Protein Signatures and Individual Circulating Proteins, including IL-6 and IL-15, Associated with Prognosis in Patients with Biliary Tract Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Characteristics
2.3. Biomarker Analyses
2.4. Statistics
3. Results
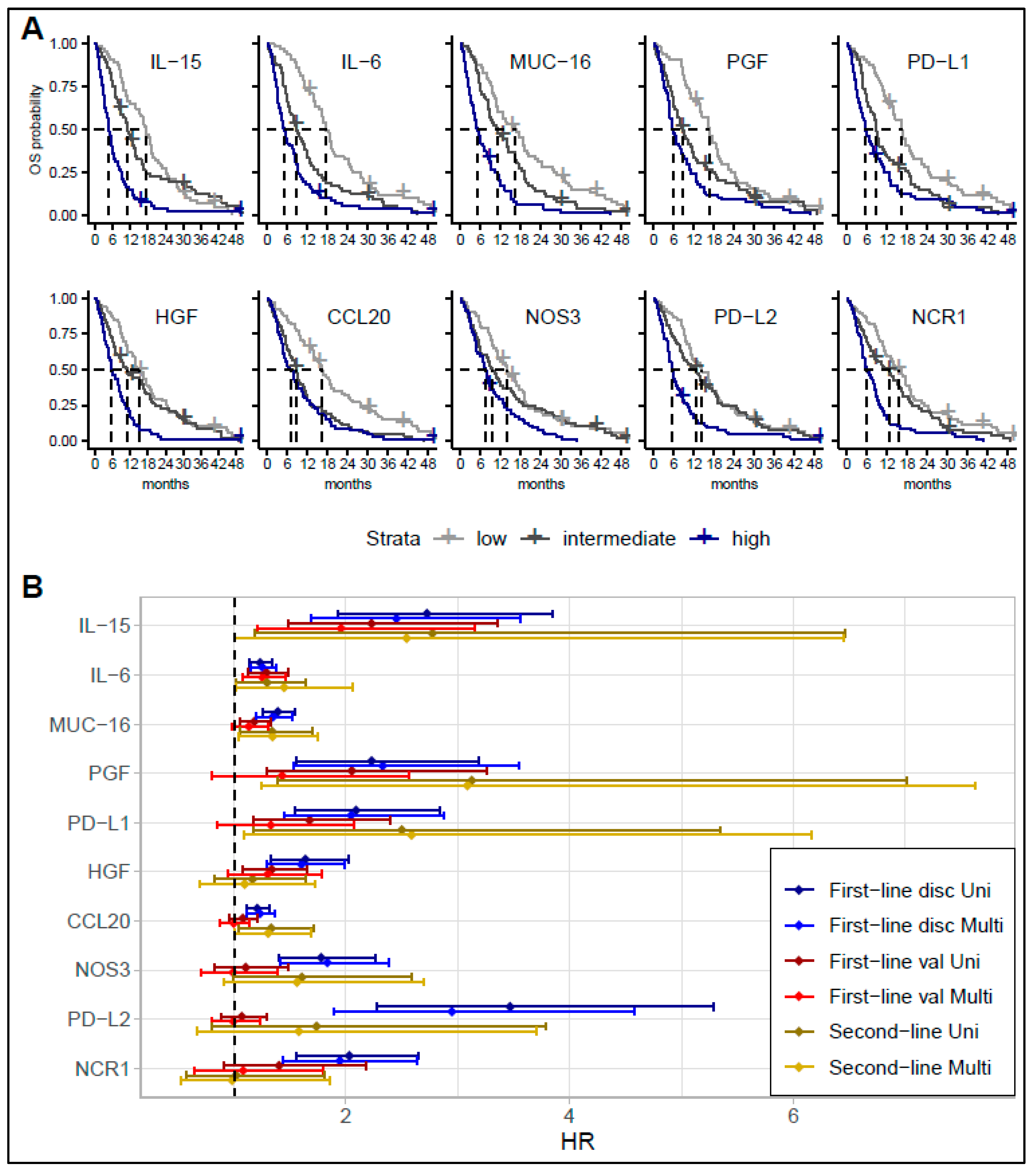
3.1. Survival Analyses
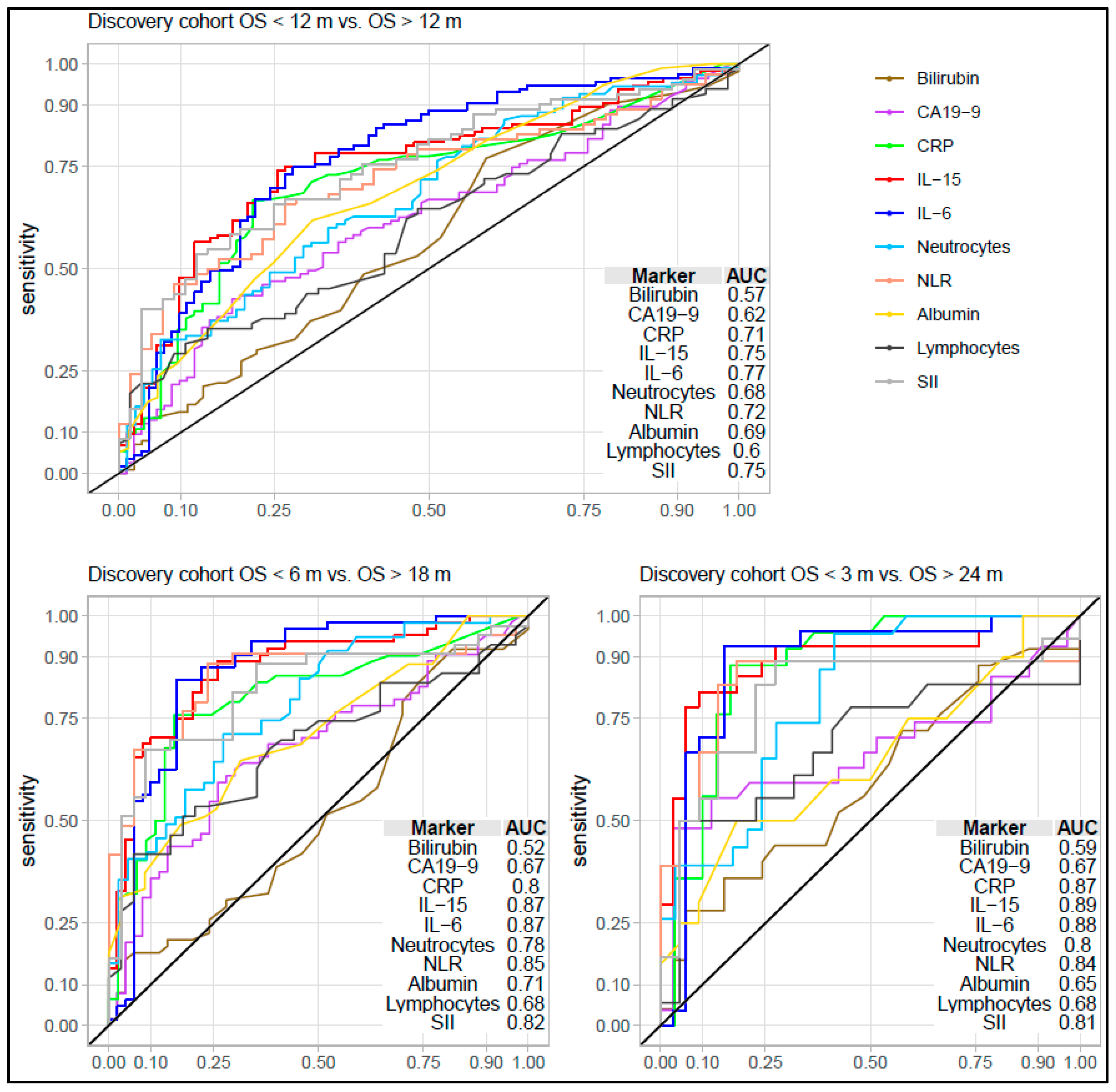
3.2. Comparing Biomarker Levels in Patients with Short and Long Survival in the Discovery Cohort
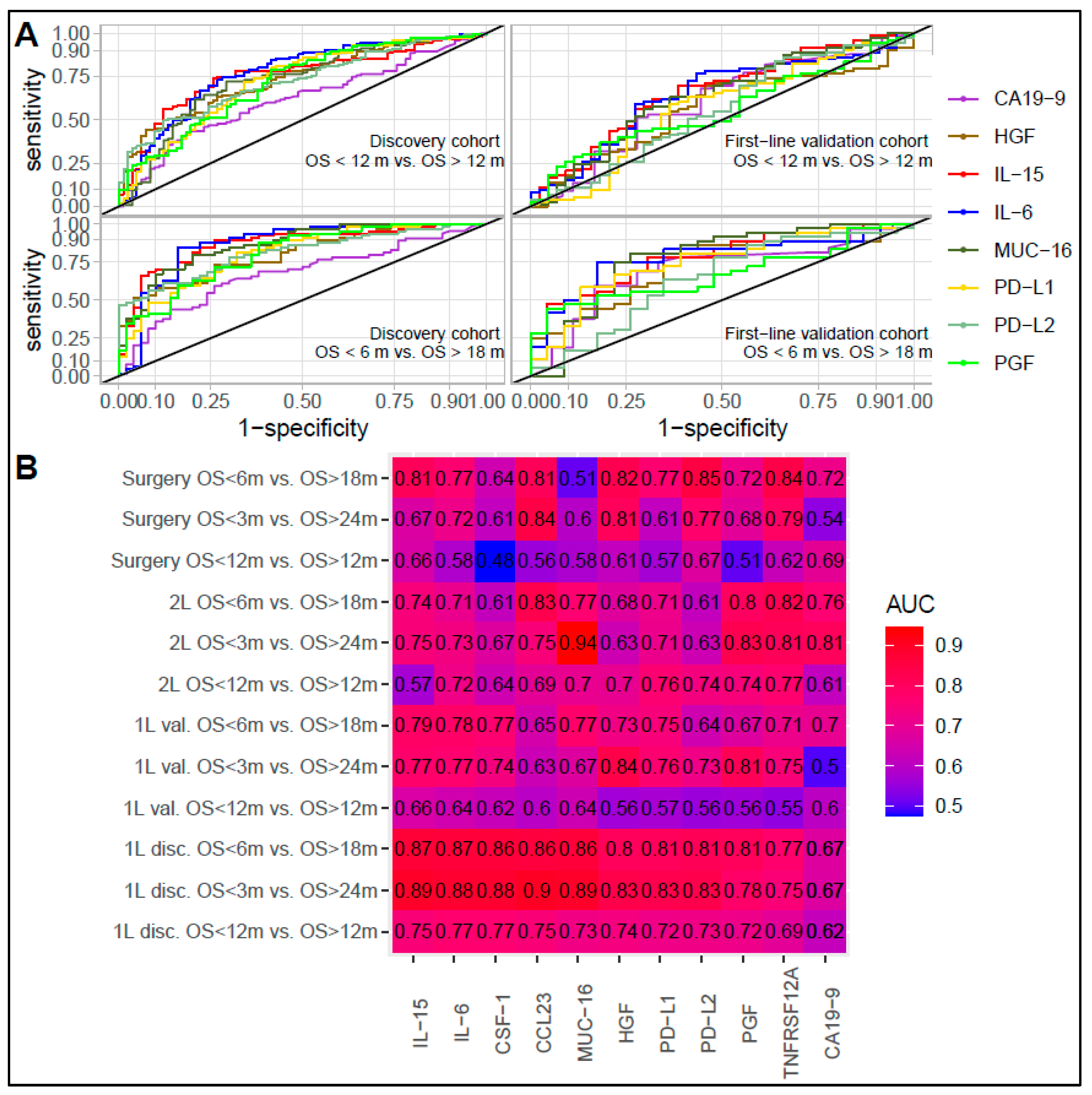
3.3. Association and Performance Compared to Other Inflammation and Prognostic Factors
3.4. Protein Signatures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Pinter, M.; Hucke, F.; Zielonke, N.; Waldhör, T.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Incidence and mortality trends for biliary tract cancers in Austria. Liver Int. 2014, 34, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-W.; Oh, C.-M.; Choi, H.Y.; Park, J.-W.; Cho, H.; Ki, M. Incidence and overall survival of biliary Tract cancers in South Korea from 2006 to 2015: Using the national health information database. Gut Liver 2019, 13, 104–113. [Google Scholar] [CrossRef]
- Kang, M.J.; Lim, J.; Han, S.-S.; Park, H.M.; Kim, S.-W.; Lee, W.J.; Woo, S.M.; Kim, T.H.; Won, Y.-J.; Park, S.-J. Distinct prognosis of biliary tract cancer according to tumor location, stage, and treatment: A population-based study. Sci. Rep. 2022, 12, 10206. [Google Scholar] [CrossRef] [PubMed]
- Alabraba, E.; Joshi, H.; Bird, N.; Griffin, R.; Sturgess, R.; Stern, N.; Sieberhagen, C.; Cross, T.; Camenzuli, A.; Davis, R.; et al. Increased multimodality treatment options has improved survival for Hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged. Eur. J. Surg. Oncol. 2019, 45, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Mishra-Kalyani, P.S.; Amiri Kordestani, L.; Rivera, D.R.; Singh, H.; Ibrahim, A.; DeClaro, R.A.; Shen, Y.; Tang, S.; Sridhara, R.; Kluetz, P.G.; et al. External control arms in oncology: Current use and future directions. Ann. Oncol. 2022, 33, 376–383. [Google Scholar] [CrossRef]
- Sinniah, R.S.; Shapses, M.S.; Ahmed, M.U.; Babiker, H.; Chandana, S.R. Novel biomarkers for cholangiocarcinoma: How can it enhance diagnosis, prognostication, and investigational drugs? Part-1. Expert Opin. Investig. Drugs 2021, 30, 1047–1056. [Google Scholar] [CrossRef]
