Inhibition of CXCR4 Enhances the Efficacy of Radiotherapy in Metastatic Prostate Cancer Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human PCa Tissue Samples
2.2. Cells
2.3. Treatment Reagents and Irradiation
2.4. Clonogenic Assay
2.5. Migration Assay
2.6. Western Blotting (WB) for Evaluating Protein Level Changes in PCa Cells, Mesenchymal Stem Cells, and Pericyte Precursor Cells after CXCR4 Inhibition In Vitro
2.7. Cell Co-Culture to Generate Vascular Network
2.8. Orthotopic PCa Models
2.9. In Vivo Irradiation and AMD3100 Treatment
2.10. Immunofluorescence (IF)
2.11. Statistical Analysis
3. Results
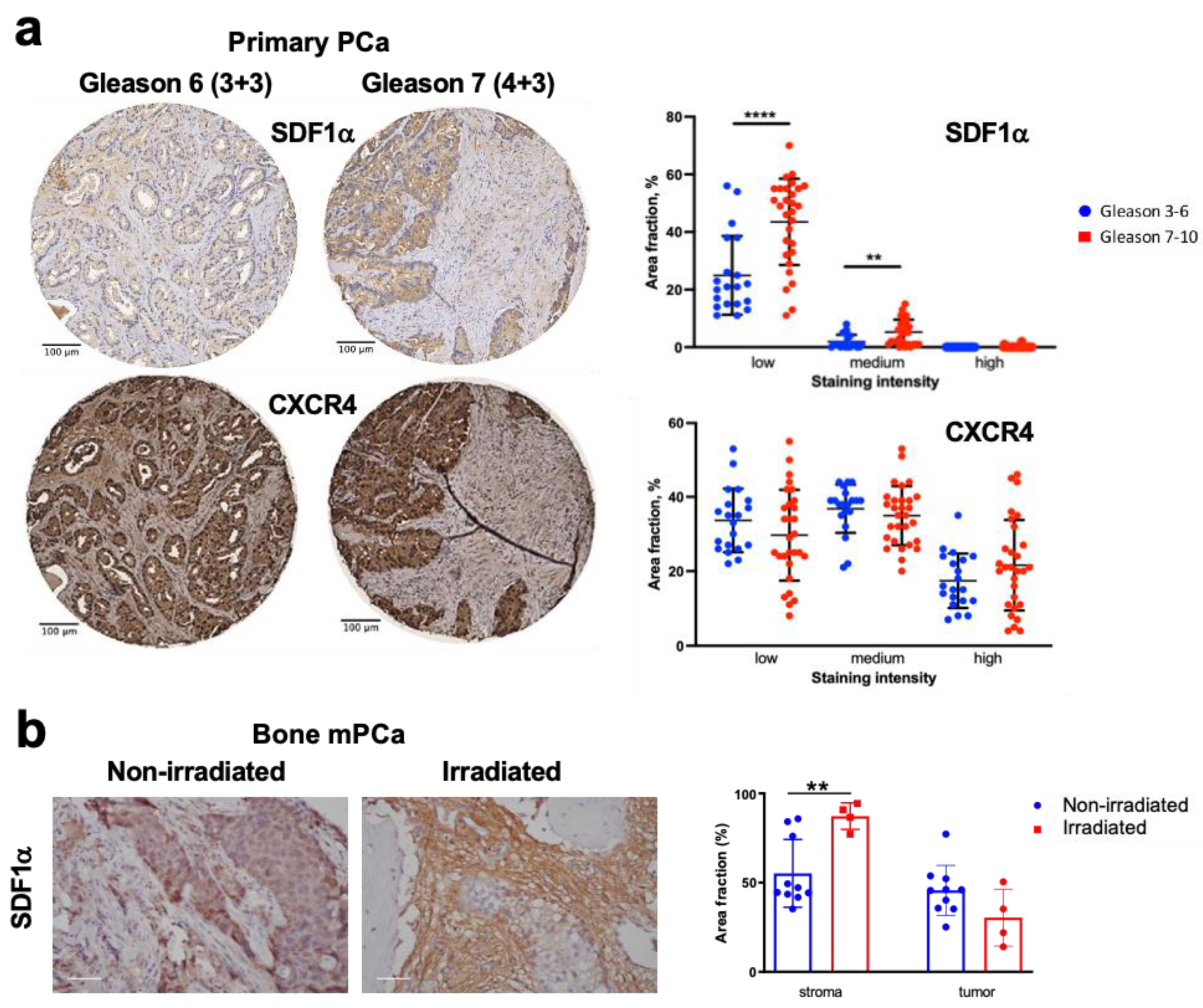
3.1. SDF1α Expression Is Increased with Tumor Progression and after RT in Human PCa Tissues
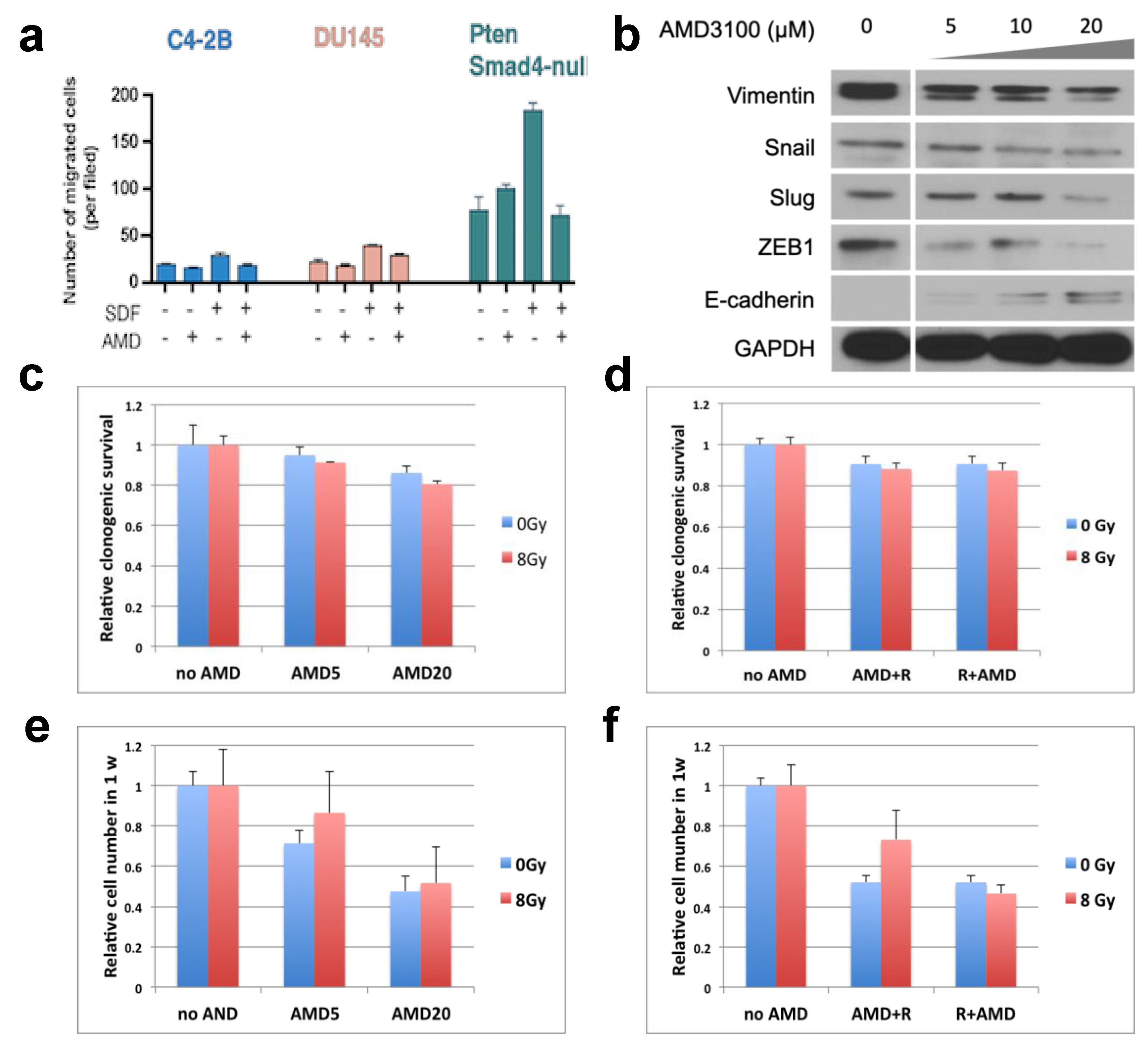
3.2. Inhibition of CXCR4 Decreases Invasion of PCa Cells and Has Only a Growth Inhibitory but Not Radiosensitization Effect In Vitro
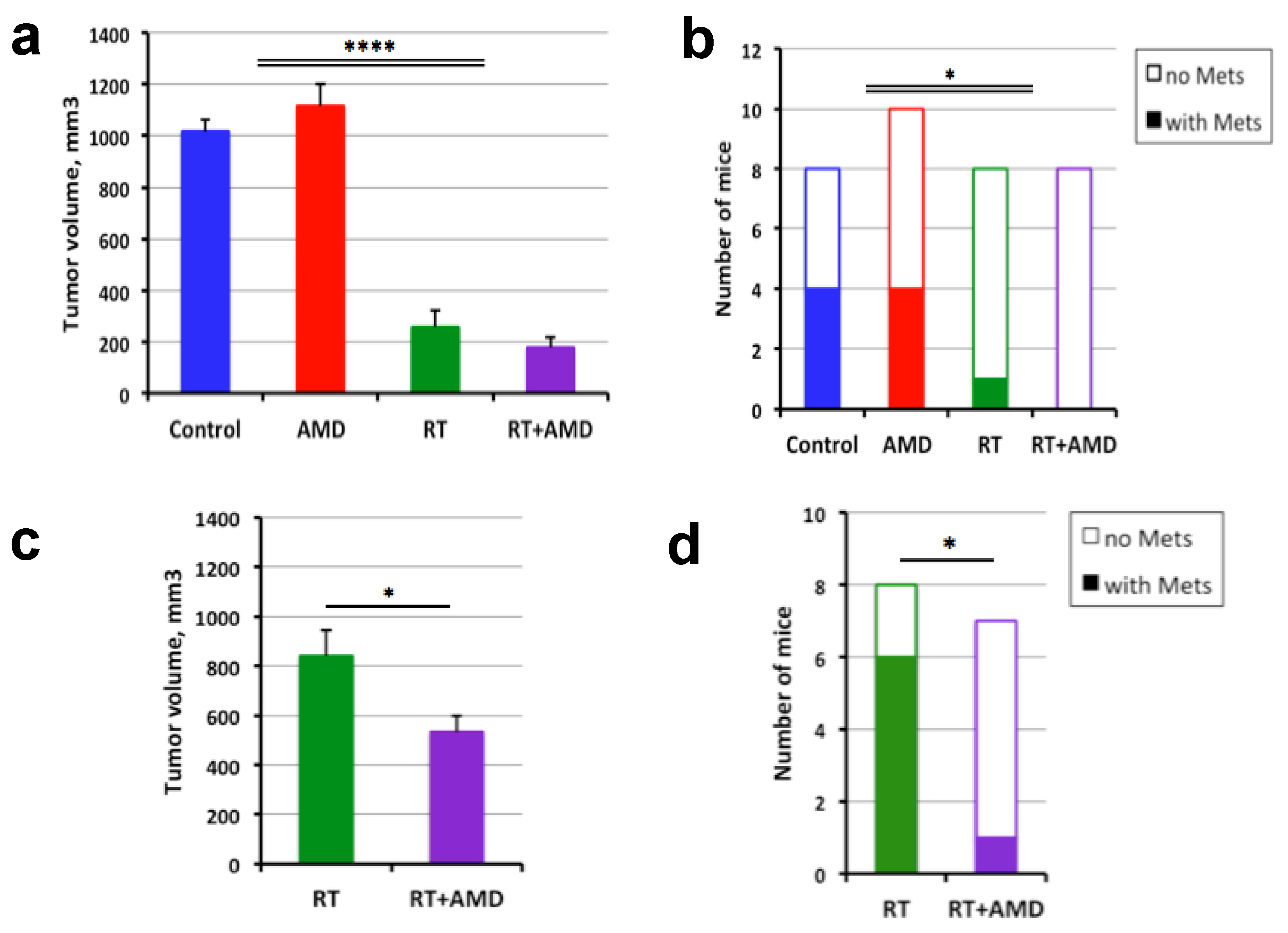
3.3. CXCR4 Inhibition after RT Delays the Regrowth and Metastasis of Orthotopic PCa Xenografts
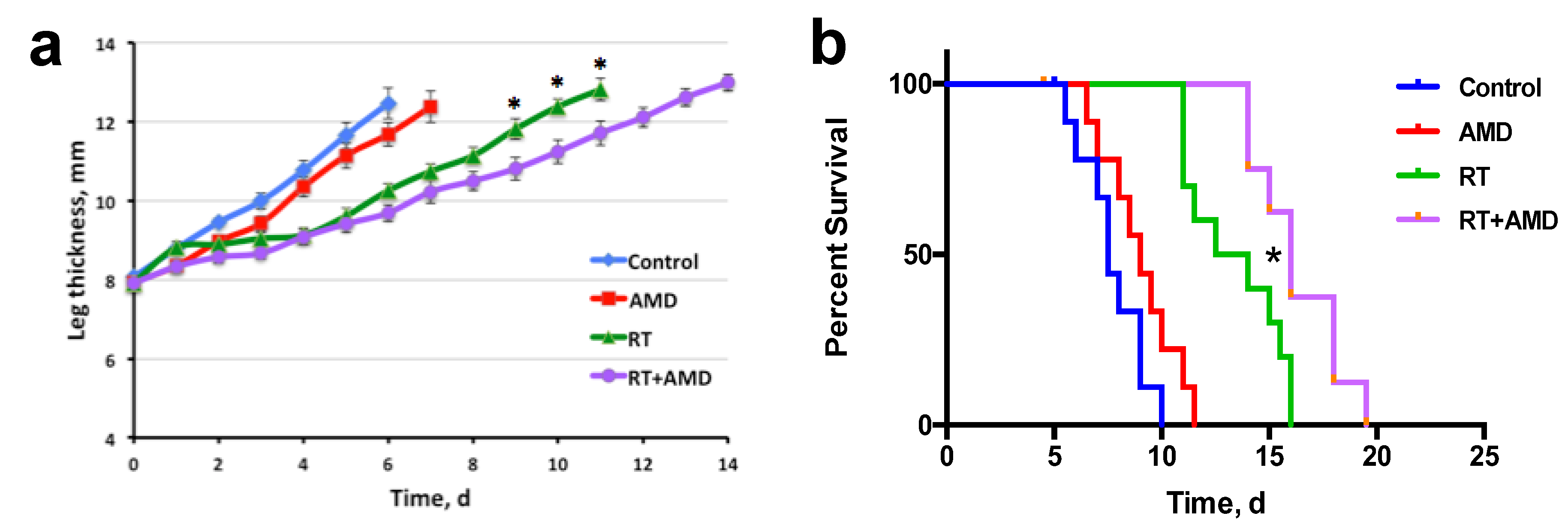
3.4. CXCR4 Inhibition after RT Delays the Regrowth of Established Bone Metastatic PCa
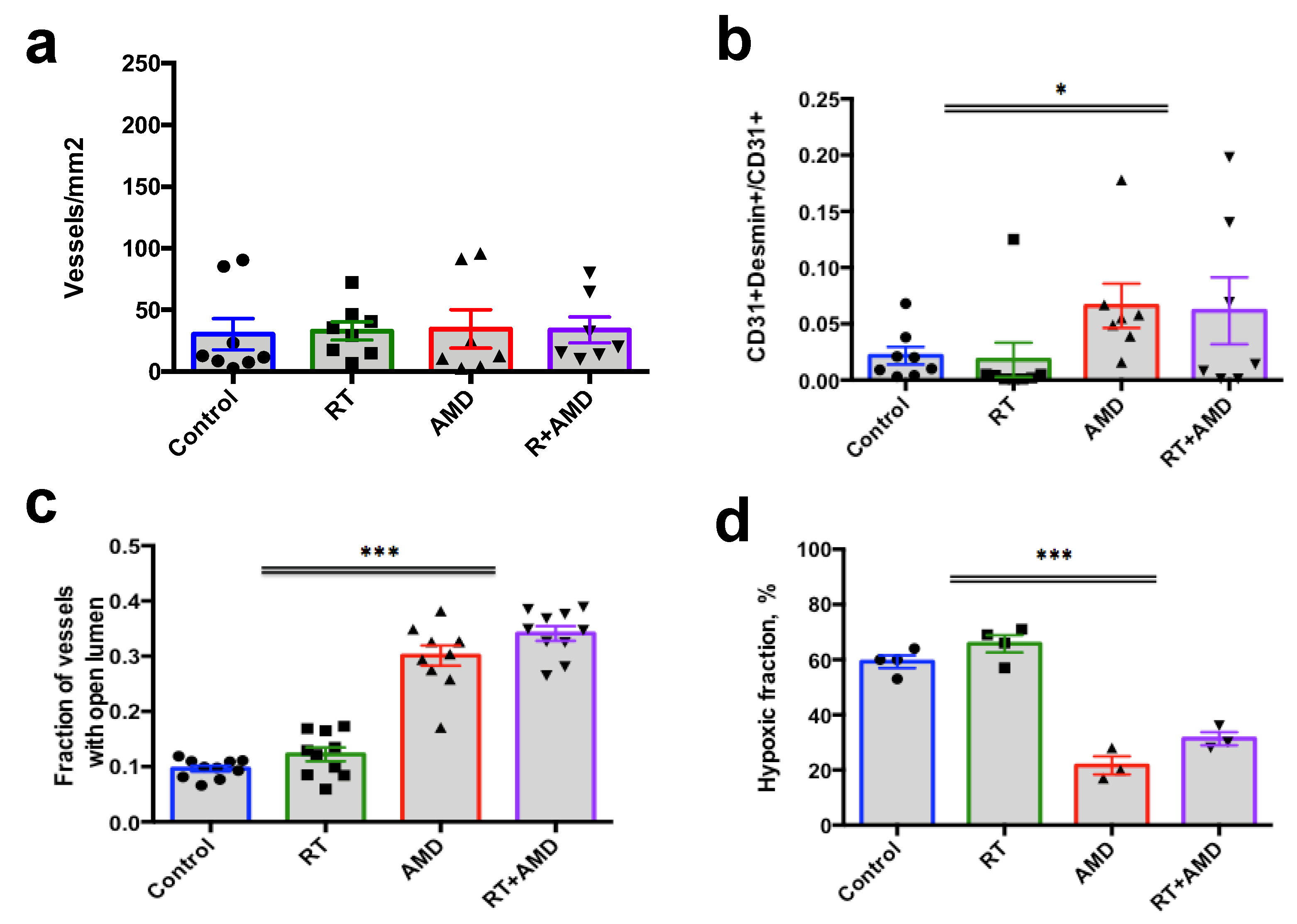
3.5. CXCR4 Inhibition Normalizes Vessel Structure and Reduces Hypoxia in Bone mPCa
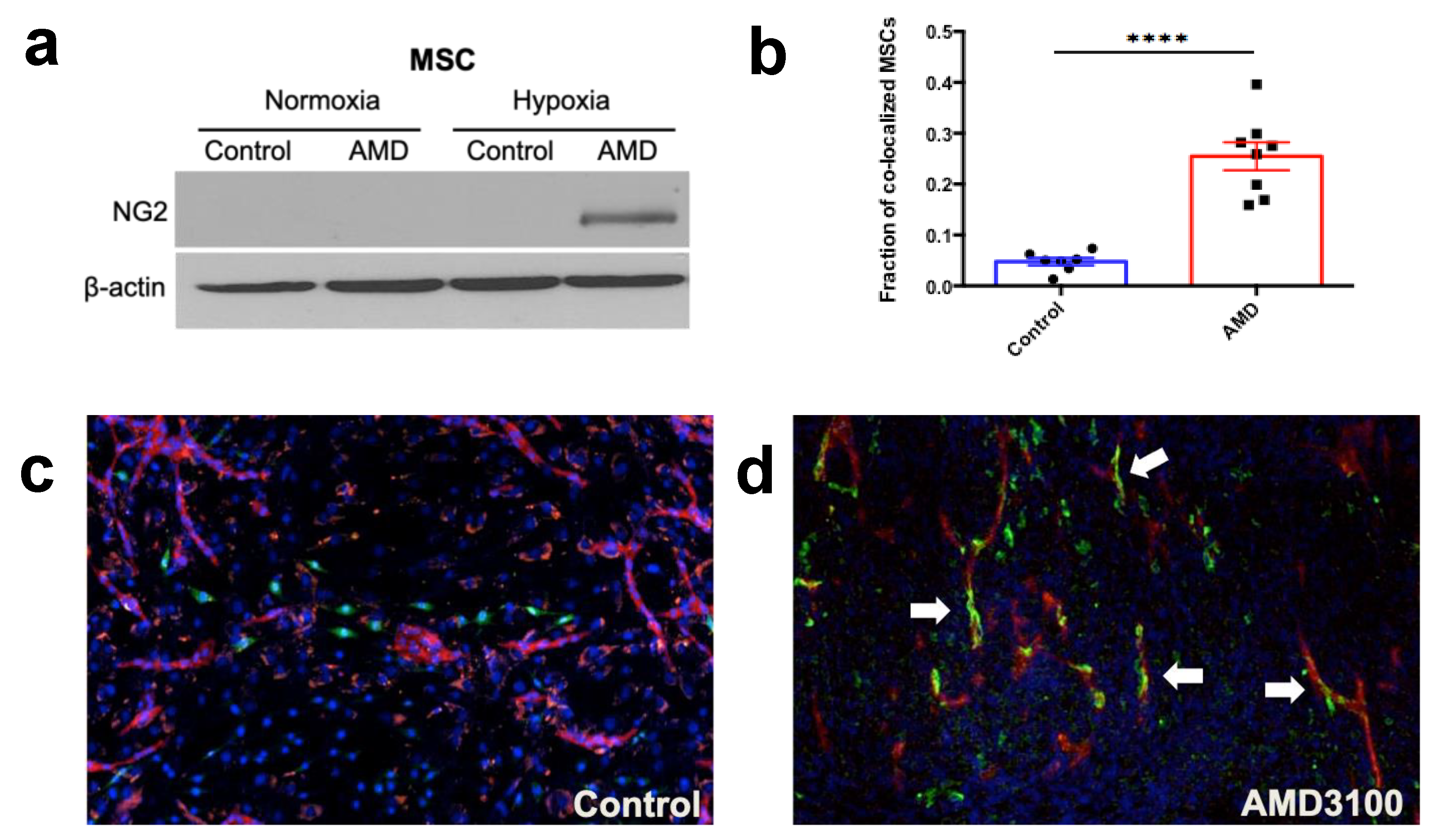
