Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, towards Prognostic Markers and New Therapeutic Targets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Tumor Characteristics
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Immunohistochemical Analysis
3.3. Biomarkers and Clinical Parameters Associated with the Diagnosis and Histological Response
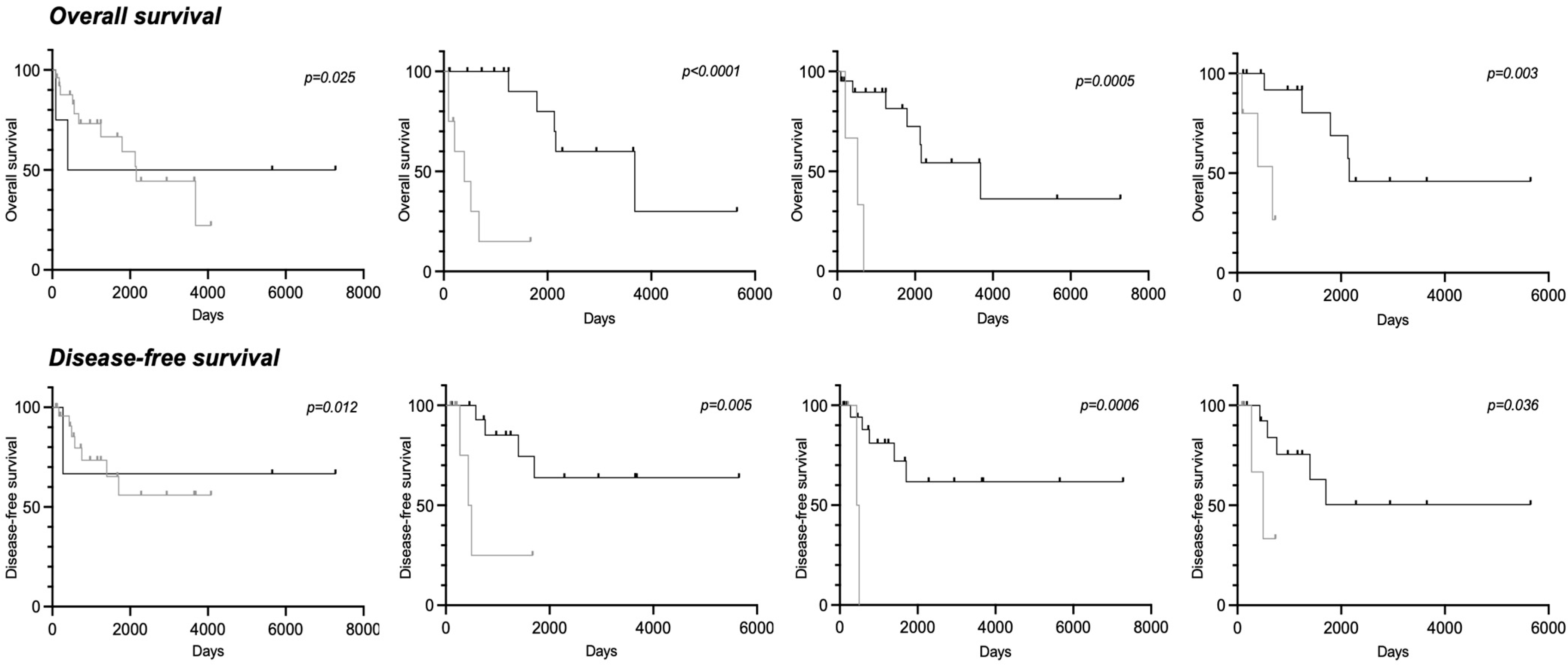
3.4. Clinical Parameters and Biomarkers Associated with Overall Survival
3.5. Clinical Parameters and Biomarkers Associated with Disease-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumhoer, D.; Brunner, P.; Eppenberger-Castori, S.; Smida, J.; Nathrath, M.; Jundt, G. Osteosarcomas of the Jaws Differ from Their Peripheral Counterparts and Require a Distinct Treatment Approach. Experiences from the DOESAK Registry. Oral Oncol. 2014, 50, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Julieron, M.; Brouchet, A.; Italiano, A.; Schouman, T.; Marcy, P.-Y.; Odin, G.; Lacout, A.; Dassonville, O.; Peyrottes-Birstwisles, I.; et al. Osteosarcomas of the Mandible: Are They Different from Other Tumor Sites? Crit. Rev. Oncol. Hematol. 2012, 82, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Boon, E.; van der Graaf, W.T.A.; Gelderblom, H.; Tesselaar, M.E.T.; van Es, R.J.J.; Oosting, S.F.; de Bree, R.; van Meerten, E.; Hoeben, A.; Smeele, L.E.; et al. Impact of Chemotherapy on the Outcome of Osteosarcoma of the Head and Neck in Adults. Head Neck 2017, 39, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kontio, R.; Hagström, J.; Lindholm, P.; Böhling, T.; Sampo, M.; Mesimäki, K.; Saarilahti, K.; Koivunen, P.; Mäkitie, A.A. Craniomaxillofacial Osteosarcoma—The Role of Surgical Margins. J. Craniomaxillofac. Surg. 2019, 47, 922–925. [Google Scholar] [CrossRef]
- Lee, R.J.; Arshi, A.; Schwartz, H.C.; Christensen, R.E. Characteristics and Prognostic Factors of Osteosarcoma of the Jaws: A Retrospective Cohort Study. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 470–477. [Google Scholar] [CrossRef]
- Van den Berg, H.; Merks, J.H.M. Incidence and Grading of Cranio-Facial Osteosarcomas. Int. J. Oral Maxillofac. Surg. 2014, 43, 7–12. [Google Scholar] [CrossRef]
- Nissanka, E.H.; Amaratunge, E.A.P.D.; Tilakaratne, W.M. Clinicopathological Analysis of Osteosarcoma of Jaw Bones. Oral Dis. 2007, 13, 82–87. [Google Scholar] [CrossRef]