- Høgdall, D.; O’Rourke, C.J.; Dehlendorff, C.; Larsen, O.F.; Jensen, L.H.; Johansen, A.Z.; Dang, H.; Factor, V.M.; Grunnet, M.; Mau-Sørensen, M.; et al. Serum IL6 as a prognostic biomarker and IL6R as a therapeutic target in biliary tract cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
- Saqib, R.; Pathak, S.; Smart, N.; Nunes, Q.; Rees, J.; Finch Jones, M.; Poston, G. Prognostic significance of pre-operative inflammatory markers in resected gallbladder cancer: A systematic review. ANZ J. Surg. 2018, 88, 554–559. [Google Scholar] [CrossRef]
- Sun, L.; Jin, Y.; Hu, W.; Zhang, M.; Jin, B.; Xu, H.; Du, S.; Xu, Y.; Zhao, H.; Lu, X.; et al. The Impacts of Systemic Immune-Inflammation Index on Clinical Outcomes in Gallbladder Carcinoma. Front. Oncol. 2020, 10, 554521. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Liu, Y.; Yi, M.; Jiao, D.; Wu, K. Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer. Front. Immunol. 2022, 13, 827921. [Google Scholar] [CrossRef]
- Ha, H.; Nam, A.R.; Bang, J.H.; Park, J.E.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Bang, Y.J.; et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016, 7, 76604–76612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Ou, Y.J.; Dai, H.S.; Wan, K.; Bie, P.; Chen, Z.Y.; Zhang, L.D.; Zhang, C.C. Elevated preoperative CA125 levels predicts poor prognosis of hilar cholangiocarcinoma receiving radical surgery. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101695. [Google Scholar] [CrossRef]
- Lindgaard, S.C.; Maag, E.; Sztupinszki, Z.; Chen, I.M.; Johansen, A.Z.; Jensen, B.V.; Bojesen, S.E.; Nielsen, D.L.; Szallasi, Z.; Johansen, J.S. Circulating Protein Biomarkers for Prognostic Use in Patients with Advanced Pancreatic Ductal Adenocarcinoma Undergoing Chemotherapy. Cancers 2022, 14, 3250. [Google Scholar] [CrossRef] [PubMed]
- Backen, A.C.; Lopes, A.; Wasan, H.; Palmer, D.H.; Duggan, M.; Cunningham, D.; Anthoney, A.; Corrie, P.G.; Madhusudan, S.; Maraveyas, A.; et al. Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial. Br. J. Cancer 2018, 119, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Høgdall, D.; Lewinska, M.; Andersen, J.B. Desmoplastic tumor microenvironment and immunotherapy in cholangiocarcinoma. Trends Cancer 2018, 4, 239–255. [Google Scholar] [CrossRef]
- Fabris, L.; Perugorria, M.J.; Mertens, J.; Björkström, N.K.; Cramer, T.; Lleo, A.; Solinas, A.; Sänger, H.; Lukacs-Kornek, V.; Moncsek, A.; et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019, 39, 63–78. [Google Scholar] [CrossRef]
