Simple Summary
Low cost, reliable predictors of benefit from PARP inhibitors (PARPi) are missing for relapsed BRCA wild-type (WT) ovarian cancer (OC). MITO 37 is a multicenter retrospective study aiming at correlating Ki67 expression at diagnosis with a clinical outcome following platinum treatment and PARPi maintenance. Clinical data were collected from 150 patients with high grade serous or endometroid BRCA WT OC treated with niraparib or rucaparib maintenance in 15 centers within MITO group. Ki67 expression was assessed by certified pathologists on tumor tissue at diagnosis and median Ki67 was used as cut-off. 136 patients were included. Median Ki67 was 45.7% (range 1.0–99.9). No statistically significant differences in response to PARPi neither in progression free survival and overall survival were identified between low and high Ki67 subgroups. High Ki-67 at diagnosis cannot discriminate responders to PARPi among OC BRCA WT patients.
Abstract
Background: There is compelling need for novel biomarkers to predict response to PARP inhibitors (PARPi) in BRCA wild-type (WT) ovarian cancer (OC). Methods: MITO 37 is a multicenter retrospective study aiming at correlating Ki67 expression at diagnosis with a clinical outcome following platinum treatment and PARPi maintenance. Clinical data were collected from high grade serous or endometroid BRCAWT OC treated with niraparib or rucaparib maintenance between 2010–2021 in 15 centers. Ki67 expression was assessed locally by certified pathologists on formalin-fixed paraffin embedded (FFPE) tissues. Median Ki67 was used as a cut-off. Results: A total of 136 patients were eligible and included in the analysis. Median Ki67 was 45.7% (range 1.0–99.9). The best response to platinum according to median Ki67 was 26.5% vs. 39.7% complete response (CR), 69.1% vs. 58.8% partial response (PR), 4.4% vs. 1.5% stable disease (SD). The best response to PARPi according to median Ki67 was 19.1% vs. 36.8% CR, 26.5% vs. 26.5% PR, 26.5 vs. 25% SD, 27.9% vs. 16.2% progressive disease (PD). No statistically significant differences in progression free survival (PFS) and overall survival (OS) were identified between low and high Ki67. PFS and OS are in line with registration trials. Conclusions: Ki67 at diagnosis did not discriminate responders to PARPi.
1. Introduction
Major clinical developments in the treatment of ovarian cancer (OC) have occurred during the past ten years, including the introduction of angiogenesis inhibitors [1] and poly-ADP ribose polymerase inhibitors (PARPi) [2,3,4,5], which have significantly increased overall survival (OS) rates [6]. Despite these advancements, OC is still the most lethal gynecologic malignancy accounting for more than 19880 new cases and 12810 deaths worldwide [7,8]. Indeed about 70% of OC treated with first-line surgery and carboplatin-paclitaxel combination will eventually relapse within two years after diagnosis [9].
At relapse, the first evaluation to be made is to determine whether a patient is a candidate for systemic anticancer therapy or surgery and is willing to continue treatment. Tumor biology, prior therapies, response to chemotherapy, therapy free interval (TFI), persistent toxicities, patient preference, and symptoms must all be considered when deciding whether to offer platinum-based therapy or non-platinum treatment [9].
Platinum-based chemotherapy followed by a PARPi maintenance could be considered for patients with a platinum-free interval (PFI) ≥ 6 months. Three PARP inhibitors are currently approved for the maintenance treatment of platinum-sensitive OC: niraparib, olaparib, and rucaparib for patients with germline or somatic BRCA 1/2 mutation, niraparib and rucaparib also for serous BRCA 1/2 wild-type (WT) patients [10,11,12,13]. The choice of the treatment depends mainly on safety profile since different trials show similar activity of the two drugs. Moreover, in Italy, endometrioid ovarian cancers can only be treated with rucaparib. No data are available on comparison between the two drugs [14,15].