- Granowski-LeCornu, M.; Chuang, S.-K.; Kaban, L.B.; August, M. Osteosarcoma of the Jaws: Factors Influencing Prognosis. J. Oral Maxillofac. Surg. 2011, 69, 2368–2375. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational Biology of Osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Laurence, V.; Occean, B.-V.; Ray-Coquard, I.; Linassier, C.; Corradini, N.; Collard, O.; Chaigneau, L.; Cupissol, D.; Kerbrat, P.; et al. Methotrexate-Etoposide-Ifosfamide Compared with Doxorubicin-Cisplatin-Ifosfamide Chemotherapy in Osteosarcoma Treatment, Patients Aged 18–25 Years. J. Adolesc. Young Adult Oncol. 2020, 9, 172–182. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Ray-Coquard, I.; Occean, B.-V.; Laurence, V.; Cupissol, D.; Perrin, C.; Penel, N.; Bompas, E.; Rios, M.; Le Cesne, A.; et al. Results of API-AI Based Regimen in Osteosarcoma Adult Patients Included in the French OS2006/Sarcome-09 Study. Int. J. Cancer 2020, 146, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Bouaoud, J.; Beinse, G.; Epaillard, N.; Amor-Sehlil, M.; Bidault, F.; Brocheriou, I.; Hervé, G.; Spano, J.-P.; Janot, F.; Boudou-Rouquette, P.; et al. Lack of Efficacy of Neoadjuvant Chemotherapy in Adult Patients with Maxillo-Facial High-Grade Osteosarcomas: A French Experience in Two Reference Centers. Oral Oncol. 2019, 95, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Khadembaschi, D.; Jafri, M.; Praveen, P.; Parmar, S.; Breik, O. Does Neoadjuvant Chemotherapy Provide a Survival Benefit in Maxillofacial Osteosarcoma: A Systematic Review and Pooled Analysis. Oral Oncol. 2022, 135, 106133. [Google Scholar] [CrossRef] [PubMed]
- Crenn, V.; Biteau, K.; Amiaud, J.; Dumars, C.; Guiho, R.; Vidal, L.; Nail, L.-R.L.; Heymann, D.; Moreau, A.; Gouin, F.; et al. Bone Microenvironment Has an Influence on the Histological Response of Osteosarcoma to Chemotherapy: Retrospective Analysis and Preclinical Modeling. Am. J. Cancer Res. 2017, 7, 2333–2349. [Google Scholar]
- Xin, S.; Wei, G. Prognostic Factors in Osteosarcoma: A Study Level Meta-Analysis and Systematic Review of Current Practice. J. Bone Oncol. 2020, 21, 100281. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Mascard, E.; Siegfried, A.; de Pinieux, G.; Gaspar, N.; Bouvier, C.; Aubert, S.; Marec-Bérard, P.; Piperno-Neumann, S.; Marie, B.; et al. Assessment of Resection Margins in Bone Sarcoma Treated by Neoadjuvant Chemotherapy: Literature Review and Guidelines of the Bone Group (GROUPOS) of the French Sarcoma Group and Bone Tumor Study Group (GSF-GETO/RESOS). Orthop. Traumatol. Surg. Res. 2019, 105, 773–780. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, F.; Zhao, T.; Wang, C.; Tao, Q. Clinical Characteristics of Radiation-Induced Sarcoma of the Head and Neck: Review of 15 Cases and 323 Cases in the Literature. J. Oral Maxillofac. Surg. 2016, 74, 283–291. [Google Scholar] [CrossRef]
- De Souza, L.L.; Pontes, H.A.R.; Santos-Silva, A.R.; Fernandes, L.A.; Batista, L.A.L.; Lopes, M.A.; Khan, W.; Pontes, F.S.C. Oral Radiation-Induced Sarcomas: Systematic Review. Head Neck 2020, 42, 2660–2668. [Google Scholar] [CrossRef]
