Re-Irradiation by Stereotactic Radiotherapy of Brain Metastases in the Case of Local Recurrence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Selection
2.2. Baseline Evaluation and Treatment
2.3. Outcome Evaluation
2.4. Statistical Methods
3. Results
3.1. Patient and Tumor Characteristics
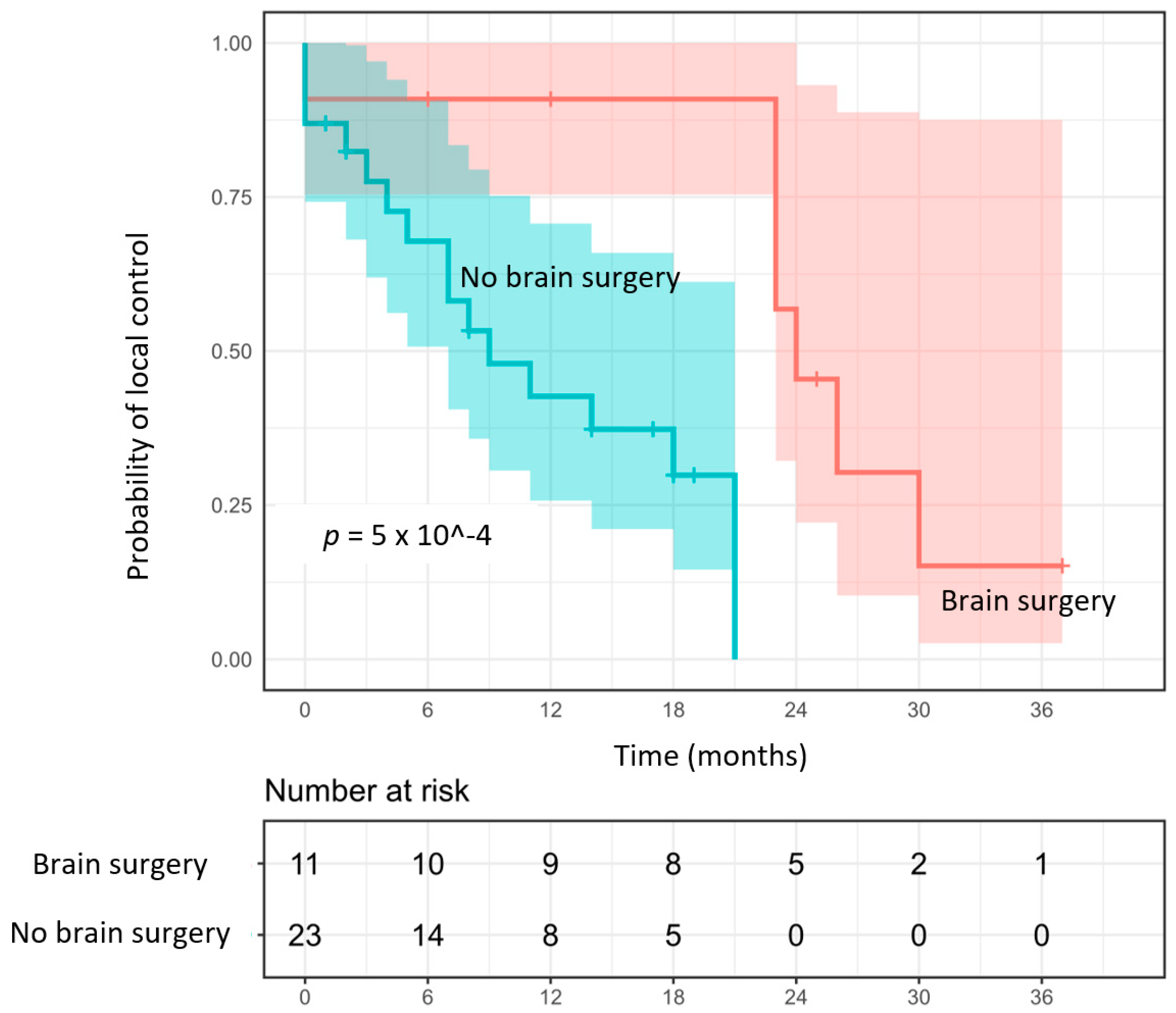
3.2. Local Control
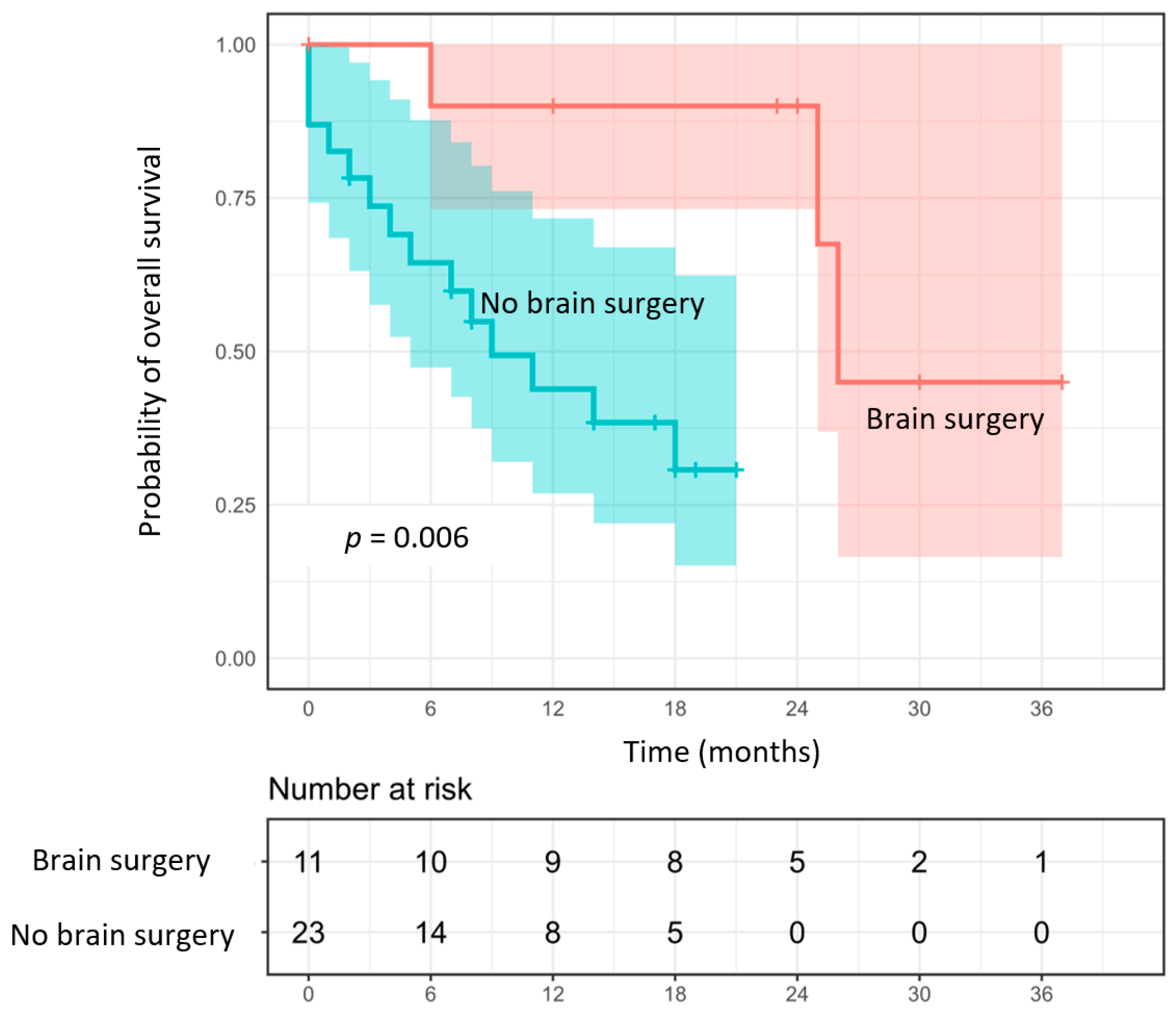
3.3. Overall Survival
3.4. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, M.P.; Tsao, M.N.; Whelan, T.J.; Morris, D.E.; Hayman, J.A.; Flickinger, J.C.; Mills, M.; Rogers, C.L.; Souhami, L. The American Society for Therapeutic Radiology and Oncology (ASTRO) Evidence-Based Review of the Role of Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 37–46. [Google Scholar] [CrossRef]
- Patrick, Y.; Wen, M.D.; Jay, S.; Loeffler, M.D. Management of Brain Metastases. Oncology 1999, 13, 957–961. [Google Scholar]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 Trials of Stereotactic Radiosurgery With or Without Whole-Brain Radiation Therapy for 1 to 4 Brain Metastases: Individual Patient Data Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 710–717. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs. Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Jagannathan, J.; Bourne, T.D.; Schlesinger, D.; Yen, C.-P.; Shaffrey, M.E.; Laws, E.R.; Sheehan, J.P. Clinical and Pathological Characteristics of Brain Metastasis Resected after Failed Radiosurgery. Neurosurgery 2010, 66, 208–217. [Google Scholar] [CrossRef]
- Ammirati, M.; Cobbs, C.S.; Linskey, M.E.; Paleologos, N.A.; Ryken, T.C.; Burri, S.H.; Asher, A.L.; Loeffler, J.S.; Robinson, P.D.; Andrews, D.W.; et al. The Role of Retreatment in the Management of Recurrent/Progressive Brain Metastases: A Systematic Review and Evidence-Based Clinical Practice Guideline. J. Neurooncol. 2010, 96, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.T.; St Clair, E.G.; Donahue, B.R.; Rush, S.C.; Miller, D.C.; Formenti, S.C.; Knopp, E.A.; Han, K.; Golfinos, J.G. Results of Surgical Resection for Progression of Brain Metastases Previously Treated by Gamma Knife Radiosurgery. Neurosurgery 2006, 59, 86–97. [Google Scholar] [CrossRef]