However, based on recent data suggesting a detrimental effect of PARPi on OS in several randomized trials in relapsed BRCA WT OC, including homologous recombination deficiency (HRD) population [16,17,18], the Food and Drug Administration (FDA) have restricted their indication at platinum sensitive relapse to BRCA-mutated patients. Although the exact scenario on the use of PARPi is still to be defined (the European Medicines Agency has maintained the current indication), it is clear that current HRD testing still present several limitations. Different approaches are currently being investigated to identify BRCA1/2 WT tumours that can benefit from DNA-damaging agents and PARPi based on the presence of HRD, that is, (1) scores capturing large genomic aberrations, so-called ‘genomic scars’, (2) analysis of mutational signatures, or (3) point mutations identified in homologus recombination repair (HRR) genes using DNA sequencing panels.
Commercial HRD tests such as Myriad and Foundation One [19] identify genomic scars and not the actual genomic status neither the mechanisms of resistance that develop during therapy and functional information of the HRD pathway’s activity, thus translating in a weak predictive value of response to PARPi [5,20,21,22,23,24]. Some limitations of these and other available tests also include the proportion of samples returned with inconclusive results, false-negative results, and high cost. This is the reason why the academic community is looking at more “functional tests” in tissues, such as RAD51 foci expression [25].
Therefore, the search for an easily accessible, low-cost, reproducible biomarker represents an urgent need in clinical practice.
In the early 1980s, Scholzer and Gerdes discovered the Ki67 antigen, which encodes two protein isoforms with molecular weights of 345 and 395 kDa [26]. Ki67 protein has a half-life of only 1 to 1.5 h. It is present in all active cell cycle phases (G1, S, G2, and M), but not in resting cells (G0) [27,28]. Ki67 expression is nuclear, and it is proportional to the mitotic count but reveals more proliferating tumor cells than the citoplasmatic expression since it marks cells not in the G0 phase.
Interestingly, although with different cut-offs varying among different studies, high Ki67 is predictive of pathologic complete response (pCR) in breast cancer undergoing neoadiuvant chemotherapy with alkylating agents, anthracyclines, and taxanes [29,30]. In the context of OC, some reports correlated high Ki67 expression to poor histological and clinical characteristics. In particular, Kritpracha et al. [31], showed that in 105 patients with locally advanced OCs, the percentage staining of Ki67 expression ranged from 0.3 to 100%, with a median of 11.9% and that Ki67 was higher in serous tumors than in other histotypes (p = 0.048). The 5-year OS was 15.1% in the high Ki67 (≥11.9%) and 36.5% in the low Ki67 (<11.9%) patients, respectively. Median overall survival (OS) in the two groups was 1.8 years and 3.0 years, respectively (p < 0.008).
Heeran et al. [32] analyzed Ki67% in 606 OC patients using 10% as the cut-off level and found that the frequency of Ki67% expression increased with an increasing International Federation of Gynecology and Obstetrics (FIGO) stage (p = 0.003) and histological grade (p > 0.0001).
Finally, Layfield et al. [33] demonstrated that Ki67 had prognostic significance in late-stage OC. The median OS of patients whose carcinoma had a high Ki67 expression (defined as >15%) was 30 months compared to 16 months in the low-expression subgroup.
The aim of our study was to investigate the role of Ki67 as a potential biomarker of response to platinum salts and PARPi in relapsed OC.
2. Material and Methods
2.1. Patients
MITO 37 is a multicenter retrospective Italian study aiming at correlating Ki67 expression with a clinical outcome following platinum treatment and PARPi maintenance. Clinical data were collected from all patients with high grade serous or endometroid BRCA WT ovarian cancer treated with niraparib or rucaparib maintenance between 2010–2021. The study has been approved by the ethical committee of participating Institutions. We collected data from 15 Italian Centers. See Table S1.