- Koshiol, J.; Gao, Y.-T.; Corbel, A.; Kemp, T.J.; Shen, M.-C.; Hildesheim, A.; Hsing, A.W.; Rashid, A.; Wang, B.; Pfeiffer, R.M.; et al. Circulating inflammatory proteins and gallbladder cancer: Potential for risk stratification to improve prioritization for cholecystectomy in high-risk regions. Cancer Epidemiol. 2018, 54, 25–30. [Google Scholar] [CrossRef]
- Christensen, T.D.; Maag, E.; Larsen, O.; Feltoft, C.L.; Nielsen, K.R.; Jensen, L.H.; Leerhøy, B.; Hansen, C.P.; Chen, I.M.; Nielsen, D.L.; et al. Development and validation of circulating protein signatures as diagnostic biomarkers for biliary tract cancer. JHEP Rep. 2023, 5, 100648. [Google Scholar] [CrossRef]
- Larsen, F.o.; Hoegdall, D.T.S.; Hoegdall, E.; Nielsen, D. Gemcitabine, capecitabine and oxaliplatin with or without cetuximab in advanced biliary tract carcinoma. Acta Oncol. 2016, 55, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Markussen, A.; Jensen, L.H.; Diness, L.V.; Larsen, F.O. Treatment of patients with advanced biliary tract cancer with either oxaliplatin, gemcitabine, and capecitabine or cisplatin and gemcitabine-a randomized phase II trial. Cancers 2020, 12, 1975. [Google Scholar] [CrossRef]
- Larsen, F.O.; Markussen, A.; Diness, L.V.; Nielsen, D. Efficacy and Safety of Capecitabine, Irinotecan, Gemcitabine, and Bevacizumab as Second-Line Treatment in Advanced Biliary Tract Cancer: A Phase II Study. Oncology 2018, 94, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.E.L.; Hansen, T.F.; Fernebro, E.; Ploen, J.; Eberhard, J.; Lindebjerg, J.; Jensen, L.H. Randomized Phase II trial of combination chemotherapy with panitumumab or bevacizumab for patients with inoperable biliary tract cancer without KRAS exon 2 mutations. Int. J. Cancer 2021, 149, 119–126. [Google Scholar] [CrossRef]
- Jensen, L.H.; Lindebjerg, J.; Ploen, J.; Hansen, T.F.; Jakobsen, A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012, 23, 2341–2346. [Google Scholar] [CrossRef]
- Jensen, L.; Andersen, R.; Byriel, L.; Fernebro, E.; Jakobsen, A.; Lindebjerg, J.; Nottelmann, L.; Pløen, J.; Hansen, T. Phase II study of gemcitabine, oxaliplatin and capecitabine in patients with KRAS exon 2 mutated biliary tract cancers. Acta Oncol. 2019, 59, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and elaboration. PLoS Med. 2012, 9, e1001216. [Google Scholar] [CrossRef]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Fiore, P.F.; Di Matteo, S.; Tumino, N.; Mariotti, F.R.; Pietra, G.; Ottonello, S.; Negrini, S.; Bottazzi, B.; Moretta, L.; Mortier, E.; et al. Interleukin-15 and cancer: Some solved and many unsolved questions. J. Immunother. Cancer 2020, 8, e001428. [Google Scholar] [CrossRef]
