Early Hospital Discharge on Day Two Post Robotic Lobectomy with Telehealth Home Monitoring: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Inclusion and Exclusion Criteria
2.3. Protocol
2.4. Telehealth Home Monitoring
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Enrolment and Drop Out
3.2. Postoperative Period
3.3. Teleconsultation
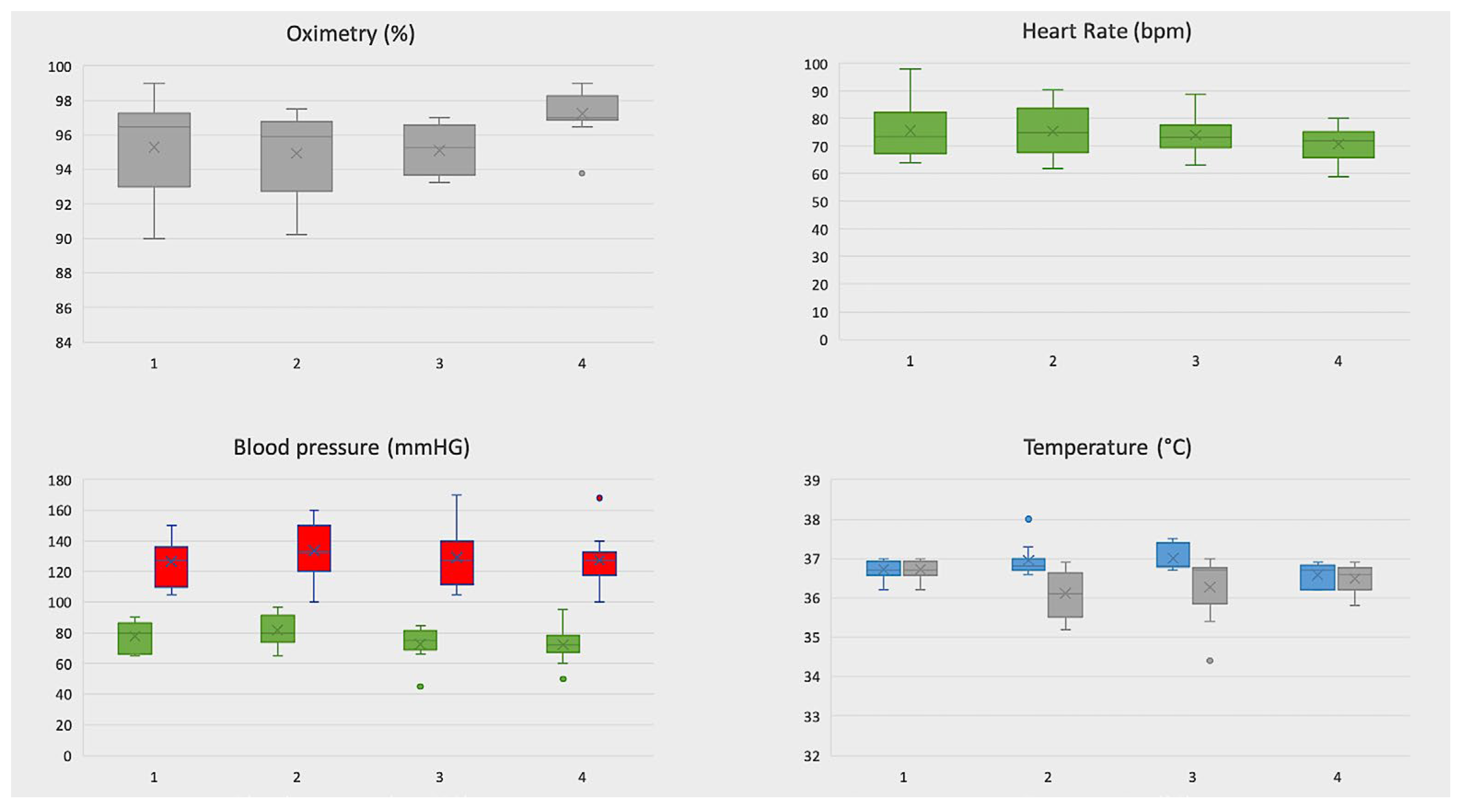
3.4. Telemonitoring of Vital Signs
3.5. Complication Rate and Readmission
3.6. Costs Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falcoz, P.-E.; Puyraveau, M.; Thomas, P.; Decaluwe, H.; Hürtgen, M.; Petersen, R.H.; Hansen, H.; Brunelli, A.; Van Raemdonck, D.; Dahan, M.; et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: A propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur. J. Cardio-Thorac. Surg. 2015, 49, 602–609. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERASVR) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Patel, Y.S.; Hanna, W.C.; Fahim, C.; Shargall, Y.; Waddell, T.K.; Yasufuku, K.; Machuca, T.N.; Pipkin, M.; Baste, J.-M.; Xie, F.; et al. RAVAL trial: Protocol of an international, multi-centered, blinded, randomized controlled trial comparing robotic-assisted versus video-assisted lobectomy for early-stage lung cancer. PLoS ONE 2022, 17, e0261767. [Google Scholar] [CrossRef]
- Yang, H.-X. Long-term survival of early-stage non-small cell lung cancer patients who underwent robotic procedure: A propensity score-matched study. Chin. J. Cancer 2016, 35, 66. [Google Scholar] [CrossRef]
- Jin, R.M.; Zheng, Y.; Yuan, Y.; Han, D.M.; Cao, Y.; Zhang, Y.M.; Li, C.M.; Xiang, J.; Zhang, Z.; Niu, Z.; et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy. Ann. Surg. 2021, 275, 295–302. [Google Scholar] [CrossRef]
- Huang, L.; Kehlet, H.; Petersen, R.H. Reasons for staying in hospital after video-assisted thoracoscopic surgery lobectomy. BJS Open 2022, 6, zrac050. [Google Scholar] [CrossRef]
- Haveman, M.E.; Jonker, L.T.; Hermens, H.J.; Tabak, M.; de Vries, J.P. Effectiveness of current perioperative telemonitoring on postoperative outcome in patients undergoing major abdominal surgery: A systematic review of controlled trials. J. Telemed. Telecare. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Light, D.; Geraci, T.C.; Chang, S.H.; Cerfolio, R.J. Novel Pre- and Postoperative Care Using Telemedicine. Front. Surg. 2020, 7, 596970. [Google Scholar] [CrossRef] [PubMed]
- Soon, S.; Svavarsdottir, H.; Downey, C.; Jayne, D.V. Wearable devices for remote vital signs monitoring in the outpatient setting: An overview of the field. BMJ Innov. 2020, 6, 55–71. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Amato, S.; Sgroi, D.; Di Giovanni, C.; Poliandri, G.; Cioffi, A.; Politi, M. La telemedicina: Uno strumento di prossemica dell’assistenza territoriale? In Telemedicine: A Proxemics Tool of Primary Care? Igiene e Sanita Pubblica: Rome, Italy, 2020; Volume 76, pp. 288–294. [Google Scholar] [PubMed]