3.6. CXCR4 Inhibition Can Induce Mesenchymal Stem Cells (MSC) and Pericyte Precursor Cell Differentiation into Perivascular Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greco, C.; Vazirani, A.; Pares, O.; Pimentel, N.; Louro, V.; Morales, J.; Nunes, B.; Vasconcelos, A.L.; Antunes, I.; Kociolek, J.; et al. The evolving role of external beam radiotherapy in localized prostate cancer. Semin. Oncol. 2019, 46, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Henry, A.; Mason, M.; Wiegel, T. The role of radiotherapy in localised and locally advanced prostate cancer. Asian J. Urol. 2019, 6, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.; Roy, S.; Morgan, S.; Malone, S. A Review on the Current Treatment Paradigm in High-Risk Prostate Cancer. Cancers 2021, 13, 4257. [Google Scholar] [CrossRef]
- Harstell, W.F.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M.; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar]
- Klusa, D.; Lohaus, F.; Furesi, G.; Rauner, M.; Benešová, M.; Krause, M.; Kurth, I.; Peitzsch, C. Metastatic Spread in Prostate Cancer Patients Influencing Radiotherapy Response. Front. Oncol. 2021, 10, 627379. [Google Scholar] [CrossRef]
- Habl, G.; Straube, C.; Schiller, K.; Duma, M.N.; Oechsner, M.; Kessel, K.A.; Eiber, M.; Schwaiger, M.; Kübler, H.; Gschwend, J.E.; et al. Oligometastases from prostate cancer: Local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer 2017, 17, 361. [Google Scholar] [CrossRef]