Eligible patients were at least 18 years of age and had platinum-sensitive (defined as PFI ≥ 6 months) relapsed cancer of the ovary, peritoneum, or fallopian tube (collectively defined as ovarian cancer), with disease progression after at least 1 line of chemotherapy. All patients had high-grade serous or endometrioid tumors that were classified at diagnosis according to the FIGO staging criteria. Before the start of PARPi, all the patients had received four to six cycles of platinum-based chemotherapy, which had resulted in a complete response (CR) or partial response (PR), according to investigator assessment. Patients receiving treatment within clinical trials were not eligible. All patients had been tested for BRCA1/2 germline and/or somatic mutations and resulted WT.
The primary endpoint was to determine, in a real-world setting, the overall response rate (ORR) to platinum-based chemotherapy and to PARPi according to the Ki67 value expressed as %. Patients were divided into 2 groups (low and high) using the median value as the cut-off (see below).
The secondary endpoints were to describe progression free survival (PFS) and OS comparing subgroups based on the Ki67 value.
PFS was defined as the time from the first day of niraparib or rucaparib administration to the date of objective disease progression on imaging according to the response evaluation criteria in solid tumors (RECIST), version 1.1, or death from any cause. Patients who did not experience progression disease (PD) were censored on the date of the last follow-up visit. OS was defined from the first day of niraparib or rucaparib administration to death for any cause or the last follow up visit. The median follow-up (FU) was calculated according to the reverse Kaplan–Meier formula. PFS and OS were calculated according to the Kaplan–Meier formula, and groups were compared by log-rank test.
Within 8 weeks after completion of the last dose of platinum-based chemotherapy, patients were assigned to receive oral niraparib or rucaparib as per clinical practice choice (according to previous toxicities, comorbidities, and patients’ choice) in 28-day cycles.
All the patients started rucaparib at a fixed dose of 600 mg twice daily, while patients starting niraparib were assigned a full dose of 300 mg daily or an individualized dose of 200 mg daily according to platelets count and weight (a 200 mg daily dose was assigned in case of a baseline body weight of less than 77 kg, a platelet count of less than 150,000/mm3, or both) [34].
The data cut-off for the analysis was 30 April 2022.
2.2. Ki67 Staining and Scoring
The Ki67 expression was assessed locally by certified pathologists on a formalin-fixed paraffin-embedded tumor tissue at diagnosis.
To overcome the possible bias of poor reproducibility, a kick-off meeting with the pathologists was performed in order to define the criteria for Ki67 evaluation.
Tissue samples from each tumor lesion were fixed for 24 h in 10% buffered formalin. The selected blocks were cut at a 3 mm thickness and immunohistochemistry (IHC) was performed using a Ki67 mouse monoclonal antibody (Mib-1 clone, monoclonal, Dako, Glostrup, Denmark), then incubated with a commercially available detection kit (EnVision FLEX; Dako, Glostrup, Denmark).
Slides were explored with a conventional light microscope by an experienced pathologist trained in Gynecopathology. A representative field for Ki67 evaluation was selected. In case of heterogenous staining, a hot spot (an area with high Ki67 expression) was chosen. Ki67 expression was analyzed for each case, and all colored nuclei were considered positive, independent of the intensity and distribution. It was performed using a quantitative method with evaluation of the ratio of stained cells compared with the total number of cells in the field. A total of 100 cells were evaluated for each case (Figure 1).
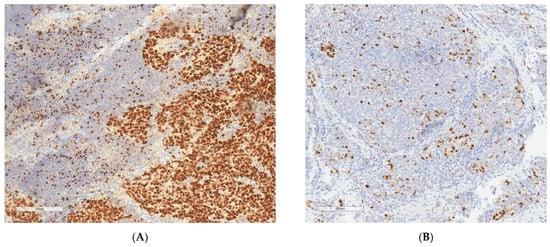
Figure 1.
Ki67 IHC staining on ovarian cancer tissue. Original magnification 200×. (A) high Ki67 (>45.7%). (B) low Ki67 (<45.7%).
3. Results
Baseline demographic and clinical characteristics of patients included in the MITO 37 study are summarized in Table 1.

Table 1.
Demographic and clinical characteristics of the patients at baseline.