- Szeifert, G.T.; Atteberry, D.S.; Kondziolka, D.; Levivier, M.; Lunsford, L.D. Cerebral Metastases Pathology after Radiosurgery: A Multicenter Study. Cancer 2006, 106, 2672–2681. [Google Scholar] [CrossRef]
- Vecil, G.G.; Suki, D.; Maldaun, M.V.C.; Lang, F.F.; Sawaya, R. Resection of Brain Metastases Previously Treated with Stereotactic Radiosurgery. J. Neurosurg. 2005, 102, 209–215. [Google Scholar] [CrossRef]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of Brain Metastases According to Molecular Subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy versus Observation after Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy versus Observation in Patients with One to Three Brain Metastases from Solid Tumors after Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef]
- Kaba, S.E.; Kyritsis, A.P.; Hess, K.; Yung, W.K.; Mercier, R.; Dakhil, S.; Jaeckle, K.A.; Levin, V.A. TPDC-FuHu Chemotherapy for the Treatment of Recurrent Metastatic Brain Tumors. J. Clin. Oncol. 1997, 15, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Bafaloukos, D.; Linardou, H.; Aravantinos, G.; Bamias, A.; Carina, M.; Klouvas, G.; Skarlos, D. Hellenic Cooperative Oncology Group Temozolomide (TMZ) Combined with Cisplatin (CDDP) in Patients with Brain Metastases from Solid Tumors: A Hellenic Cooperative Oncology Group (HeCOG) Phase II Study. J. Neurooncol. 2005, 71, 61–65. [Google Scholar] [CrossRef]
- Yamanaka, K.; Iwai, Y.; Yasui, T.; Nakajima, H.; Komiyama, M.; Nishikawa, M.; Morikawa, T.; Kishi, H. Gamma Knife Radiosurgery for Metastatic Brain Tumor: The Usefulness of Repeated Gamma Knife Radiosurgery for Recurrent Cases. Ster. Funct. Neurosurg. 1999, 72 (Suppl. 1), 73–80. [Google Scholar] [CrossRef]
- Shultz, D.B.; Modlin, L.A.; Jayachandran, P.; Von Eyben, R.; Gibbs, I.C.; Choi, C.Y.H.; Chang, S.D.; Harsh, G.R.; Li, G.; Adler, J.R.; et al. Repeat Courses of Stereotactic Radiosurgery (SRS), Deferring Whole-Brain Irradiation, for New Brain Metastases After Initial SRS. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 993–999. [Google Scholar] [CrossRef]