- Duda, D.G.; Kozin, S.V.; Kirkpatrick, N.D.; Xu, L.; Fukumura, D.; Jain, R.K. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: An emerging sensitizer for anticancer therapies? Clin. Cancer Res. 2011, 17, 2074–2080. [Google Scholar] [CrossRef]
- Trautmann, F.; Cojoc, M.; Kurth, I.; Melin, N.; Bouchez, L.C.; Dubrovska, A.; Peitzsch, C. CXCR4 as biomarker for radioresistant cancer stem cells. Int. J. Radiat. Biol. 2014, 90, 687–699. [Google Scholar] [CrossRef]
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018, 9, 3018. [Google Scholar] [CrossRef]
- Cojoc, M.; Peitzsch, C.; Trautmann, F.; Polishchuk, L.; Telegeev, G.D.; Dubrovska, A. Emerging targets in cancer management: Role of the CXCL12/CXCR4 axis. Onco Targets Ther. 2013, 6, 1347–1361. [Google Scholar]
- Brown, J.M. Radiation Damage to Tumor Vasculature Initiates a Program That Promotes Tumor Recurrences. Int. J. Radiat. Oncol. 2020, 108, 734–744. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J., 2nd; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharm. 2020, 11, 574667. [Google Scholar] [CrossRef]
- Thomas, R.P.; Nagpal, S.; Iv, M.; Soltys, S.G.; Bertrand, S.; Pelpola, J.S.; Ball, R.; Yang, J.; Sundaram, V.; Chernikova, S.B.; et al. Macrophage Exclusion after Radiation Therapy (MERT): A First in Human Phase I/II Trial using a CXCR4 Inhibitor in Glioblastoma. Clin. Cancer Res. 2019, 25, 6948–6957. [Google Scholar] [CrossRef]