- Eltahir, M.; Isaksson, J.; Mattsson, J.S.M.; Kärre, K.; Botling, J.; Lord, M.; Mangsbo, S.M.; Micke, P. Plasma Proteomic Analysis in Non-Small Cell Lung Cancer Patients Treated with PD-1/PD-L1 Blockade. Cancers 2021, 13, 3116. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.C.; Bouchaud, G.G.; Agueznay, N.E.H.; Mortier, E.; Hans, S.P.; Gey, A.; Fernani, F.; Peyrard, S.V.; Puig, P.L.; Bruneval, P.; et al. The Soluble α Chain of Interleukin-15 Receptor: A Proinflammatory Molecule Associated with Tumor Progression in Head and Neck Cancer. Cancer Res. 2008, 68, 3907–3914. [Google Scholar] [CrossRef]
- Seike, M.; Yanaihara, N.; Bowman, E.D.; Zanetti, K.A.; Budhu, A.; Kumamoto, K.; Mechanic, L.E.; Matsumoto, S.; Yokota, J.; Shibata, T.; et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J. Natl. Cancer Inst. 2007, 99, 1257–1269. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Sasso, M.S.; Obenauf, A.C.; Fredriksen, T.; Lafontaine, L.; Bilocq, A.M.; Kirilovsky, A.; Tosolini, M.; et al. Functional Network Pipeline Reveals Genetic Determinants Associated with in Situ Lymphocyte Proliferation and Survival of Cancer Patients. Sci. Transl. Med. 2014, 6, 228ra37. [Google Scholar] [CrossRef]
- Knudson, K.M.; Hicks, K.C.; Alter, S.; Schlom, J.; Gameiro, S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer 2019, 7, 82. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Velcheti, V.; Patel, M.R.; Garrett-Mayer, E.; Hill, E.G.; Ravenel, J.G.; Miller, J.S.; Farhad, M.; Anderton, K.; Lindsey, K.; et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018, 19, 694–704. [Google Scholar] [CrossRef]
- Chamie, K.; Chang, S.S.; Gonzalgo, M.; Kramolowsky, E.V.; Sexton, W.J.; Bhar, P.; Reddy, S.K.; Soon-Shiong, P. Final clinical results of pivotal trial of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive CIS and papillary nonmuscle-invasive bladder cancer (NMIBC). J. Clin. Oncol. 2022, 40, 4508. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Hong, C.; Schubert, M.; Tijhuis, A.E.; Requesens, M.; Roorda, M.; van den Brink, A.; Ruiz, L.A.; Bakker, P.L.; van der Sluis, T.; Pieters, W.; et al. cGAS–STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature 2022, 607, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef]