- Lucia, F.; Touati, R.; Crainic, N.; Dissaux, G.; Pradier, O.; Bourbonne, V.; Schick, U. Efficacy and Safety of a Second Course of Stereotactic Radiation Therapy for Locally Recurrent Brain Metastases: A Systematic Review. Cancers 2021, 13, 4929. [Google Scholar] [CrossRef]
- Wilke, L.; Andratschke, N.; Blanck, O.; Brunner, T.B.; Combs, S.E.; Grosu, A.-L.; Moustakis, C.; Schmitt, D.; Baus, W.W.; Guckenberger, M. ICRU Report 91 on Prescribing, Recording, and Reporting of Stereotactic Treatments with Small Photon Beams: Statement from the DEGRO/DGMP Working Group Stereotactic Radiotherapy and Radiosurgery. Strahlenther. Onkol. 2019, 195, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor Control Probability of Radiosurgery and Fractionated Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 53–67. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Andratschke, N.; Willmann, J.; Appelt, A.L.; Alyamani, N.; Balermpas, P.; Baumert, B.G.; Hurkmans, C.; Høyer, M.; Langendijk, J.A.; Kaidar-Person, O.; et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus on Re-Irradiation: Definition, Reporting, and Clinical Decision Making. Lancet Oncol. 2022, 23, e469–e478. [Google Scholar] [CrossRef]
- Qian, J.M.; Yu, J.B.; Kluger, H.M.; Chiang, V.L.S. Timing and Type of Immune Checkpoint Therapy Affect the Early Radiographic Response of Melanoma Brain Metastases to Stereotactic Radiosurgery. Cancer 2016, 122, 3051–3058. [Google Scholar] [CrossRef]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic Radiosurgery for Melanoma Brain Metastases in Patients Receiving Ipilimumab: Safety Profile and Efficacy of Combined Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 368–375. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Zorro, O.; Lobato-Polo, J.; Flickinger, J.C.; Lunsford, L.D. The Results of Resection after Stereotactic Radiosurgery for Brain Metastases. J. Neurosurg. 2009, 111, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Rae, A.; Gorovets, D.; Rava, P.; Ebner, D.; Cielo, D.; Kinsella, T.J.; DiPetrillo, T.A.; Hepel, J.T. Management Approach for Recurrent Brain Metastases Following Upfront Radiosurgery May Affect Risk of Subsequent Radiation Necrosis. Adv. Radiat. Oncol. 2016, 1, 294–299. [Google Scholar] [CrossRef]