- Parker, R.S.; Le, J.; Doan, A.; Aguayo-Hiraldo, P.; Pannaraj, P.S.; Rushing, T.; Malvar, J.; O’Gorman, M.R.; Bard, J.D.; Parekh, C. COVID -19 outcomes in children, adolescents and young adults with cancer. Int. J. Cancer 2022, 151, 1913–1924. [Google Scholar] [CrossRef]
- Scarci, M.; Raveglia, F.; Bortolotti, L.; Benvenuti, M.; Merlo, L.; Petrella, L.; Cardillo, G.; Rocco, G. COVID-19 After Lung Resection in Northern Italy. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, E.; Vozzella, E.A.; Iervolino, A.; Egidio, R.; Buonocore, G.; Perrone, A.; Toscano, G.; Tremante, R.; Cesaro, F.; Sommella, V.; et al. Telemedicine: A cornerstone of healthcare assistance during the SARS-Cov2 pandemic outbreak but also a great opportunity for the near future. Smart Health 2022, 26, 100324. [Google Scholar] [CrossRef]
- Van Der Meij, E.; Anema, J.R.; Otten, R.H.J.; Huirne, J.A.F.; Schaafsma, F.G. The Effect of Perioperative E-Health Interventions on the Postoperative Course: A Systematic Review of Randomised and Non-Randomised Controlled Trials. PLoS ONE 2016, 11, e0158612. [Google Scholar] [CrossRef]
- Pickens, R.; Cochran, A.; Tezber, K.; Berry, R.; Bhattacharya, E.; Koo, D.; King, L.; Iannitti, D.A.; Martinie, J.B.; Baker, E.H.; et al. Using a mobile application for real-time collection of patient-reported outcomes in hepatopancreatobiliary surgery within an ERAS pathway. Am. Surg. 2019, 85, 909–917. [Google Scholar] [CrossRef]
- Graetz, I.; Anderson, J.N.; McKillop, C.N.; Stepanski, E.J.; Paladino, A.J.; Tillmanns, T.D. Use of a web-based app to improve postoperative outcomes for patients receiving gynecological oncology care: A randomized controlled feasibility trial. Gynecol. Oncol. 2018, 150, 311–317. [Google Scholar] [CrossRef]
- Bouwsma, E.V.A.; Noordegraaf, A.V.; Szlavik, Z.; Brölmann, H.A.M.; Emanuel, M.H.; Lips, J.P.; Van Mechelen, W.; Mozes, A.; Thurkow, A.L.; Huirne, J.A.F.; et al. Process evaluation of a multidisciplinary care program for patients undergoing gynaecological surgery. J. Occup. Rehabil. 2013, 24, 425–438. [Google Scholar] [CrossRef]
- Dorrell, R.D.; Vermillion, S.A.; Clark, C.J. Feasibility of real time location systems in monitoring recov-ery after major abdominal surgery. Surg. Endosc. 2017, 31, 5457–5462. [Google Scholar] [CrossRef]
- Faiz, O.; Nachiappan, S.; Anele, C.; Roberts, E.J.; Baker, C. An observational study to assess the feasibility of remote monitoring of patients in the early postoperative period after elective surgery. Digit. Med. 2018, 4, 133. [Google Scholar] [CrossRef]