- Sun, Y.X.; Schneider, A.; Jung, Y.; Wang, J.; Dai, J.; Wang, J.; Taichman, R.S. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Min. Res. 2005, 20, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Mancini, A.; Muzi, P.; Ventura, L.; Biordi, L.; Ricevuto, E.; Pompili, S.; Mattei, C.; Di Cesare, E.; Jannini, E.A.; et al. CXCR4 pharmacogical inhibition reduces bone and soft tissue metastatic burden by affecting tumor growth and tumorigenic potential in prostate cancer preclinical models. Prostate 2015, 75, 1227–1246. [Google Scholar] [CrossRef] [PubMed]
- Domanska, U.M.; Timmer-Bosscha, H.; Nagengast, W.B.; Munnink, T.H.O.; Kruizinga, R.C.; Ananias, H.J.; Kliphuis, N.M.; Huls, G.; De Vries, E.G.; de Jong, I.J.; et al. CXCR4 Inhibition with AMD3100 Sensitizes Prostate Cancer to Docetaxel Chemotherapy. Neoplasia 2012, 14, 709–718. [Google Scholar] [CrossRef]
- Wang, J.; Shiozawa, Y.; Wang, J.; Wang, Y.; Jung, Y.; Pienta, K.J.; Mehra, R.; Loberg, R.; Taichman, R.S. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J. Biol. Chem. 2008, 283, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Darash-Yahana, M.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Pal, B.; Karplus, R.; Beider, K.; Avniel, S.; Kasem, S.; Galun, E.; et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004, 18, 1240–1242. [Google Scholar] [CrossRef]