- Thariat, J.; Schouman, T.; Brouchet, A.; Sarini, J.; Miller, R.C.; Reychler, H.; Ray-Coquard, I.; Italiano, A.; Verite, C.; Sohawon, S.; et al. Osteosarcomas of the Mandible: Multidisciplinary Management of a Rare Tumor of the Young Adult a Cooperative Study of the GSF-GETO, Rare Cancer Network, GETTEC/REFCOR and SFCE. Ann. Oncol. 2013, 24, 824–831. [Google Scholar] [CrossRef]
- Heymann, M.-F.; Lézot, F.; Heymann, D. The Contribution of Immune Infiltrates and the Local Microenvironment in the Pathogenesis of Osteosarcoma. Cell. Immunol. 2019, 343, 103711. [Google Scholar] [CrossRef]
- Guise, T.A. The Vicious Cycle of Bone Metastases. J. Musculoskelet. Neuronal Interact. 2002, 2, 570–572. [Google Scholar] [PubMed]
- Wittrant, Y.; Théoleyre, S.; Chipoy, C.; Padrines, M.; Blanchard, F.; Heymann, D.; Rédini, F. RANKL/RANK/OPG: New Therapeutic Targets in Bone Tumours and Associated Osteolysis. Biochim. Biophys. Acta 2004, 1704, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Wada, T.; Akatsuka, T.; Kawaguchi, S.; Nagoya, S.; Shindoh, M.; Higashino, F.; Mezawa, F.; Okada, F.; Ishii, S. Vascular Endothelial Growth Factor Expression in Untreated Osteosarcoma Is Predictive of Pulmonary Metastasis and Poor Prognosis. Clin. Cancer Res. 2000, 6, 572–577. [Google Scholar] [PubMed]
- Rossi, B.; Schinzari, G.; Maccauro, G.; Scaramuzzo, L.; Signorelli, D.; Rosa, M.A.; Fabbriciani, C.; Carlo, B. Neoadjuvant Multidrug Chemotherapy Including High-Dose Methotrexate Modifies VEGF Expression in Osteosarcoma: An Immunohistochemical Analysis. BMC Musculoskelet. Disord. 2010, 11, 34. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, J.; Jiang, T.; Zhao, H.; Gao, Y.; Zheng, C.; Shi, X. Difference in Pre- and Postchemotherapy Vascular Endothelial Growth Factor Levels as a Prognostic Indicator in Osteosarcoma. J. Int. Med. Res. 2011, 39, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.-F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-Positive Tumor-Associated Macrophages and CD8-Positive Cytotoxic Lymphocytes Are Powerful Diagnostic Markers for the Therapeutic Stratification of Osteosarcoma Patients: An Immunohistochemical Analysis of the Biopsies Fromthe French OS2006 Phase 3 Trial. Oncoimmunology 2017, 6, e1331193. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic Significance of CD68+ and CD163+ Tumor Associated Macrophages in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef]
- Costa Arantes, D.A.; Gonçalves, A.S.; Jham, B.C.; Duarte, E.C.B.; de Paula, É.C.; de Paula, H.M.; Mendonça, E.F.; Batista, A.C. Evaluation of HLA-G, HLA-E, and PD-L1 Proteins in Oral Osteosarcomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, e188–e196. [Google Scholar] [CrossRef]
