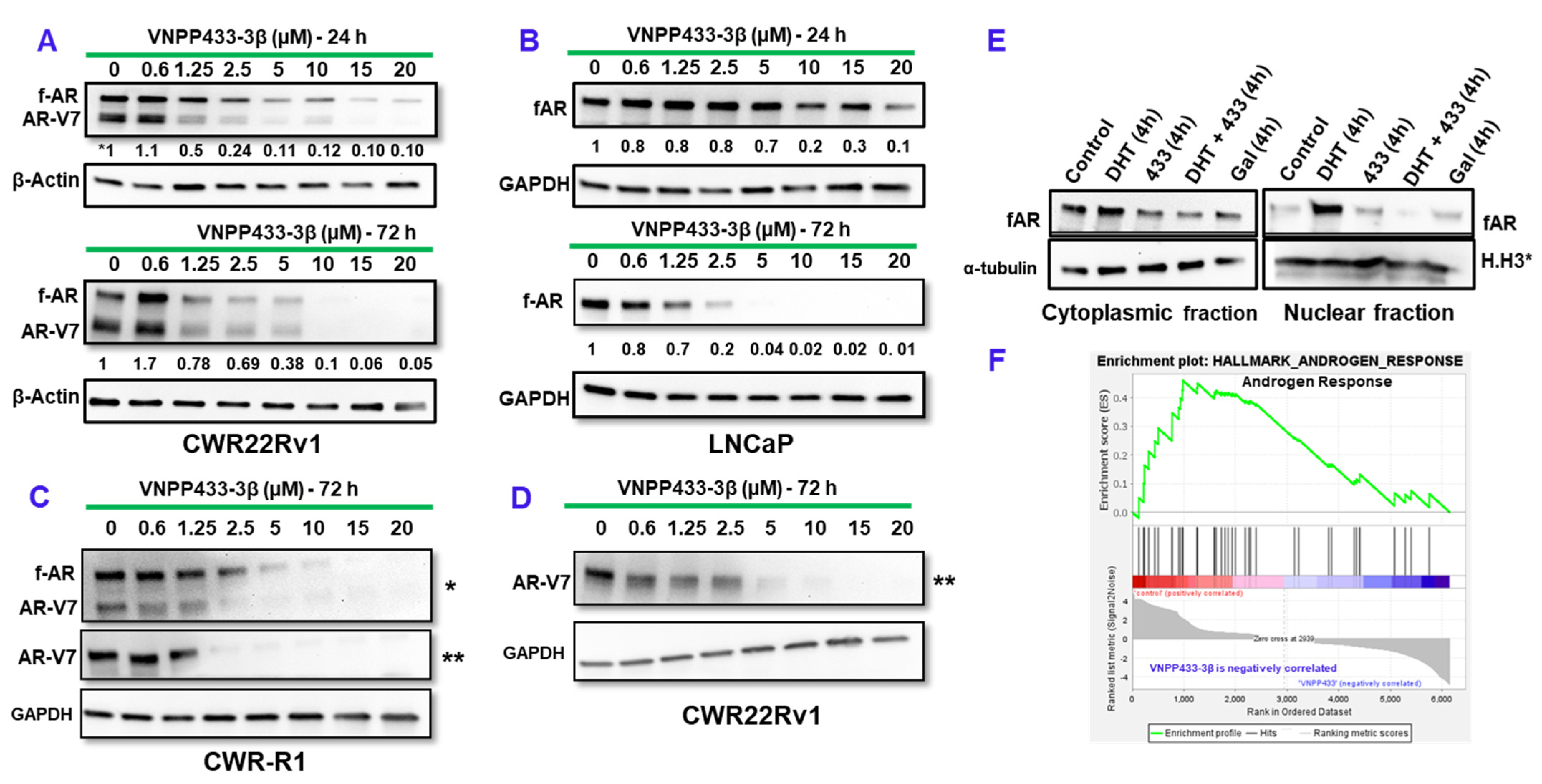
3.1. VNPP433-3β Induces Targeted Degradation of AR/AR-V7
3.2. Degradation of f-AR by VNPP433-3β Leads to Its Decreased Nuclear Translocation
3.3. Decrease in f-AR/AR-V7 by VNPP433-3β Is Due to Enhanced Proteasomal Degradation
3.4. Real Time Quantitative Assay of VNPP433-3β-Induced AR Degradation in Live CWR22Rv1 Cells
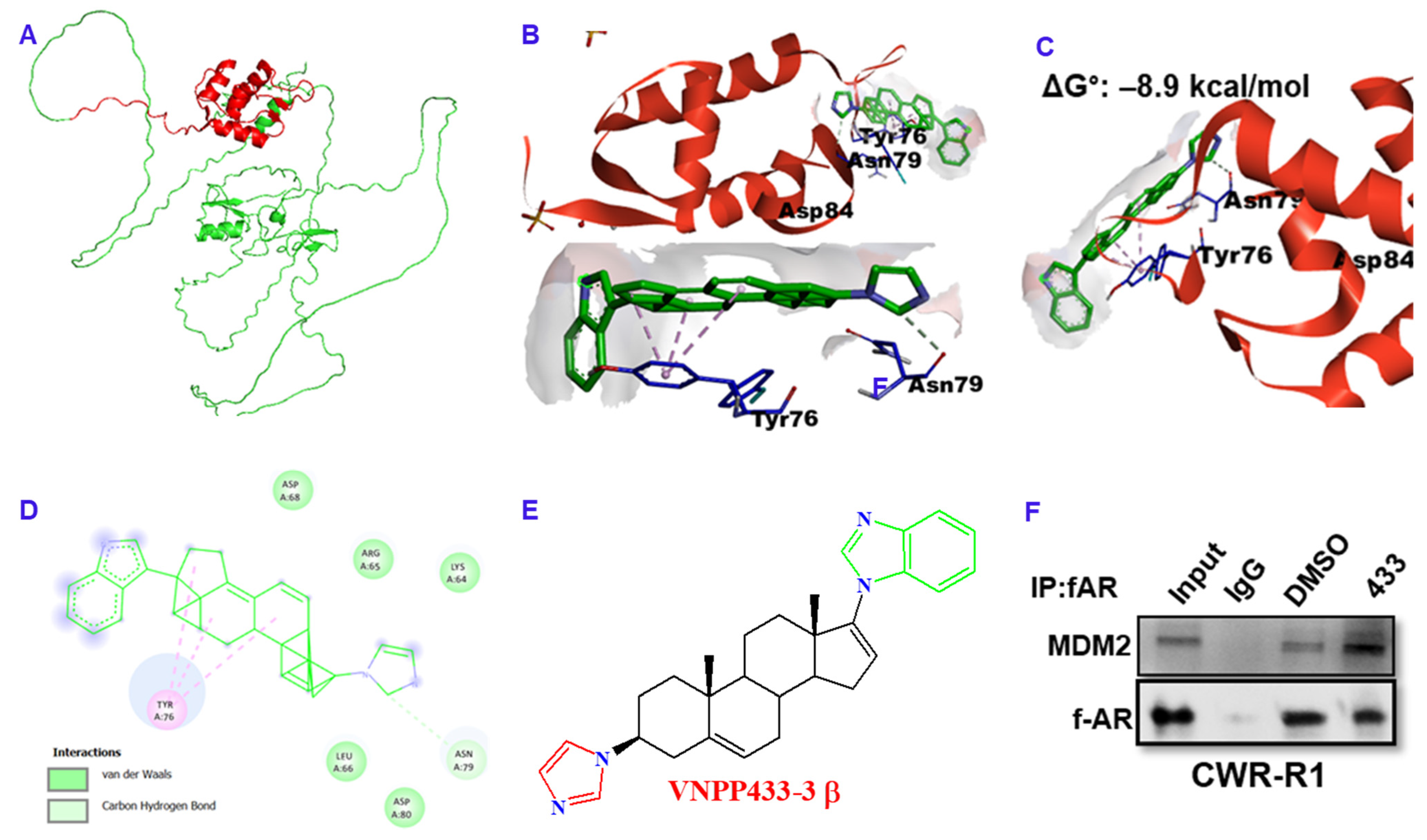
3.5. VNPP433-3β Acts as Molecular Glue between AR and MDM2
3.6. RNA Sequencing Shows Transcriptional Downregulation of Several AR-Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mullard, A. Targeted protein degraders crowd into the clinic. Nat. Rev. Drug Discov. 2021, 20, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.Z.; Chory, E.J.; Crabtree, G.R. Chemically induced proximity in biology and medicine. Science 2018, 359, eaao5902. [Google Scholar] [CrossRef]
- Gerry, C.J.; Schreiber, S.L. Unifying principles of bifunctional, proximity-inducing small molecules. Nat. Chem. Biol. 2020, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Ding, Y.; He, S.; Sheng, C. Molecular Glues for Targeted Protein Degradation: From Serendipity to Rational Discovery. J. Med. Chem. 2021, 64, 10606–10620. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Avgeris, I.; Pliatsika, D.; Nikolaropoulos, S.S.; Fousteris, M.A. Targeting androgen receptor for prostate cancer therapy: From small molecules to PROTACs. Bioorg. Chem. 2022, 128, 106089. [Google Scholar] [CrossRef]
- Beretta, G.L.; Zaffaroni, N. Androgen Receptor-Directed Molecular Conjugates for Targeting Prostate Cancer. Front. Chem. 2019, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Han, X. Targeting androgen receptor degradation with PROTACs from bench to bedside. Biomed. Pharmacother. 2023, 158, 114112. [Google Scholar] [CrossRef]