Three patients were excluded because they received PARPi as a first-line maintenance treatment and not at relapse and one patient was excluded because she received olaparib. At diagnosis for 10 patients, a tumor tissue was not available for Ki67 assessment. Therefore, the final efficacy analysis was performed on 136 patients (Figure 2).
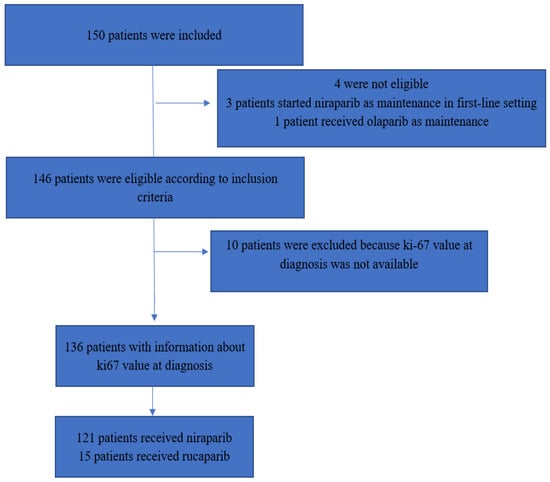
Figure 2.
Flowchart of the study.
The median age at diagnosis was 63 years (range 38–84). All patients were somatic and/or germline BRCA wild-type as per the inclusion criteria. In particular, the germline BRCA 1–2 wild-type status was known in 87.5% and the somatic BRCA1/2 wild-type status was known in 61.8%.
At diagnosis, the majority of the patients (82.3%) had advanced disease (stage FIGO III-IV), high grade serous ovarian cancer (HGSOC) histology (92.6%), while the remaining patients had OC with endometrioid histology (5.9%) or mixed (serous and endometrioid) histology (1.5%).
Most patients (76.5%) had an elevated CA-125 serum level at diagnosis.
More than half of the patients (66.2%) underwent upfront debulking surgery and had no residual disease (R0, 70.6%).
Initial PFI (time between the last cycle of platinum during first line and evidence of disease progression) was ≥12 months in 75.7% of the cases and between 6 and 12 months in 22.8%.
The CA-125 serum level was normalized before starting PARPi maintenance in the majority (68.4%).
Most of the patients (72.1%) received PARPi (niraparib or rucaparib) at first recurrence, after a second line of platinum-based chemotherapy, while the remaining received PARPi after three or more lines of platinum-based chemotherapy.
Most patients had received bevacizumab as maintenance at first line or in a line before starting PARPi (67.6%).
The two most used platinum-based regimens at relapse were carboplatin-liposomal doxorubicine and carboplatin-paclitaxel, respectively, 34.6% and 33.1%.
About one third (33.1%) of the patients achieved CR with platinum-based chemotherapy and 64% achieved PR, while 2.9% had a stable disease (SD) at imaging after platinum-based chemotherapy, but they were considered eligible to start PARPi in a clinical practice, due to a CA-125 level decrease.
About one third of the patients (33.8%) underwent surgery at relapse (considering any relapse before PARPi).
The median FU was 29.1 months (range 24.7–33.5).
As shown in Table 2, the majority of the patients (90.0%) started niraparib as maintenance after platinum-based chemotherapy; among these, 38 patients (31.4%) started at a full dose of 300 mg daily, while 83 patients (68.6%) started an individualized dose of 200 mg daily according to platelets count and weight.

Table 2.
PARPi clinical outcomes.
All patients in the rucaparib group started at a full dose of 600 mg twice daily. About half of the patients reduced their dose of PARPi during maintenance due to toxicities (48.5%), and at the data cut-off, 22.6% of patients were still ongoing with maintenance therapy. The best response to PARPi was CR (30.9%); however, 24.3% experienced PD at first evaluation. The majority of the patients discontinued maintenance therapy due to PD (72.8%).
The median treatment duration was 9.3 months (95%, CI 7.9–10.7 months).
As for patients who experienced disease progression after PARPi, the subsequent chemotherapy line was mainly platinum-based chemotherapy (32.4%) and, at the data cut-off, 61.8% of the patients were alive.