- Chen, F.H.; Fu, S.Y.; Yang, Y.C.; Wang, C.C.; Chiang, C.S.; Hong, J.H. Combination of vessel-targeting agents and fractionated radiation therapy: The role of the SDF-1/CXCR4 pathway. Int. J. Radiat Oncol Biol. Phys. 2013, 86, 777–784. [Google Scholar] [CrossRef]
- Domańska, U.; Boer, J.; Timmer-Bosscha, H.; Van Vugt, M.A.T.M.; Hoving, H.D.; Kliphuis, N.M.; Rosati, S.; Van Der Poel, H.G.; De Jong, I.J.; de Vries, E.; et al. CXCR4 inhibition enhances radiosensitivity, while inducing cancer cell mobilization in a prostate cancer mouse model. Clin. Exp. Metastasis 2014, 31, 829–839. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kamoun, W.S.; Huang, Y.; Dawson, M.R.; Jain, R.K.; Duda, D.G. Recruitment of Myeloid but not Endothelial Precursor Cells Facilitates Tumor Regrowth after Local Irradiation. Cancer Res. 2010, 70, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 2010, 120, 694–705. [Google Scholar] [CrossRef]
- Wang, H.-H.; Cui, Y.-L.; Zaorsky, N.G.; Lan, J.; Deng, L.; Zeng, X.-L.; Wu, Z.-Q.; Tao, Z.; Guo, W.-H.; Wang, Q.-X.; et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016, 375, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Kim, A.-R.; Nam, J.-K.; Kim, J.-M.; Kim, J.-Y.; Seo, H.R.; Lee, H.-J.; Cho, J.; Lee, Y.-J. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat. Commun. 2018, 9, 5108. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wu, C.