- Donelli, M.G.; Zucchetti, M.; D’Incalci, M. Do Anticancer Agents Reach the Tumor Target in the Human Brain? Cancer Chemother. Pharm. 1992, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus Whole-Brain Irradiation: A Randomised Controlled Trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Morin, C.; Liscak, R.; Vladyka, V.; Kano, H.; Jacobs, R.C.; Lunsford, L.D.; Cohen-Inbar, O.; Sheehan, J.; Emad, R.; Karim, K.A.; et al. Repeat Stereotactic Radiosurgery for Progressive or Recurrent Vestibular Schwannomas. Neurosurgery 2019, 85, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Tata, A.; Moosa, S.; Lee, C.-C.; Sheehan, J.P. Stereotactic Radiosurgery in the Treatment of Parasellar Meningiomas: Long-Term Volumetric Evaluation. J. Neurosurg. 2018, 128, 362–372. [Google Scholar] [CrossRef]
- Sankey, E.W.; Tsvankin, V.; Grabowski, M.M.; Nayar, G.; Batich, K.A.; Risman, A.; Champion, C.D.; Salama, A.K.S.; Goodwin, C.R.; Fecci, P.E. Operative and Peri-Operative Considerations in the Management of Brain Metastasis. Cancer Med. 2019, 8, 6809–6831. [Google Scholar] [CrossRef]
- Telera, S.; Fabi, A.; Pace, A.; Vidiri, A.; Anelli, V.; Carapella, C.M.; Marucci, L.; Crispo, F.; Sperduti, I.; Pompili, A. Radionecrosis Induced by Stereotactic Radiosurgery of Brain Metastases: Results of Surgery and Outcome of Disease. J. Neurooncol. 2013, 113, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated Volume as a Predictor of Brain Radionecrosis after Linear Accelerator Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic Radiosurgery for Brain Metastases: Analysis of Outcome and Risk of Brain Radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Minniti, G.; D’Angelillo, R.M.; Scaringi, C.; Trodella, L.E.; Clarke, E.; Matteucci, P.; Osti, M.F.; Ramella, S.; Enrici, R.M.; Trodella, L. Fractionated Stereotactic Rad.diosurgery for Patients with Brain Metastases. J. Neurooncol. 2014, 117, 295–301. [Google Scholar] [CrossRef]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral Radiation Necrosis: Incidence, Outcomes, and Risk Factors with Emphasis on Radiation Parameters and Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef]