- Mohler, M.L.; Sikdar, A.; Ponnusamy, S.; Hwang, D.J.; He, Y.; Miller, D.D.; Narayanan, R. An Overview of Next-Generation Androgen Receptor-Targeted Therapeutics in Development for the Treatment of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 2124. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, S. Therapeutic Strategies to Target the Androgen Receptor. J. Med. Chem. 2022, 65, 8772–8797. [Google Scholar] [CrossRef]
- Kwegyir-Afful, A.K.; Ramalingam, S.; Ramamurthy, V.P.; Purushottamachar, P.; Murigi, F.N.; Vasaitis, T.S.; Huang, W.; Kane, M.A.; Zhang, Y.; Ambulos, N.; et al. Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3beta Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo. Cancers 2019, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Purushottamachar, P.; Thomas, E.; Thankan, R.S.; Rudchenko, V.; Huang, G.; Njar, V.C.O. Large-scale synthesis of galeterone and lead next generation galeterone analog VNPP433-3beta. Steroids 2022, 185, 109062. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Huang, W.; Kane, M.A.; Zhang, Y.; Ambulos, N.P.; Weber, D.J.; Njar, V.C.O. Novel AR/AR-V7 and Mnk1/2 Degrader, VNPP433-3beta: Molecular Mechanisms of Action and Efficacy in AR-Overexpressing Castration Resistant Prostate Cancer In Vitro and In Vivo Models. Cells 2022, 11, 2699. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Huang, W.; Kane, M.A.; Zhang, Y.; Ambulos, N.; Weber, D.J.; Njar, V.C.O. Transcriptome profiling reveals that VNPP433-3beta, the lead next-generation galeterone analog inhibits prostate cancer stem cells by downregulating epithelial-mesenchymal transition and stem cell markers. Mol. Carcinog. 2022, 61, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G.; et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2019, 129, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Gopalakrishnan, V.; Somasagara, R.R.; Choudhary, B.; Raghavan, S.C. Extract of Vernonia condensata, Inhibits Tumor Progression and Improves Survival of Tumor-allograft Bearing Mouse. Sci. Rep. 2016, 6, 23255. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cai, C.; Gao, S.; Simon, N.I.; Shen, H.C.; Balk, S.P. Galeterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptor. Clin. Cancer Res. 2014, 20, 4075–4085. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Gopalakrishnan, V.; Hegde, M.; Kumar, S.; Karki, S.S.; Raghavan, S.C.; Choudhary, B. A Novel Resveratrol Based Tubulin Inhibitor Induces Mitotic Arrest and Activates Apoptosis in Cancer Cells. Sci. Rep. 2016, 6, 34653. [Google Scholar] [CrossRef]
- Riching, K.M.; Mahan, S.; Corona, C.R.; McDougall, M.; Vasta, J.D.; Robers, M.B.; Urh, M.; Daniels, D.L. Quantitative Live-Cell Kinetic Degradation and Mechanistic Profiling of PROTAC Mode of Action. ACS Chem. Biol. 2018, 13, 2758–2770. [Google Scholar] [CrossRef]
- D’Abronzo, L.S.; Bose, S.; Crapuchettes, M.E.; Beggs, R.E.; Vinall, R.L.; Tepper, C.G.; Siddiqui, S.; Mudryj, M.; Melgoza, F.U.; Durbin-Johnson, B.P.; et al. The androgen receptor is a negative regulator of eIF4E phosphorylation at S209: Implications for the use of mTOR inhibitors in advanced prostate cancer. Oncogene 2017, 36, 6359–6373. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, K.Z.; Xu, Y.; Dar, J.A.; Eisermann, K.; Pascal, L.E.; Parrinello, E.; Ai, J.; Johnston, P.A.; Nelson, J.B.; Wipf, P.; et al. Inhibition of Androgen Receptor Nuclear Localization and Castration-Resistant Prostate Tumor Growth by Pyrroloimidazole-based Small Molecules. Mol. Cancer Ther. 2017, 16, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Fan, L.; Hussain, A. Implications of ubiquitin ligases in castration-resistant prostate cancer. Curr. Opin. Oncol. 2015, 27, 172–176. [Google Scholar] [CrossRef]