-J.; Chu, G.C.; Xiao, Y.; Ho, D.; Zhang, J.; Perry, S.R.; Labrot, E.S.; Wu, X.; Lis, R.; et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 2011, 470, 269–273. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D.; Maimon, N.; Gruionu, G.; Munn, L.L. Self-assembly of vascularized tissue to support tumor explants in vitro. Integr. Biol. (Camb) 2016, 8, 1301–1311. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Reiberger, T.; Duyverman, A.M.; Huang, P.; Samuel, R.; Hiddingh, L.; Roberge, S.; Koppel, C.; Lauwers, G.Y.; et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology 2014, 59, 1435–1447. [Google Scholar] [CrossRef]
- Kamoun, W.S.; Ley, C.D.; Farrar, C.T.; Duyverman, A.M.; Lahdenranta, J.; Lacorre, D.A.; Batchelor, T.T.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol 2009, 27, 2542–2552. [Google Scholar] [CrossRef]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; Tomaso, E.D.; Jain, R.K. Pathology: Cancer cells compress intratumour vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef]
- Chaudary, N.; Pintilie, M.; Jelveh, S.; Lindsay, P.; Hill, R.P.; Milosevic, M. Plerixafor Improves Primary Tumor Response and Reduces Metastases in Cervical Cancer Treated with Radio-Chemotherapy. Clin. Cancer Res. 2017, 23, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Lecavalier-Barsoum, M.; Chaudary, N.; Han, K.; Pintilie, M.; Hill, R.P.; Milosevic, M. Targeting CXCL12/CXCR4 and myeloid cells to improve