Ki67 Assessment and Predictive Value
The median Ki67 expression was 45.7% (range 1–99.9).
The patients’ characteristics according to the median Ki67 (low ≤ 45.7% vs. high > 45.7%) are summarized in Table 3 and Table 4.

Table 3.
Characteristics of the patients according to the median Ki67 value.

Table 4.
PARPi description according to the Ki67 median value.
The two groups (≤45.7% vs. >45.7%) were well balanced in terms of baseline characteristics with the exception of bevacizumab use: patients with high Ki67 had received bevacizumab more frequently than those with low Ki67.
The median PFS in the whole cohort was 11.4 months (95% confidence interval [CI] 8.2–14.6). No difference according to median Ki67 value was detected: for patients with a low Ki67 value, the median PFS was 11.4 months (95%CI 8.2–14.6); for patients with a high Ki67 value, the median PFS was 10.6 months (95%CI 6.4–16.4), HR 1.31 (95% CI 0.89–1.94), p-value= 0.18 (Figure 3).
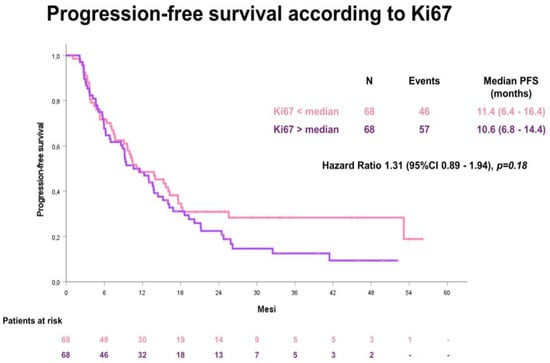
Figure 3.
Progression-free survival (PFS) according to the median Ki67 value.
The median OS was 37.1 months (95% CI 37.1–42.6). Also for OS, no statistical significant difference according to the median Ki67 value was observed between the two groups: for patients with a low Ki67 value ≤ 45.7, the median OS was 34.9 months (95% CI 21.5–48.3); for patients with a high Ki67 value > 45.7, the median OS was not reached (Figure 4).
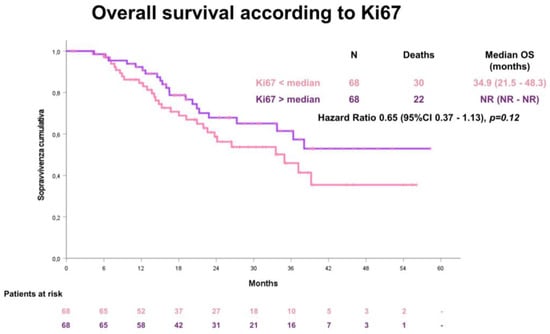
Figure 4.
Overall survival (OS) according to the median Ki67 value.
4. Discussion
The MITO 37 study investigated the predictive role of Ki67 IHC expression in serous or endometrioid OC patients receiving PARPi (niraparib and rucaparib) at platinum-sensitive recurrence.
To date, no data are available about the potential role of Ki67 IHC expression in predicting PARPi response, but several reports showed that OC patients who have a high proliferation index (PI) are more likely to have poor prognostic factors, including an advanced FIGO stage, a higher tumor grade, a bulk residual tumor, and a poor response to chemotherapy, and also have a less favorable 5-year survival compared with those with a low PI [31,32,33].
In our study, in the absence of a universally accepted cut-off, we used the median value to divide the population into two categories (low vs. high Ki67).
Unfortunately, we were not able to identify significant differences between low and high Ki67 cancers in terms of: age at diagnosis, FIGO stage, grading or histological subtype, CA-125 level at diagnosis, type of surgery at diagnosis (upfront, IDS, and no surgery) and residual disease at first surgery, CA-125 before starting PARPi, BRCA status, first PFI, number of previous lines of chemotherapy, clinical response to platinum, number of surgeries before PARPi, and residual disease at last surgery. Interestingly, and perhaps due to its earlier approval, niraparib was clearly predominant for the treatment of the patients included in the study.