| Characteristics | Group | |
|---|---|---|
| n = 32 | % | |
Gender
| 17 15 | 53 47 |
| Age Median (Range) | 63 (42–79) | |
Prior WBRT
| 5 27 | 16 84 |
Primary
| 6 2 18 15 1 1 13 1 2 5 1 1 1 | 19 6 56 47 3 3 41 3 6 16 3 3 3 |
Number of BMs
| 31 1 | 97 3 |
Extra Cranial Metastases
| 19 13 | 59 41 |
| DS-GPA Score Median (Range) | 2 (1–4) | |
| KPS Median (Range) | 90 (60–100) | |
RPA Class
| 14 16 2 | 44 50 6 |
Neurologic Symptoms
| 10 22 | 31 69 |
Resection Before SRT1
| 6/34 27/34 | 18 82 |
Resection Before SRT2
| 11/34 23/34 | 32 68 |
Other SRT
| 13 19 | 41 59 |
| Concomitant Systemic Treatment | ||
| Yes | 9/34 | 26 |
| Chemotherapy | 4 | 11 |
| Hormonotherapy | 1 | 3 |
| Immunotherapy | 2 | 6 |
| Anti-HER-2 | 2 | 6 |
| Anti-EGFR | 0 | 0 |
| Anti-ALK | 0 | 0 |
| No | 25/34 | 74 |
| Interval Between Treatments (Months) Median (Range) | 12 | 3–65 |
| Dosimetric Parameters | 34 Metastases (n = 34) Median (Range) % |
|---|---|
| GTV volume | 3.8 (0.14–67.51) |
| PTV volume median | 8.28 (0.68–97.79) |
| Dmin GTV | 26.09 Gy (21.08–36.14) |
| Dmax GTV | 32.88 (22.22–41.59) |
| Dmin PTV | 21.21 (15.03–36.02) |
| Dmax PTV | 32.88 (22.22–41.59) |
| Prescription isodose line | 81.11% (64.14–97.31) |
| Target coverage GTV | 100 (51.82–100) |
| Target coverage PTV | 98.93 (51.82–99.45) |
| Delivered dose GTV | 23.1 (15.55–39.80) |
| Delivered dose GTV BED | 40.89 (24.87–50.36) |
| Delivered dose PTV | 23.01 (15.55–37.73) |
| Delivered dose PTV BED | 40.71 (24.87–48.00) |
| V5 brain | 10.21 (2.35–82.92) |
| V10 brain | 4.22 (0.97–54.1) |
| V15 brain | 2.15 (0.39–31.70) |
| V20 brain | 1.23 (0.00–16.37) |
| V25 brain | 0.48 (0.00–8.32) |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Histology (lung vs. others) | 3.57 | 1.43–8.89 | 0.005 | 1.66 | 0.59–4.72 | 0.34 |
| Age | 1.04 | 0.99–1.09 | 0.09 | 1.03 | 0.98–1.09 | 0.23 |
| Gender (female vs. male) | 0.48 | 0.19–1.22 | 0.11 | - | - | - |