the therapeutic ratio in patient-derived cervical cancer models treated with radio-chemotherapy. Br. J. Cancer 2019, 121, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Kashiwagi, S.; Yuan, J.; Santosuosso, M.; Leblanc, P.; Ingraham, R.; Poznansky, M.C. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res. 2011, 71, 5522–5534. [Google Scholar] [CrossRef]
- Conley-LaComb, M.K.; Semaan, L.; Singareddy, R.; Li, Y.; Heath, E.I.; Kim, S.; Chinni, S.R. Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol. Cancer 2016, 15, 68. [Google Scholar] [CrossRef]
- Porvasnik, S.; Sakamoto, N.; Kusmartsev, S.; Eruslanov, E.; Kim, W.-J.; Cao, W.; Urbanek, C.; Wong, D.; Goodison, S.; Rosser, C.J. Effects of CXCR4 antagonist CTCE-9908 on prostate tumor growth. Prostate 2009, 69, 1460–1469. [Google Scholar] [CrossRef]
- Wang, Q.; Diao, X.; Sun, J.; Chen, Z. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol. Int. 2011, 35, 897–904. [Google Scholar] [CrossRef]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1–CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R.K. Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.-S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef]
- Dings, R.P.; Loren, M.; Heun, H.; McNiel, E.; Griffioen, A.W.; Mayo, K.H.; Griffin, R.J. Scheduling of Radiation with Angiogenesis Inhibitors Anginex and Avastin Improves Therapeutic Outcome via Vessel