The median PFS in our study was 11.7 months and was consistent with the NOVA and ARIEL-3 studies. In the NOVA trial [15], in fact, the median PFS was 9.3 months and in ARIEL-3 [14], it was 13.7 months. No differences according to the median Ki67 value have been detected also for PFS.
The present study contains both limitations and strengths. Limitations include those associated with the retrospective nature and the intrinsic risk of confounding the relatively small number of patients included.
Ki67 was assessed mostly at diagnosis and not from a tumor tissue at relapse, just before PARPi was started. Considering tumor clones’ evolution during the natural history of the disease, our data might not reflect the Ki67 value at the time of relapse.
Furthermore, such information comes from analyses of single sites of tumor growth. Considering the biology of ovarian cancer and its extremely heterogeneous nature, both spatially and temporally, it is legitimate to state that coexistence of several different clones at diagnosis render unpredictable which ones would be prevalent at relapse [35,36].
In conclusion, our study was not able to demonstrate a potential role for Ki67 expression to predict response to PARPi at diagnosis nor to identify different populations in terms of clinical features.
5. Conclusions
This work is an example of how the MITO and other collaborative groups could collaborate efficiently and address important clinical issues such as the identification of reliable biomarkers of response to PARPi for BRCA WT-relapsed ovarian cancer patients. Although the MITO 37 was a negative study, we believe we should increase the recognition among all clinicians that negative studies are also necessary to the advancement of clinical practice. Publication of negative results is particularly useful to avoid publication bias, which could affect not only studies of interventions but also studies exploring the performance of diagnostic, prognostic, and predictive factors.
Moving into perspective, we are planning to centralize Ki67 readings and to enrich the study including an analysis of tumor tissues at relapse and possibly multiple sites.
Finally, this work reminds us of the complex nature of OC and highlights the urgent and still-unmet need for early and accessible in-tissue biomarkers to predict the response to PARPi and avoid unnecessary toxicities to patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15041032/s1, Table S1: Clinical sites participating in the MITO 37 study.
Author Contributions
Conceptualization, V.T., E.G. and G.V.; methodology, M.D.M.; validation, V.T., E.G., G.V. and M.D.M.; formal analysis, M.D.M.; investigation, V.T., E.G., S.P., E.P., F.R., C.A., E.M., G.A., S.M., L.Z., A.S., N.B., A.P. (Alessandro Parisi), V.D.M., C.C., G.C., M.M., A.P. (Andrea Puppo), B.P., L.B., G.S., M.T., D.S., M.D.M. and G.V.; data curation, V.T., E.G., G.V. and M.D.M.; writing—original draft preparation, V.T., E.G., G.V. and M.D.M.; writing—review and editing, V.T., E.G., S.P., E.P., F.R., C.A., E.M., G.A., S.M., L.Z., A.S., N.B., A.P. (Alessandro Parisi), V.D.M., C.C., G.C., M.M., A.P. (Andrea Puppo), B.P., L.B., G.S., M.T., D.S., M.D.M. and G.V.; supervision, V.T., E.G., S.P., E.P., F.R., C.A., E.M., G.A., S.M., L.Z., A.S., N.B., A.P. (Alessandro Parisi), V.D.M., C.C., G.C., M.M., A.P. (Andrea Puppo), B.P., L.B., G.S., M.T., D.S., M.D.M. and G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by Ricerca Locale (ex-60%), Università di Torino 2020, to DS and GV.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Città della Scienza e della Salute di Torino, A.O. Ordine Mauriziano di Torino—A.S.L Città di Torino (protocol code 0083816, pratica n. 359/2022, date of approval 28/JUL/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the current manuscript “Ki67 as a Predictor of Response to PARP Inhibitors in Platinum Sensitive BRCA Wild Type Ovarian Cancer: The MITO 37 Retrospective Study”.