- Schwinn, M.K.; Machleidt, T.; Zimmerman, K.; Eggers, C.T.; Dixon, A.S.; Hurst, R.; Hall, M.P.; Encell, L.P.; Binkowski, B.F.; Wood, K.V. CRISPR-Mediated Tagging of Endogenous Proteins with a Luminescent Peptide. ACS Chem. Biol. 2018, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Wang, L.; Hu, Y.C.; Altuwaijri, S.; Chang, C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002, 21, 4037–4048. [Google Scholar] [CrossRef]
- Gaughan, L.; Logan, I.R.; Neal, D.E.; Robson, C.N. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005, 33, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Vummidi Giridhar, P.; Williams, K.; VonHandorf, A.P.; Deford, P.L.; Kasper, S. Constant Degradation of the Androgen Receptor by MDM2 Conserves Prostate Cancer Stem Cell Integrity. Cancer Res. 2019, 79, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Fasan, R.; Dias, R.L.; Moehle, K.; Zerbe, O.; Obrecht, D.; Mittl, P.R.; Grutter, M.G.; Robinson, J.A. Structure-activity studies in a family of beta-hairpin protein epitope mimetic inhibitors of the p53-HDM2 protein-protein interaction. Chembiochem 2006, 7, 515–526. [Google Scholar] [CrossRef]
- Wu, D.; Terrian, D.M. Regulation of caveolin-1 expression and secretion by a protein kinase cepsilon signaling pathway in human prostate cancer cells. J. Biol. Chem. 2002, 277, 40449–40455. [Google Scholar] [CrossRef]
- Jin, H.J.; Kim, J.; Yu, J. Androgen receptor genomic regulation. Transl. Androl. Urol. 2013, 2, 157–177. [Google Scholar] [CrossRef]
- Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Njar, V.C. Mechanistic insights on the effects of the lead next generation galeterone analog, VNPP433-3β in castration resistant prostate cancer. Molecular Cancer Therapeutics 20. Mechanistic insights on the effects of the lead next generation galeterone analog, VNPP433-3β in castration resistant prostate cancer. Mol. Cancer Ther. 2021, 20, LBA027. [Google Scholar]
- Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Guo, J.; Parise, R.A.; Beumer, J.H.; Njar, V.C.O. Murine Toxicology and Pharmacokinetics of Lead Next Generation Galeterone Analog, VNPP433-3beta. Steroids 2023, 192, 109184. [Google Scholar] [CrossRef] [PubMed]
- Cato, L.; de Tribolet-Hardy, J.; Lee, I.; Rottenberg, J.T.; Coleman, I.; Melchers, D.; Houtman, R.; Xiao, T.; Li, W.; Uo, T.; et al. ARv7 Represses Tumor-Suppressor Genes in Castration-Resistant Prostate Cancer. Cancer Cell 2019, 35, 401–413.e6. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]


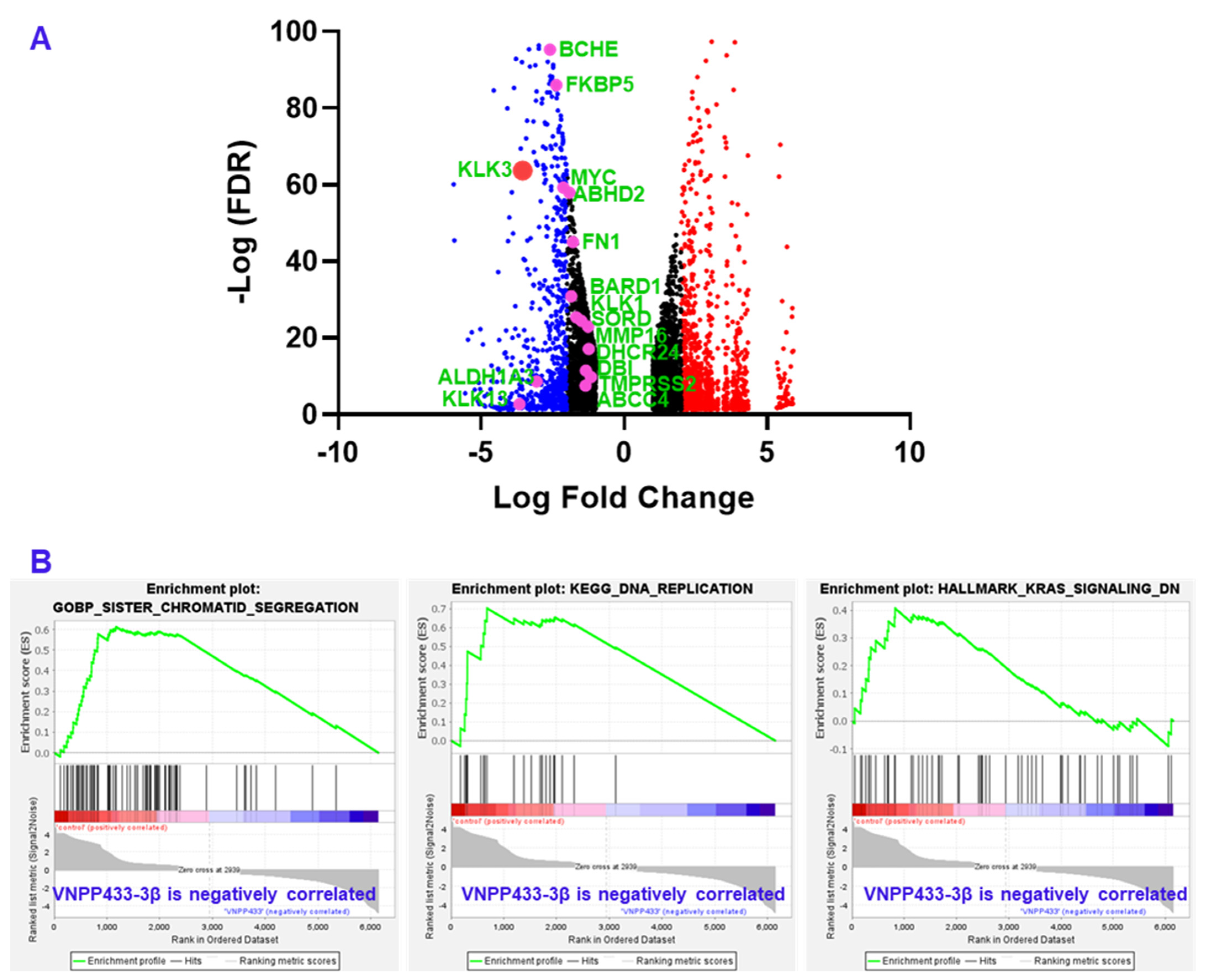

| Gene | LFC | −Log (FDR) | Remarks |
|---|---|---|---|
| KLK3 | −3.549878878 | 63.74105 | Prostate specific antigen (PSA) |
| KLK1 | −1.499349751 | 24.47005 | Serine protease; Oncoprotein |
| KLK13 | −3.661495531 | 2.685301 | Serine protease; Oncoprotein |
| NKX3-1 | −2.987220702 | 106.1704 | Transcription factor in prostate epithelium, tumor suppressor |
| DHCR24 | −1.242758268 | 17.15381 | Flavin adenine dinucleotide-dependent oxidoreductase; cholesterol biosynthesis |
| DBI | −1.340163994 | 11.44401 | acyl-CoA binding protein, cell signaling, steroid synthesis |
| MMP16 | −1.264127518 | 22.95686 | Cancer metastasis, matrix metalloproteinase |
| FKBP5 | −2.367045284 | 86.07973 | Co-chaperone that regulates steroid hormone receptors, immunoregulation |
| ALDH1A3 | −3.05609345 | 8.603198 | Tumorigenesis, detoxification of aldehydes and lipid peroxidation |
| BCHE | −2.590390622 | 95.29679 | Nonspecific cholinesterase |
| ABHD2 | −1.915809808 | 57.9697 | Enables nuclear steroid receptor activity, highly expressed in spermatozoa |
| SORD | −1.673855387 | 25.39808 | Carbohydrate metabolism |
| ABCC4 | −1.351567948 | 7.512045 | Multidrug resistance, regulates intracellular yclic nucleotide levels, cAMP-dependent signaling to the nucleus |