- Paul, J.E.; Chong, M.A.; Buckley, N.; Harsha, P.; Shanthanna, H.; Tidy, A.; Buckley, D.; Clarke, A.; Young, C.; Wong, T.; et al. Vital sign monitoring with continuous pulse oximetry and wire-less clinical notification after surgery (the VIGILANCE pilot study)—A randomized controlled pilot trial. Pilot Feasibility Stud. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gunter, R.L.; Fernandes-Taylor, S.; Rahman, S.; Awoyinka, L.; Bennett, K.M.; Weber, S.M.; Greenberg, C.C.; Kent, C.K. Feasibility of an image-based mobile health protocol for postoperative wound monitoring. J. Am. Coll. Surg. 2018, 226, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sun, V.; Dumitra, S.; Ruel, N.; Lee, B.; Melstrom, L.; Melstrom, K.; Woo, Y.; Sentovich, S.; Singh, G.; Fong, Y. Wireless monitoring program of patient-centered outcomes and recov-ery before and after major abdominal cancer surgery. JAMA Surg. 2017, 152, 852–859. [Google Scholar] [CrossRef]
- Jonker, L.T.; Lahr, M.M.H.; Oonk, M.H.M.; de Bock, G.H.; van Leeuwen, B.L. Post-discharge Telemonitoring of Physical Activity, Vital Signs, and Patient-Reported Symptoms in Older Patients Undergoing Cancer Surgery. Ann. Surg. Oncol. 2021, 28, 6512–6522. [Google Scholar] [CrossRef]
- Mangiameli, G.; Cioffi, U.; Testori, A. Lung Cancer Treatment: From Tradition to Innovation. Front. Oncol. 2022, 12, 858242. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Topol, E.J. Telemedicine 2020 and the next decade. Lancet 2020, 395, 859. [Google Scholar] [CrossRef]
- Bouwsma, A.E.V.; Huirne, J.A.F.; Van De Ven, P.M.; Noordegraaf, A.V.; Schaafsma, F.G.; Koops, S.E.S.; Van Kesteren, P.J.M.; Brölmann, H.A.M.; Anema, J.R. Effectiveness of an internet-based perioperative care programme to enhance postoperative recovery in gynaecological patients: Cluster controlled trial with randomised steppedwedge implementation. BMJ Open 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Noordegraaf, A.V.; Anema, J.; Van Mechelen, W.; Knol, D.; Van Baal, W.; Van Kesteren, P.; Brölmann, H.; Huirne, J. A personalised eHealth programme reduces the duration until return to work after gynaecological surgery: Results of a multicentre randomised trial. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1127–1135. [Google Scholar] [CrossRef]

| Patient | Age | Sex | ASA | BMI | Smoke | cTNM | pTNM | Histology | Surgery | Surgical Time | Chest Tube Removal (POD) | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | III | 23 | Active | T1N0 | T1cN0 | Spino | RUL | 139 | IV | 0 |
| 2 | 70 | M | III | 24 | Former | T1N0 | T1bN0 | ADK | RUL | 169 | IV | 0 |
| 3 | 74 | F | IV | 14 | Active | T1N0 | T1bN0 | ADK | RUL | 101 | IV | 0 |
| 4 | 73 | F | III | 27 | Former | T2N0 | T2aN0 | ADK | RLL | 115 | IV | 0 |