| DS-GPA | 0.88 | 0.55–1.42 | 0.61 | - | - | - |
| RPA | 1.67 | 0.75–3.74 | 0.21 | - | - | - |
| Number of BMs | 1.01 | 0.78–1.33 | 0.90 | - | - | - |
| ECM (yes vs. no) | 0.86 | 0.48–1.55 | 0.61 | - | - | - |
| KPS | 0.07 | 0.01–4.67 | 0.22 | - | - | - |
| Systemic treatment concomitant | 0.83 | 0.36–1.90 | 0.66 | - | - | - |
| Chemotherapy (yes vs. no) | 0.89 | 0.31–1.45 | 0.75 | - | - | - |
| Immunotherapy (yes vs. no) | 2.34 | 0.81–4.11 | 0.15 | - | - | - |
| Targeted Therapy (yes vs. no) | 1.14 | 0.72–1.61 | 0.41 | - | - | - |
| GTV volume SRT1 | 0.98 | 0.89–1.08 | 0.72 | - | - | - |
| PTV volume SRT1 | 0.98 | 0.92–1.04 | 0.48 | - | - | - |
| GTV volume SRT2 | 1.00 | 0.98–1.02 | 0.94 | - | - | - |
| PTV volume SRT2 | 1.00 | 0.99–1.01 | 0.91 | - | - | - |
| Interval treatment time | 1.03 | 0.98–1.08 | 0.32 | - | - | - |
| Brain surgery SRT2 (yes vs. no) | 19.47 | 2.36–160.37 | <0.0001 | 12.76 | 1.48–110.01 | 0.002 |
| Brain surgery SRT1 (yes vs. no) | 1.02 | 0.23–4.52 | 0.98 | - | - | - |
| Delivered dose GTV SRT1 | 0.47 | 0.17–1.32 | 0.23 | - | - | - |
| Delivered dose GTV SRT1 BED | 0.74 | 0.49–1.12 | 0.23 | - | - | - |
| Delivered dose PTV SRT1 | 0.86 | 0.49–1.51 | 0.61 | - | - | - |
| Delivered dose PTV SRT1 BED | 0.90 | 0.67–1.21 | 0.50 | - | - | - |
| Delivered dose GTV SRT2 | 1.00 | 0.93–1.07 | 0.94 | - | - | - |
| Delivered dose GTV SRT2 BED | 1.01 | 0.94–1.09 | 0.69 | - | - | - |
| Delivered dose PTV SRT2 | 1.01 | 0.93–1.09 | 0.88 | - | - | - |
| Delivered dose PTV SRT2 BED | 1.03 | 0.95–1.11 | 0.45 | - | - | - |
| Delivered dose GTV total | 0.96 | 0.87–1.06 | 0.42 | - | - | - |
| Delivered dose GTV total BED | 0.99 | 0.89–1.09 | 0.82 | - | - | - |
| Delivered dose PTV total | 0.97 | 0.89–1.07 | 0.59 | - | - | - |
| Delivered dose PTV total BED | 1.01 | 0.92–1.11 | 0.83 | - | - | - |
| Dmax SRT1 | 0.99 | 0.88–1.10 | 0.82 | - | - | - |
| Dmax SRT2 | 1.01 | 0.91–1.11 | 0.88 | - | - | - |
| Dmax total | 0.99 | 0.94–1.05 | 0.83 | - | - | - |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Histology (lung vs. others) | 1.45 | 0.20–10.58 | 0.71 | - | - | - |
| Age | 0.94 | 0.85–1.04 | 0.22 | - | - | - |