- Rubio, R.; Abarrategi, A.; Garcia-Castro, J.; Martinez-Cruzado, L.; Suarez, C.; Tornin, J.; Santos, L.; Astudillo, A.; Colmenero, I.; Mulero, F.; et al. Bone Environment Is Essential for Osteosarcoma Development from Transformed Mesenchymal Stem Cells. Stem Cells 2014, 32, 1136–1148. [Google Scholar] [CrossRef]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone Microenvironment Signals in Osteosarcoma Development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Bertin, H.; Gomez-Brouchet, A.; Rédini, F. Osteosarcoma of the Jaws: An Overview of the Pathophysiological Mechanisms. Crit. Rev. Oncol. Hematol. 2020, 156, 103126. [Google Scholar] [CrossRef]
- Stricker, E.; Reed, D.R.; Schabath, M.B.; Sok, P.; Scheurer, M.E.; Lupo, P.J. Trends in Overall Survival among Patients Treated for Sarcoma at a Large Tertiary Cancer Center between 1986 and 2014. Cancers 2023, 15, 514. [Google Scholar] [CrossRef]
- Jawad, S.N.; Abdullah, B.H. Proliferative, Apoptotic and Angiogenic Potentials in Jaws and Long Bones Osteosarcomas: A Comparative Immunohistochemical Study. J. Oral Pathol. Med. 2010, 39, 681–686. [Google Scholar] [CrossRef]
- Junior, A.T.; de Abreu Alves, F.; Pinto, C.A.L.; Carvalho, A.L.; Kowalski, L.P.; Lopes, M.A. Clinicopathological and Immunohistochemical Analysis of Twenty-Five Head and Neck Osteosarcomas. Oral Oncol. 2003, 39, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wei, R.; Wu, J. Identification and Functional Analysis of EPOR+ Tumor-Associated Macrophages in Human Osteosarcoma Lung Metastasis. J. Immunol. Res. 2020, 2020, 9374240. [Google Scholar] [CrossRef]
- Weber, M.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Distel, L.; Ries, J.; Amann, K.; Neukam, F.W.; Wehrhan, F. Small Oral Squamous Cell Carcinomas with Nodal Lymphogenic Metastasis Show Increased Infiltration of M2 Polarized Macrophages--an Immunohistochemical Analysis. J. Craniomaxillofac. Surg. 2014, 42, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, D.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Iriki, T.; Tsuboki, J.; Cheng, P.; Nakagata, N.; Mizuta, H.; Bekki, H.; et al. CD163 Is Required for Protumoral Activation of Macrophages in Human and Murine Sarcoma. Cancer Res. 2018, 78, 3255–3266. [Google Scholar] [CrossRef]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of Macrophage Polarization Is Associated with the Metastatic Process in Osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef]
- Weber, M.; Söder, S.; Sander, J.; Ries, J.; Geppert, C.; Kesting, M.; Wehrhan, F. Craniofacial Osteosarcoma-Pilot Study on the Expression of Osteobiologic Characteristics and Hypothesis on Metastasis. Front. Oncol. 2020, 10, 745. [Google Scholar] [CrossRef]