- Aoki, S.; Inoue, K.; Klein, S.; Halvorsen, S.; Chen, J.; Matsui, A.; Nikmaneshi, M.R.; Kitahara, S.; Hato, T.; Chen, X.; et al. Placental growth factor promotes tumour desmoplasia and treatment resistance in intrahepatic cholangiocarcinoma. Gut 2022, 71, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Olink User Manual, v. 1.9.5; Olink Proteomics: Uppsala, Sweden, 2018.
- Christensen, T.D.; Maag, E.; Madsen, K.; Lindgaard, S.C.; Nielsen, D.; Johansen, J.S. Determination of temporal reproducibility and variability of cancer biomarkers in serum and EDTA plasma samples using a proximity extension assay. Clin. Proteom. 2022, 19, 39. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Narasimhan, B.; Chu, G. impute: Imputation for Microarray Data, R package Version 1.58.0. 2019.
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Leerhøy, B.; Shabanzadeh, D.M.; Nordholm-Carstensen, A.; Novovic, S.; Hansen, M.B.; Jørgensen, L.N. Pancreatic function following post-endoscopic retrograde cholangiopancreatography pancreatitis: A controlled cohort study with long-term follow-up. United Eur. Gastroent. J. 2018, 6, 586–594. [Google Scholar] [CrossRef] [PubMed]
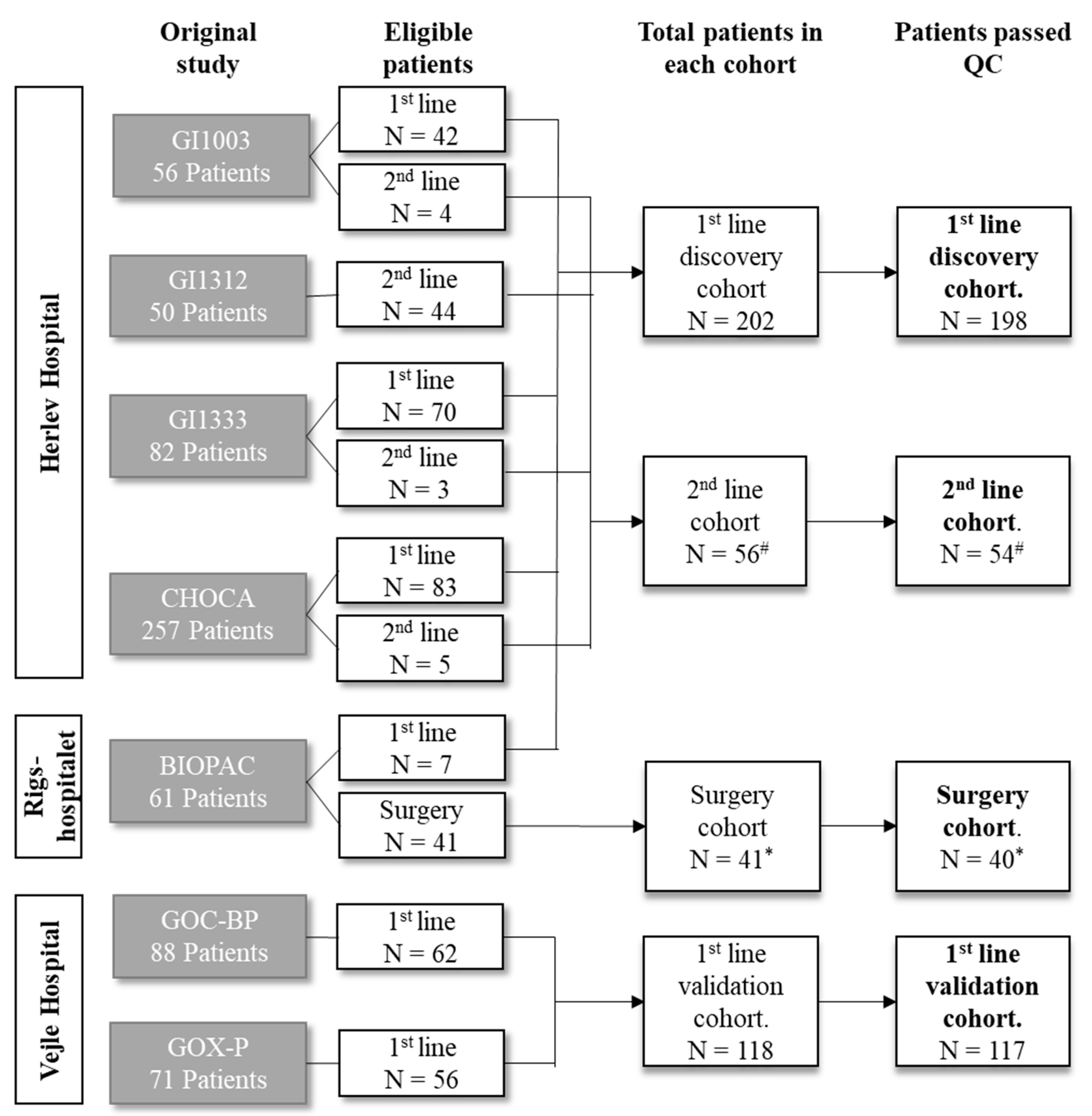
| First-Line Discovery Cohort | First-Line Validation Cohort | Surgery Cohort | Second-Line Cohort | ||
|---|---|---|---|---|---|
| No. of patients after removing samples that failed quality control | 198 | 117 | 40 | 54 | |
| Sex (%) | Female | 115 (58.1) | 75 (64.1) | 19 (47.5) | 31 (57.4) |
| Male | 83 (41.9) | 42 (35.9) | 21 (52.5) | 23 (42.6) | |
| Age—years (median [IQR]) | 67 (59, 72) | 66 (59, 73) | 67 (56, 70) | 67 (59, 71) | |
| Performance status (%) | 0 | 106 (53.5) | 41 (35.0) | 16 (40.0) | 32 (59.3) |
| 1 | 78 (39.4) | 52 (44.4) | 12 (30.0) | 20 (37.0) | |
| 2 | 10 (5.1) | 24 (20.5) | 1 (2.5) | 2 (3.7) | |
| Unknown | 4 (2.0) | 0 (0.0) | 11 (27.5) | 0 (0.0) | |
| Stage (%) | Resectable | 0 (0.0) | 0 (0.0) | 40 (100.0) | 0 (0.0) |
| Locally advanced | 87 (43.9) | 24 (20.5) | 0 (0.0) | 6 (11.1) | |
| Metastatic | 111 (56.1) | 93 (79.5) | 0 (0.0) | 48 (88.9) | |
| Location (%) | iCC | 108 (54.5) | 46 (39.3) | 0 (0.0) | 29 (53.7) |
| pCC | 28 (14.1) | 20 (17.1) | 0 (0.0) | 10 (18.5) | |
| GBC | 40 (20.2) | 23 (19.7) | 1 (2.5) | 13 (24.1) | |