Normalization. Clin. Cancer Res. 2007, 13, 3395–3402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Ochiai, H.; Hoshino, Y.; Klein, S.; Zustin, J.; Ramjiawan, R.R.; Kitahara, S.; Maimon, N.; Bazou, D.; Chiang, S.; et al. Inhibition of CXCR4 Enhances the Efficacy of Radiotherapy in Metastatic Prostate Cancer Models. Cancers 2023, 15, 1021. https://doi.org/10.3390/cancers15041021
Gupta N, Ochiai H, Hoshino Y, Klein S, Zustin J, Ramjiawan RR, Kitahara S, Maimon N, Bazou D, Chiang S, et al. Inhibition of CXCR4 Enhances the Efficacy of Radiotherapy in Metastatic Prostate Cancer Models. Cancers. 2023; 15(4):1021. https://doi.org/10.3390/cancers15041021
Chicago/Turabian StyleGupta, Nisha, Hiroki Ochiai, Yoshinori Hoshino, Sebastian Klein, Jozef Zustin, Rakesh R. Ramjiawan, Shuji Kitahara, Nir Maimon, Despina Bazou, Sarah Chiang, and et al. 2023. "Inhibition of CXCR4 Enhances the Efficacy of Radiotherapy in Metastatic Prostate Cancer Models" Cancers 15, no. 4: 1021. https://doi.org/10.3390/cancers15041021
APA StyleGupta, N., Ochiai, H., Hoshino, Y., Klein, S., Zustin, J., Ramjiawan, R. R., Kitahara, S., Maimon, N., Bazou, D., Chiang, S., Li, S., Schanne, D. H., Jain, R. K., Munn, L. L., Huang, P., Kozin, S. V., & Duda, D. G. (2023). Inhibition of CXCR4 Enhances the Efficacy of Radiotherapy in Metastatic Prostate Cancer Models. Cancers, 15(4), 1021. https://doi.org/10.3390/cancers15041021