- Finkelman, R.D.; Eason, A.L.; Rakijian, D.R.; Tutundzhyan, Y.; Hardesty, R.A. Elevated IGF-II and TGF-Beta Concentrations in Human Calvarial Bone: Potential Mechanism for Increased Graft Survival and Resistance to Osteoporosis. Plast. Reconstr. Surg. 1994, 93, 732–738. [Google Scholar] [CrossRef]
- Wehrhan, F.; Hyckel, P.; Ries, J.; Stockmann, P.; Nkenke, E.; Schlegel, K.A.; Neukam, F.W.; Amann, K. Expression of Msx-1 Is Suppressed in Bisphosphonate Associated Osteonecrosis Related Jaw Tissue-Etiopathology Considerations Respecting Jaw Developmental Biology-Related Unique Features. J. Transl. Med. 2010, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.A.; Silvan, U. On the Biomechanical Properties of Osteosarcoma Cells and Their Environment. Int. J. Dev. Biol. 2019, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dzamukova, M.; Brunner, T.M.; Miotla-Zarebska, J.; Heinrich, F.; Brylka, L.; Mashreghi, M.-F.; Kusumbe, A.; Kühn, R.; Schinke, T.; Vincent, T.L.; et al. Mechanical Forces Couple Bone Matrix Mineralization with Inhibition of Angiogenesis to Limit Adolescent Bone Growth. Nat. Commun. 2022, 13, 3059. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Brouchet, A.; Gilhodes, J.; Acker, N.V.; Brion, R.; Bouvier, C.; Assemat, P.; Gaspar, N.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; et al. Characterization of Macrophages and Osteoclasts in the Osteosarcoma Tumor Microenvironment at Diagnosis: New Perspective for Osteosarcoma Treatment? Cancers 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.J.; Dutton, P.; Bruland, Ø.; Gelderblom, H.; Faleti, A.; Bühnemann, C.; van Maldegem, A.; Johnson, H.; Poulton, L.; Love, S.; et al. Outcomes from a Mechanistic Biomarker Multi-Arm and Randomised Study of Liposomal MTP-PE (Mifamurtide) in Metastatic and/or Recurrent Osteosarcoma (EuroSarc-Memos Trial). BMC Cancer 2022, 22, 629. [Google Scholar] [CrossRef]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of Mifamurtide in Addition to Standard Chemotherapy for High-Grade Osteosarcoma: A Systematic Review. JBI Database System Rev. Implement. Rep. 2017, 15, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; de Arruda, J.A.A.; Arantes, D.A.C.; Costa, S.F.S.; Souza, L.L.; Pontes, H.A.R.; Fonseca, F.P.; Mesquita, R.A.; Nonaka, C.F.W.; Mendonça, E.F.; et al. Evaluation of Tumor-Infiltrating Lymphocytes in Osteosarcomas of the Jaws: A Multicenter Study. Virchows Arch. 2019, 474, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.M.; Almeida, J.-S.; Reith, J.D.; Sousa, L.M.; Fonseca, R.; Freitas-Tavares, P.; Santos-Rosa, M.; Rodrigues-Santos, P. Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival. Cancers 2021, 13, 6075. [Google Scholar] [CrossRef]