| dCC | 22 (11.1) | 19 (16.2) | 39 (97.5) | 2 (3.7) | |
| Unknown | 0 (0.0) | 9 (7.7) | 0 (0.0) | 0 (0.0) | |
| History of liver disease * | No | 190 (96.0) | 0 (0.0) | 40 (100.0) | 45 (83.3) |
| Yes | 8 (4.0) | 0 (0.0) | 0 (0.0) | 4 (7.4) | |
| Unknown | 0 (0.0) | 117 (100.0) | 0 (0.0) | 5 (9.3) | |
| Resection of primary tumor | No | 169 (85.4) | 103 (88.0) | 0 (0.0) | 39 (72.2) |
| Yes | 29 (14.6) | 14 (12.0) | 40 (100.0) | 14 (25.9) | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | |
| Adjuvant chemotherapy | No | 178 (89.9) | 0 (0.0) | 9 (22.5) | 48 (88.9) |
| Yes | 20 (10.1) | 0 (0.0) | 31 (77.5) | 6 (11.1) | |
| Unknown | 0 (0.0) | 117 (100.0) | 0 (0.0) | 0 (0.0) | |
| First-line treatment | No | 8 (4.0) | 5 (4.3) | 9 (22.5) | 0 (0.0) |
| Gem | 11 (5.6) | 0 (0.0) | 1 (2.5) | 0 (0.0) | |
| Other | 7 (3.5) | 0 (0.0) | 8 (20.0) | 1 (1.9) | |
| GemCapOx | 48 (24.2) | 14 (12.0) | 3 (7.5) | 37 (68.5) | |
| GemCapOx + mAB | 44 (22.2) | 98 (83.8) | 0 (0.0) | 7 (13.0) | |
| GemCis | 80 (40.4) | 0 (0.0) | 4 (10.0) | 8 (14.8) | |
| Unknown | 0 (0.0) | 0 (0.0) | 15 (37.5) | 1 (1.9) | |
| Second-line treatment | None | 119 (60.1) | 0 (0.0) | 34 (85.0) | 4 (7.4) |
| GemCapIri | 25 (12.6) | 0 (0.0) | 0 (0.0) | 4 (7.4) | |
| GemCapIri + mAB | 21 (10.6) | 0 (0.0) | 1 (2.5) | 42 (77.8) | |
| Other | 33 (16.7) | 0 (0.0) | 5 (12.5) | 4 (7.4) | |
| Unknown | 0 (0.0) | 117 (100.0) | 0 (0.0) | 0 (0.0) | |
| OS status | Alive | 8 (4.0) | 4 (3.4) | 9 (22.5) | 0 (0.0) |
| Dead | 190 (96.0) | 113 (96.6) | 31 (77.5) | 54 (100.0) | |
| OS | <90 days | 27 (13.6) | 17 (14.5) | 3 (7.5) | 13 (24.1) |
| 90–179 days | 37 (18.7) | 19 (16.2) | 2 (5.0) | 19 (35.2) | |
| 180–364 days | 52 (26.3) | 37 (31.6) | 7 (17.5) | 8 (14.8) | |
| 365–547 days | 32 (16.2) | 21 (17.9) | 4 (10.0) | 8 (14.8) | |
| 548–729 days | 17 (8.6) | 11 (9.4) | 5 (12.5) | 2 (3.7) | |
| >730 days | 33 (16.7) | 12 (10.3) | 19 (47.5) | 4 (7.4) | |
| CA19-9, kU/L (median [IQR]) | 177 (56, 1918) | 161 (39, 1823) | 135 (29, 330) | 171 (39, 749) | |
| ALAT, U/L (median [IQR]) | 41 (28, 66) | NA | 60 (34, 123) | 48 (32, 119) | |
| ASAT U/L (median [IQR]) | 52 (37, 84) | NA | NA | 81 (74, 133) | |
| ALP U/L (median [IQR]) | 220 (126, 382) | NA | 189 (122, 276) | 182 (81, 392) | |
| Bilirubin µmol/L (median [IQR]) | 13 (9, 20) | NA | 31 (15, 91) | 8 (7, 28) | |
| Sig * | n | AUC Disc Det | AUC Disc Rep | AUC First-Line Val | AUC Surgery | AUC Second-Line |