Acknowledgments
We thank the patients, their families, the MITO group, and the investigational site members.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poveda, A.M.; Selle, F.; Hilpert, F.; Reuss, A.; Savarese, A.; Vergote, I.; Witteveen, P.; Bamias, A.; Scotto, N.; Mitchell, L.; et al. Bevacizumab Combined with Weekly Paclitaxel, Pegylated Liposomal Doxorubicin, or Topotecan in Platinum-Resistant Recurrent Ovarian Cancer: Analysis by Chemotherapy Cohort of the Randomized Phase III AURELIA Trial. J. Clin. Oncol. 2015, 33, 3836–3838. [Google Scholar] [CrossRef] [PubMed]
- Penson, R.T.; Valencia, R.V.; Cibula, D.; Colombo, N.; Leath, C.A.; Bidziński, M.; Kim, J.W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib versus Nonplatinum Chemotherapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020, 38, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Halverson, J.L.; Martinez-Donate, A.P.; Palta, M.; Leal, T.; Lubner, S.; Walsh, M.C.; Strickland, J.S.; Smith, P.D.; Trentham-Dietz, A. Health Literacy and Health-Related Quality of Life Among a Population-Based Sample of Cancer Patients. J. Health Commun. 2015, 20, 1320–1329. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2022, 41, 609–617. [Google Scholar] [CrossRef]
- National Institutes of Health; National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer; National Cancer Institute (NIH): Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 12 December 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Kim, G.; Ison, G.; McKee, A.E.; Zhang, H.; Tang, S.; Gwise, T.; Sridhara, R.; Lee, E.; Tzou, A.; Philip, R.; et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin. Cancer Res. 2015, 21, 4257–4261. [Google Scholar] [CrossRef]
- Gogineni, V.; Morand, S.; Staats, H.; Royfman, R.; Devanaboyina, M.; Einloth, K.; Dever, D.; Stanbery, L.; Aaron, P.; Manning, L.; et al. Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J. Cancer 2021, 12, 38–53. [Google Scholar] [CrossRef]
- Drew, Y.; Kristeleit, R.S.; Oaknin, A.; Ray-Coquard, I.; Haris, N.; Swisher, E.M. Real-World Delivery of Rucaparib to Patients with Ovarian Cancer: Recommendations Based on an Integrated Safety Analysis of ARIEL2 and Study 10. Oncology 2019, 25, e109–e119. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Pignata, S.; Ledermann, J. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann. Oncol. 2018, 29, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Overall survival results from ARIEL3: A phase 3 randomized, double-blind study of Rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma. Int. J. Gynecol. Cancer 2022. [Google Scholar]
- Matulonis, U.H.J.; Oza, A.; Mahner, S.; Redondo, A.; Berton, D.; Berek, J.; Lund, B.; Marme, F.; Gonzales-Martin, A.; Tinker, A.; et al. Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecol. Oncol. 2021, 162 (Suppl. S1), S24–S25. [Google Scholar] [CrossRef]
- Penson, R.V.; Colombo, N.; Leath, C.; Bidzinski, M.; Kim, J.-W.; Nam, J.-H.; Madry, R.; Hernández, C.; Mora, P.; Ryu, S.-Y.; et al. Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1- and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer. Gynecol. Oncol. 2022, 166 (Suppl. S1), S19–S20. [Google Scholar] [CrossRef]
- Book Foundation Medicine. Available online: https://www.foundationmedicine.it/our-services/cdx.html (accessed on 12 December 2022).