- Wen, Y.; Tang, F.; Tu, C.; Hornicek, F.; Duan, Z.; Min, L. Immune Checkpoints in Osteosarcoma: Recent Advances and Therapeutic Potential. Cancer Lett. 2022, 547, 215887. [Google Scholar] [CrossRef]
- Sisay, M.; Mengistu, G.; Edessa, D. The RANK/RANKL/OPG System in Tumorigenesis and Metastasis of Cancer Stem Cell: Potential Targets for Anticancer Therapy. OncoTargets Ther. 2017, 10, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Pazzaglia, L.; Cevolani, L.; Pratelli, L.; Pierini, M.; Quattrini, I.; Carretta, E.; Manara, M.C.; Pasello, M.; Frega, G.; et al. Bone Turnover Marker (BTM) Changes after Denosumab in Giant Cell Tumors of Bone (GCTB): A Phase II Trial Correlative Study. Cancers 2022, 14, 2863. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Richard, P.; Wittrant, Y.; Battaglia, S.; Pilet, P.; Trichet, V.; Blanchard, F.; Gouin, F.; Pitard, B.; Heymann, D.; et al. Therapeutic Relevance of Osteoprotegerin Gene Therapy in Osteosarcoma: Blockade of the Vicious Cycle between Tumor Cell Proliferation and Bone Resorption. Cancer Res. 2007, 67, 7308–7318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL Blockade Prevents and Treats Aggressive Osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197. [Google Scholar] [CrossRef]
- Punzo, F.; Tortora, C.; Argenziano, M.; Pinto, D.D.; Pota, E.; Martino, M.D.; Paola, A.D.; Rossi, F. Can Denosumab Be Used in Combination with Doxorubicin in Osteosarcoma? Oncotarget 2020, 11, 2763–2773. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Le Deley, M.-C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.-C.; Entz-Werlé, N.; et al. Zoledronate in Combination with Chemotherapy and Surgery to Treat Osteosarcoma (OS2006): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Zheng, M.; Yu, S.; Zhang, X.; Xu, X. CD146 Is Closely Associated with the Prognosis and Molecular Features of Osteosarcoma: Guidance for Personalized Clinical Treatment. Front. Genet. 2022, 13, 1025306. [Google Scholar] [CrossRef]
- Taylor, R.M.; Kashima, T.G.; Knowles, H.J.; Athanasou, N.A. VEGF, FLT3 Ligand, PlGF and HGF Can Substitute for M-CSF to Induce Human Osteoclast Formation: Implications for Giant Cell Tumour Pathobiology. Lab. Investig. 2012, 92, 1398–1406. [Google Scholar] [CrossRef]
- Kumta, S.M.; Huang, L.; Cheng, Y.Y.; Chow, L.T.C.; Lee, K.M.; Zheng, M.H. Expression of VEGF and MMP-9 in Giant Cell Tumor of Bone and Other Osteolytic Lesions. Life Sci. 2003, 73, 1427–1436. [Google Scholar] [CrossRef]
- De Vita, A.; Vanni, S.; Miserocchi, G.; Fausti, V.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Liverani, C.; Calabrese, C.; Casadei, R.; et al. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines 2022, 10, 372. [Google Scholar] [CrossRef]