|---|---|---|---|---|---|---|
| Set 1, Sig. 1 | 90 | 0.80 (0.67–0.92) | 0.73 (0.63–0.83) | 0.64 (0.54–0.75) | 0.70 (0.54–0.86) | 0.73 (0.58–0.87) |
| Set 1, Sig. 2 | 54 | 0.83 (0.71–0.94) | 0.72 (0.61–0.82) | 0.65 (0.55–0.76) | 0.72 (0.57–0.88) | 0.71 (0.56–0.86) |
| Set 1, Sig. 3 | 32 | 0.87 (0.76–0.97) | 0.71 (0.61–0.81) | 0.67 (0.57–0.77) | 0.67 (0.50–0.84) | 0.68 (0.51–0.84) |
| Set 1, Sig. 4 | 16 | 0.86 (0.75–0.97) | 0.74 (0.63–0.84) | 0.65 (0.55–0.75) | 0.63 (0.44–0.82) | 0.67 (0.50–0.84) |
| Set 1, Sig. 5 | 10 | 0.85 (0.73–0.97) | 0.72 (0.62–0.82) | 0.66 (0.55–0.76) | 0.66 (0.49–0.84) | 0.70 (0.54–0.86) |
| Set 1, Sig. 7 | 6 | 0.85 (0.72–0.97) | 0.73 (0.63–0.83) | 0.65 (0.55–0.76) | 0.65 (0.48–0.83) | 0.70 (0.54–0.86) |
| Set 1, Sig. 8 | 5 | 0.84 (0.71–0.97) | 0.75 (0.65–0.84) | 0.66 (0.56–0.77) | 0.68 (0.51–0.85) | 0.69 (0.53–0.86) |
| Set 1, Sig. 9 | 4 | 0.84 (0.71–0.97) | 0.74 (0.64–0.84) | 0.66 (0.56–0.76) | 0.65 (0.46–0.83) | 0.76 (0.62–0.89) |
| Set 1, Sig. 10 | 3 | 0.84 (0.71–0.97) | 0.71 (0.60–0.81) | 0.64 (0.54–0.74) | 0.70 (0.52–0.88) | 0.74 (0.58–0.90) |
| Set 1, Sig. 13 | 2 | 0.80 (0.67–0.93) | 0.63 (0.52–0.75) | 0.59 (0.49–0.70) | 0.70 (0.53–0.87) | 0.64 (0.48–0.80) |
| Set 1, Sig. 14 | 1 | 0.67 (0.51–0.84) | 0.58 (0.46–0.69) | 0.55 (0.44–0.66) | 0.61 (0.44–0.79) | 0.46 (0.29–0.64) |
| Set 2, Sig. 1 | 90 | 0.91 (0.81–1.00) | 0.86 (0.77–0.96) | 0.79 (0.68–0.90) | 0.85 (0.71–0.99) | 0.85 (0.71–0.98) |
| Set 2, Sig. 2 | 39 | 0.96 (0.91–1.00) | 0.88 (0.80–0.96) | 0.82 (0.70–0.93) | 0.81 (0.64–0.98) | 0.88 (0.77–0.99) |
| Set 2, Sig. 3 | 23 | 0.99 (0.98–1.00) | 0.89 (0.81–0.97) | 0.82 (0.71–0.93) | 0.85 (0.71–0.98) | 0.89 (0.78–0.99) |
| Set 2, Sig. 4 | 19 | 1.00 (1.00–1.00) | 0.89 (0.80–0.97) | 0.82 (0.71–0.93) | 0.83 (0.67–0.98) | 0.90 (0.80–0.99) |
| Set 2, Sig. 5 | 11 | 1.00 (1.00–1.00) | 0.90 (0.82–0.98) | 0.83 (0.72–0.93) | 0.85 (0.71–0.98) | 0.86 (0.74–0.98) |
| Set 2, Sig. 6 | 9 | 1.00 (1.00–1.00) | 0.91 (0.83–0.99) | 0.82 (0.71–0.93) | 0.85 (0.71–0.98) | 0.86 (0.75–0.98) |
| Set 2, Sig. 7 | 6 | 0.99 (0.98–1.00) | 0.91 (0.83–0.98) | 0.82 (0.72–0.93) | 0.85 (0.71–0.98) | 0.87 (0.75–0.99) |
| Set 2, Sig. 9 | 4 | 0.99 (0.98–1.00) | 0.90 (0.83–0.98) | 0.84 (0.74–0.95) | 0.79 (0.62–0.95) | 0.86 (0.71–1.00) |
| Set 2, Sig. 12 | 3 | 1.00 (1.00–1.00) | 0.89 (0.80–0.98) | 0.85 (0.74–0.95) | 0.76 (0.56–0.96) | 0.81 (0.61–1.00) |