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Arora, S.; Balasubramaniam, S.; Zhang, H.; Berman, T.; Narayan, P.; Suzman, D.; Bloomquist, E.; Tang, S.; Gong, Y.; Sridhara, R.; et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist 2020, 26, e164–e172. [Google Scholar] [CrossRef]
- Bradley, W.; Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed, advanced ovarian cancer and a BRCA mutation: 5-year follow-up from SOLO1. Gynecol. Oncol. 2021, 162, S25–S26. [Google Scholar] [CrossRef]
- Mirza, M.R.; Coleman, R.L.; González-Martín, A.; Moore, K.N.; Colombo, N.; Ray-Coquard, I.; Pignata, S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- Capoluongo, E.D.; Pellegrino, B.; Arenare, L.; Califano, D.; Scambia, G.; Beltrame, L.; Serra, V.; Scaglione, G.L.; Spina, A.; Cecere, S.C.; et al. Alternative academic approaches for testing homologous recombination deficiency in ovarian cancer in the MITO16A/MaNGO-OV2 trial. ESMO Open 2022, 7, 100585. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Shirendeb, U.; Hishikawa, Y.; Moriyama, S.; Win, N.; Thu, M.M.; Mar, K.S.; Khatanbaatar, G.; Masuzaki, H.; Koji, T. Human papillomavirus infection and its possible correlation with p63 expression in cervical cancer in Japan, Mongolia, and Myanmar. Acta Histochem. Cytochem. 2009, 42, 181–190. [Google Scholar] [CrossRef]
- Hooghe, B.; Hulpiau, P.; Van Roy, F.; De Bleser, P. ConTra: A promoter alignment analysis tool for identification of transcription factor binding sites across species. Nucleic Acids Res. 2008, 36, W128–W132. [Google Scholar] [CrossRef] [PubMed]
- Derouane, F.; van Marcke, C.; Berlière, M.; Gerday, A.; Fellah, L.; Leconte, I.; Van Bockstal, M.R.; Galant, C.; Corbet, C.; Duhoux, F.P. Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine. Cancers 2022, 14, 3876. [Google Scholar] [CrossRef]
- Klauschen, F.; Wienert, S.; Schmitt, W.D.; Loibl, S.; Gerber, B.; Blohmer, J.U.; Huober, J.; Rüdiger, T.; Erbstößer, E.; Mehta, K.; et al. Standardized Ki67 Diagnostics Using Automated Scoring—Clinical Validation in the GeparTrio Breast Cancer Study. Clin. Cancer Res. 2015, 21, 3651–3657. [Google Scholar] [CrossRef]
- Kritpracha, K.; Hanprasertpong, J.; Chandeying, V.; Dechsukhum, C.; Geater, A. Survival analysis in advanced epithelial ovarian carcinoma in relation to proliferative index of MIB-1 immunostaining. J. Obstet. Gynaecol. Res. 2005, 31, 268–276. [Google Scholar] [CrossRef]
- Heeran, M.C.; Høgdall, C.K.; Kjaer, S.K.; Christensen, L.; Jensen, A.; Blaakaer, J.; Christensen, I.J.; Høgdall, E.V. Prognostic value of tissue protein expression levels of MIB-1 (Ki-67) in Danish ovarian cancer patients. From the ‘MALOVA’ ovarian cancer study. APMIS 2013, 121, 1177–1186. [Google Scholar] [CrossRef]
- Layfield, L.J.; Saria, E.A.; Berchuck, A.; Dodge, R.K.; Thompson, J.K.; Conlon, D.H.; Kerns, B.-J.M. Prognostic value of MIB-1 in advanced ovarian carcinoma as determined using automated immunohistochemistry and quantitative image analysis. J. Surg. Oncol. 1997, 66, 230–237. [Google Scholar] [CrossRef]
- Berek, J.S.; Matulonis, U.A.; Peen, U.; Ghatage, P.; Mahner, S.; Redondo, A.; Lesoin, A.; Colombo, N.; Vergote, I.; Rosengarten, O.; et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 2018, 29, 1784–1792. [Google Scholar] [CrossRef]
- Dangaj Laniti, D.; Coukos, G. Genetics and anatomy sculpt immune-cell partners of ovarian cancer. Nature 2022, 612, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, I.; Uhlitz, F.; Ceglia, N.; Lim, J.L.P.; Wu, M.; Mohibullah, N.; Niyazov, J.; Ruiz, A.E.B.; Boehm, K.M.; Bojilova, V.; et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature 2022, 612, 778–786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).