| BARD1 | −1.850384403 | 30.88807 | DNA repair, E3 ubiquitin ligase, transcriptional regulation to maintain genomic stability |
| TMPRSS2 | −1.160624783 | 9.802972 | Transmembrane Serine Protease 2, upregulated by androgenic hormones in prostate cancer, facilitates virus entry |
| FN1 | −1.795774135 | 45.01869 | Component of extracellular matrix, upregulated in multiple cancers |
| MYC | −2.129324177 | 59.28253 | Oncoprotein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, E.; Thankan, R.S.; Purushottamachar, P.; Weber, D.J.; Njar, V.C.O. Targeted Degradation of Androgen Receptor by VNPP433-3β in Castration-Resistant Prostate Cancer Cells Implicates Interaction with E3 Ligase MDM2 Resulting in Ubiquitin-Proteasomal Degradation. Cancers 2023, 15, 1198. https://doi.org/10.3390/cancers15041198
Thomas E, Thankan RS, Purushottamachar P, Weber DJ, Njar VCO. Targeted Degradation of Androgen Receptor by VNPP433-3β in Castration-Resistant Prostate Cancer Cells Implicates Interaction with E3 Ligase MDM2 Resulting in Ubiquitin-Proteasomal Degradation. Cancers. 2023; 15(4):1198. https://doi.org/10.3390/cancers15041198
Chicago/Turabian StyleThomas, Elizabeth, Retheesh S. Thankan, Puranik Purushottamachar, David J. Weber, and Vincent C. O. Njar. 2023. "Targeted Degradation of Androgen Receptor by VNPP433-3β in Castration-Resistant Prostate Cancer Cells Implicates Interaction with E3 Ligase MDM2 Resulting in Ubiquitin-Proteasomal Degradation" Cancers 15, no. 4: 1198. https://doi.org/10.3390/cancers15041198
APA StyleThomas, E., Thankan, R. S., Purushottamachar, P., Weber, D. J., & Njar, V. C. O. (2023). Targeted Degradation of Androgen Receptor by VNPP433-3β in Castration-Resistant Prostate Cancer Cells Implicates Interaction with E3 Ligase MDM2 Resulting in Ubiquitin-Proteasomal Degradation. Cancers, 15(4), 1198. https://doi.org/10.3390/cancers15041198