| Set 2, Sig. 18 | 2 | 0.99 (0.98–1.00) | 0.89 (0.80–0.98) | 0.83 (0.72–0.94) | 0.62 (0.30–0.94) | 0.80 (0.60–1.00) |
| Set 2, Sig. 19 | 1 | 0.89 (0.78–1.00) | 0.84 (0.73–0.94) | 0.77 (0.63–0.91) | 0.50 (0.18–0.82) | 0.76 (0.53–1.00) |
| Set 3, Sig. 1 | 90 | 0.78 (0.54–1.00) | 0.92 (0.83–1.00) | 0.74 (0.55–0.93) | 0.71 (0.47–0.96) | 0.75 (0.45–1.00) |
| Set 3, Sig. 2 | 19 | 0.94 (0.83–1.00) | 0.92 (0.84–1.00) | 0.75 (0.57–0.94) | 0.73 (0.50–0.96) | 0.80 (0.53–1.00) |
| Set 3, Sig. 3 | 12 | 1.00 (1.00–1.00) | 0.93 (0.84–1.00) | 0.74 (0.55–0.93) | 0.70 (0.45–0.95) | 0.82 (0.55–1.00) |
| Set 3, Sig. 4 | 7 | 1.00 (1.00–1.00) | 0.96 (0.91–1.00) | 0.76 (0.57–0.95) | 0.73 (0.49–0.97) | 0.84 (0.62–1.00) |
| Set 3, Sig. 5 | 3 | 1.00 (1.00–1.00) | 0.92 (0.83–1.00) | 0.81 (0.65–0.97) | 0.70 (0.39–1.00) | 0.92 (0.78–1.00) |
| Set 3, Sig. 13 | 2 | 0.96 (0.88–1.00) | 0.92 (0.83–1.00) | 0.82 (0.67–0.98) | 0.59 (0.18–1.00) | 0.96 (0.87–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, T.D.; Madsen, K.; Maag, E.; Larsen, O.; Jensen, L.H.; Hansen, C.P.; Markussen, A.; Høgdall, D.T.S.; Chen, I.M.; Nielsen, D.; et al. Protein Signatures and Individual Circulating Proteins, including IL-6 and IL-15, Associated with Prognosis in Patients with Biliary Tract Cancer. Cancers 2023, 15, 1062. https://doi.org/10.3390/cancers15041062
Christensen TD, Madsen K, Maag E, Larsen O, Jensen LH, Hansen CP, Markussen A, Høgdall DTS, Chen IM, Nielsen D, et al. Protein Signatures and Individual Circulating Proteins, including IL-6 and IL-15, Associated with Prognosis in Patients with Biliary Tract Cancer. Cancers. 2023; 15(4):1062. https://doi.org/10.3390/cancers15041062
Chicago/Turabian StyleChristensen, Troels D., Kasper Madsen, Emil Maag, Ole Larsen, Lars Henrik Jensen, Carsten P. Hansen, Alice Markussen, Dan T. S. Høgdall, Inna M. Chen, Dorte Nielsen, and et al. 2023. "Protein Signatures and Individual Circulating Proteins, including IL-6 and IL-15, Associated with Prognosis in Patients with Biliary Tract Cancer" Cancers 15, no. 4: 1062. https://doi.org/10.3390/cancers15041062
APA StyleChristensen, T. D., Madsen, K., Maag, E., Larsen, O., Jensen, L. H., Hansen, C. P., Markussen, A., Høgdall, D. T. S., Chen, I. M., Nielsen, D., & Johansen, J. S. (2023). Protein Signatures and Individual Circulating Proteins, including IL-6 and IL-15, Associated with Prognosis in Patients with Biliary Tract Cancer. Cancers, 15(4), 1062. https://doi.org/10.3390/cancers15041062