- Gaspar, N.; Venkatramani, R.; Hecker-Nolting, S.; Melcon, S.G.; Locatelli, F.; Bautista, F.; Longhi, A.; Lervat, C.; Entz-Werle, N.; Casanova, M.; et al. Lenvatinib with Etoposide plus Ifosfamide in Patients with Refractory or Relapsed Osteosarcoma (ITCC-050): A Multicentre, Open-Label, Multicohort, Phase 1/2 Study. Lancet Oncol. 2021, 22, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and Safety of Regorafenib in Adult Patients with Metastatic Osteosarcoma: A Non-Comparative, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone Sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | |
|---|---|
| Age, n (years) ± S.D. (min–max) | 47.8 ± 19.9 (17.3–83.9) |
| Gender: F (%)/M (%) | 22 (44.0)/28 (56.0) |
| Grade of malignity, n (%) | |
| High-grade | 40 (80.0) |
| Intermediate grade | 7 (14.0) |
| Unknown | 3 (6.0) |
| Histological subtype, n (%) | |
| Chondroblastic | 23 (46.0) |
| Osteoblastic | 16 (32.0) |
| Fibroblastic | 9 (18.0) |
| Undifferentiated | 2 (4.0) |
| Response to the neoadjuvant chemotherapy, n (%) | |
| Poor | 20 (40.0) |
| Good | 6 (12.0) |
| Unknown | 24 (48.0) |
| Progression of the disease, n (%) | |
| Local recurrence | 6 (12.0) |
| Metastases | 7 (14.0) |
| Overall survival, n (days) ± S.D. | 1600 ± 1737 |
| Disease-free survival, n (days) ± S.D. | 1469 ± 1745 |
| Biomarker Staining Results | |||||||
|---|---|---|---|---|---|---|---|
| Antibody | Nb Tested | Mean Positive Cells, n ± S.D. (Min–Max) | Nb ≥ 50% Positive Cells (%) | Nb ≥ 10% Positive Cells (%) | Nb ≥ 1% Positive Cells (%) | ||
| RANK | 27 | 62.9 ± 33.7 (0–90) | - | 22 (81.5%) | - | ||
| RANKL | 35 | 70.0 ± 29.5 (10–100) | - | 30 (85.7%) | - | ||
| CD146 | 35 | - | 20 (71.4%) | - | - | ||
| CD163 | 28 | 37.7 ± 21.6 (1–70) | 9 (32.1%) | - | - | ||
| CD68 | 28 | 21.4 ± 17.9 (1–70) | 3 (10.7%) | - | - | ||
| CD4+ | 24 | 13.7 ± 19.8 (0–50) | - | 8 (33.3%) | - | ||
| CD8+ | 25 | 8.9 ± 15.2 (1–50) | - | - | 7 (28%) | ||
| PD-1 | 27 | 0.1 ± 0.4 (0–1) | - | - | 4 (14.8%) | ||
| PD-L1 | 24 | 2.1 ± 10.2 (0–50) | - | - | 1 (4.2%) | ||
| Correlations between Biomarkers | |||||||
| RANK | RANKL | CD163 | CD68 | CD4+ | CD8+ | PD-1 | |
| RANKL | 0.1232 a 0.5404 b | ||||||
| CD163 | 0.0526 a 0.8115 b | −0.3261 a 0.0904 b | |||||
| CD68 | −0.0110 a 0.9582 b | −0.1714 a 0.3833 b | 0.6956 a <0.0001 b | ||||
| CD4+ | 0.4718 a 0.0308 b | −0.3373 a 0.1069 b | 0.5011 a 0.0149 b | 0.4607 a 0.0269 b | |||
| CD8+ | 0.1377 a 0.5627 b | −0.2687 a 0.1940 b | 0.6715 a 0.0003 b | 0.3619 a 0.0897 b | 0.2971 a 0.1586 b | ||
| PD-1 | 0.1300 a 0.5545 b | −0.1641 a 0.4134 b | 0.3576 a 0.0793 b | 0.3207 a 0.1265 b | 0.5781 a 0.0031 b | 0.2872 a 0.1736 b | |
| PD-L1 | 0.0569 a 0.8064 b | −0.3234 a 0.1232 b | 0.1939 a 0.3753 b | 0.0816 a 0.7113 b | 0.2927 a 0.1652 b | 0.3788 a 0.0747 b | −0.0932 a 0.6647 b |