| 5 | 67 | M | III | 38 | Former | T2N0 | T2aN0 | ADK | RLL | 166 | V | 0 |
| 6 | 71 | M | I | 22 | Former | T2N0 | T2aN0 | ADK | RUL | 118 | IV | 0 |
| 7 | 70 | F | I | 29 | No | T1N0 | T1bN0 | ADK | RUL | 157 | IV | 0 |
| 8 | 65 | M | III | 33 | Active | T2N0 | T1aN0 | ADK | RUL | 111 | VI | AF (POD II) Desaturation (POD II-III) |
| 9 | 58 | M | I | 26 | No | T1N0 | T1bN0 | Typical Carcinoid | LUL | 252 | II | 0 |
| 10 | 69 | M | I | 30 | Former | T1N0 | T2aN0 | ADK | RUL | 245 | VII | Desaturation (POD II) |
| 11 | 71 | F | I | 22 | Former | T1N0 | T1bN0 | ADK | RUL | 90 | IV | 0 |
| 12 | 53 | M | III | 26 | Active | T1N0 | T1bN0 | ADK | LUL | 170 | IV | 0 |
| Items | Robotic Group (n = 10) | VATS Group (n = 45) |
|---|---|---|
| Average stay (days) | 3.00 | 7·69 |
| Blood products (n°) | 0 (0%) | 25 ± 134 (0.3%) |
| Drugs (including VAT) | 309 ± 11 (4.9%) | 361 ± 94 (5.3%) |
| Medical device (including VAT) | 2878 ± 0 (45.6%) | 1647 ± 0 (24.4%) |
| Costs related to medical assistance (euros) | 560 ± 45 (8.9%) | 855 ± 235 (12.7%) |
| Hospitalization costs (days of stay) | 528 ± 0 (8.4%) | 1416 ± 768 (21.0%) |
| Diagnostic exams | 859 ± 235 (13.6%) | 1168 ± 609 (17.3%) |
| Operating room | 1174 ± 156 (18.6%) | 1285 ± 150 (19.0%) |
| Average costs | 6309 (100.0%) | 6756 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottoni, E.; Mangiameli, G.; Testori, A.; Piccioni, F.; Giudici, V.M.; Voulaz, E.; Ruggieri, N.; Dalla Corte, F.; Crepaldi, A.; Goretti, G.; et al. Early Hospital Discharge on Day Two Post Robotic Lobectomy with Telehealth Home Monitoring: A Pilot Study. Cancers 2023, 15, 1146. https://doi.org/10.3390/cancers15041146
Bottoni E, Mangiameli G, Testori A, Piccioni F, Giudici VM, Voulaz E, Ruggieri N, Dalla Corte F, Crepaldi A, Goretti G, et al. Early Hospital Discharge on Day Two Post Robotic Lobectomy with Telehealth Home Monitoring: A Pilot Study. Cancers. 2023; 15(4):1146. https://doi.org/10.3390/cancers15041146
Chicago/Turabian StyleBottoni, Edoardo, Giuseppe Mangiameli, Alberto Testori, Federico Piccioni, Veronica Maria Giudici, Emanuele Voulaz, Nadia Ruggieri, Francesca Dalla Corte, Alessandro Crepaldi, Giulia Goretti, and et al. 2023. "Early Hospital Discharge on Day Two Post Robotic Lobectomy with Telehealth Home Monitoring: A Pilot Study" Cancers 15, no. 4: 1146. https://doi.org/10.3390/cancers15041146
APA StyleBottoni, E., Mangiameli, G., Testori, A., Piccioni, F., Giudici, V. M., Voulaz, E., Ruggieri, N., Dalla Corte, F., Crepaldi, A., Goretti, G., Vanni, E., Pisarra, M., Cariboni, U., Alloisio, M., & Cecconi, M. (2023). Early Hospital Discharge on Day Two Post Robotic Lobectomy with Telehealth Home Monitoring: A Pilot Study. Cancers, 15(4), 1146. https://doi.org/10.3390/cancers15041146