| Overall Survival | Disease-Free Survival | |||
|---|---|---|---|---|
| HR 95% CI | p | HR 95% CI | p | |
| Age ≥ 50 y | 1.44 [0.72–2.88] | 0.27 | 0.67 [0.33–1.35] | 0.23 |
| Sex, female vs. male | 1.37 [0.67–2.82] | 0.35 | 1.48 [0.70–3.12] | 0.25 |
| Histological subtype | ||||
| Chondro vs. osteo | 0.88 [0.38–2.02] | 0.74 | 0.82 [0.35–1.92] | 0.61 |
| Chondro vs. other | 0.89 [0.45–1.75] | 0.72 | 0.61 [0.28–1.34] | 0.23 |
| Histopathologic grade | ||||
| High vs. intermediate | 0.72 [0.32–1.61] | 0.42 | 1.44 [0.66–3.15] | |
| PR vs. GR | 2.78 [1.13–6.83] | 0.02 | 3.02 [1.19–7.61] | 0.01 |
| Progression of the disease | 1.45 [0.62–3.37] | 0.31 | 1.66 [0.66–4.21] | 0.19 |
| RANK ≥ 10% | 1.32 [0.49–3.56] | 0.60 | 2.88 [1.24–6.68] | 0.03 |
| RANKL ≥ 10% | 2.11 [0.92–4.85] | 0.08 | 2.88 [1.24–6.68] | 0.03 |
| CD146 ≥ 50% | 1.27 [0.62–2.60] | 0.46 | 1.47 [0.71–3.24] | 0.28 |
| CD163 ≥ 50% | 3.62 [1.06–12.36] | 0.0006 | 3.24 [0.91–27.55] | 0.003 |
| CD68 ≥ 50% | 3.03 [0.44–20.84] | 0.04 | 3.21 [0.44–23.28] | 0.04 |
| CD4+ ≥ 10% | 4.09 [0.78–21.60] | 0.002 | 4.25 [0.77–23.50] | 0.001 |
| CD8+ ≥ 1% | 1.84 [0.55–6.19] | 0.21 | 1.55 [0.44–5.52] | 0.66 |
| PD-1 ≥ 1% | 3.02 [0.44–20.72] | 0.05 | 2.65 [0.43–16.27] | 0.09 |
| PD-L1 ≥ 1% | 3.71 [0.09–15.3] | 0.16 | 3.71 [0.09–15.3] | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, H.; Peries, S.; Amiaud, J.; Van Acker, N.; Perrot, B.; Bouvier, C.; Aubert, S.; Marie, B.; Larousserie, F.; De Pinieux, G.; et al. Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, towards Prognostic Markers and New Therapeutic Targets. Cancers 2023, 15, 1004. https://doi.org/10.3390/cancers15041004
Bertin H, Peries S, Amiaud J, Van Acker N, Perrot B, Bouvier C, Aubert S, Marie B, Larousserie F, De Pinieux G, et al. Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, towards Prognostic Markers and New Therapeutic Targets. Cancers. 2023; 15(4):1004. https://doi.org/10.3390/cancers15041004
Chicago/Turabian StyleBertin, Hélios, Sophie Peries, Jérôme Amiaud, Nathalie Van Acker, Bastien Perrot, Corinne Bouvier, Sébastien Aubert, Béatrice Marie, Frédérique Larousserie, Gonzague De Pinieux, and et al. 2023. "Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, towards Prognostic Markers and New Therapeutic Targets" Cancers 15, no. 4: 1004. https://doi.org/10.3390/cancers15041004
APA StyleBertin, H., Peries, S., Amiaud, J., Van Acker, N., Perrot, B., Bouvier, C., Aubert, S., Marie, B., Larousserie, F., De Pinieux, G., Crenn, V., Rédini, F., & Gomez-Brouchet, A. (2023). Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, towards Prognostic Markers and New Therapeutic Targets. Cancers, 15(4), 1004. https://doi.org/10.3390/cancers15041004