| Gender (female vs. male) | 1.09 | 0.15–7.76 | 0.93 | - | - | - |
| DS-GPA | 1.50 | 0.47–4.82 | 0.48 | - | - | - |
| RPA | 0.25 | 0.03–2.23 | 0.16 | - | - | - |
| Number of BMs | 1.37 | 0.78–2.40 | 0.30 | - | - | - |
| ECM (yes vs. no) | 1.66 | 0.33–8.21 | 0.51 | - | - | - |
| KPS | 4.09 | 0.004–37.52 | 0.76 | - | - | - |
| Concomitant systemic treatment | 1.36 × 109 | 0–inf | 0.028 | 2.22 × 109 | 0–inf | 0.04 |
| Chemotherapy (yes vs. no) | 2.89 | 0.67–8.32 | 0.09 | - | - | - |
| Immunotherapy (yes vs. no) | 2.14 | 0.43–7.54 | 0.21 | - | - | - |
| Targeted therapy (yes vs. no) | 1.12 | 0.37–7.71 | 0.53 | - | - | - |
| Prior WBRT | 2.00 | 0.16–24.33 | 0.59 | - | - | - |
| GTV volume SRT1 | 0.88 | 0.54–1.43 | 0.53 | - | - | - |
| PTV volume SRT1 | 0.94 | 0.73–1.21 | 0.56 | - | - | - |
| GTV volume SRT2 | 0.89 | 0.65–1.22 | 0.27 | - | - | - |
| PTV volume SRT2 | 0.93 | 0.78–1.12 | 0.27 | - | - | - |
| Interval treatment time | 1.00 | 0.88–1.13 | 0.98 | - | - | - |
| Brain surgery SRT2 (no vs. yes) | 1.19 | 0.17–8.50 | 0.86 | - | - | - |
| Delivered dose GTV SRT1 | 3381.54 | 0–inf | 0.78 | - | - | - |
| Delivered dose GTV SRT1 BED | 26.79 | 0–inf | 0.78 | - | - | - |
| Delivered dose PTV SRT1 | 7.12 × 1015 | 3.56 × 10−5–1.42 × 1036 | 0.0002 | - | - | - |
| Delivered dose PTV SRT1 BED | 8.98 × 108 | 0.003–2.61 × 1020 | 0.0002 | 28,249.20 | 0.008–1.01 × 1011 | 0.003 |
| Delivered dose GTV SRT2 | 1.10 | 0.93–1.31 | 0.30 | - | - | - |
| Delivered dose GTV SRT2 BED | 1.30 | 0.95–1.79 | 0.06 | - | - | - |
| Delivered dose PTV SRT2 | 1.13 | 0.94–1.36 | 0.25 | - | - | - |
| Delivered dose PTV SRT2 BED | 1.36 | 0.93–2.00 | 0.04 | 1.32 | 0.49–3.51 | 0.47 |
| Delivered dose GTV total | 1.12 | 0.90–1.39 | 0.37 | - | - | - |
| Delivered dose GTV total BED | 1.37 | 0.88–2.13 | 0.11 | - | - | - |
| Delivered dose PTV total | 1.14 | 0.90–1.44 | 0.31 | - | - | - |
| Delivered dose PTV total BED | 1.44 | 0.82–2.55 | 0.09 | - | - | - |
| Dmax SRT1 | 1.77 | 0.22–14.30 | 0.29 | - | - | - |
| Dmax SRT2 | 0.90 | 0.71–1.14 | 0.41 | - | - | - |
| Dmax total | 1.10 | 0.83–1.45 | 0.50 | - | - | - |
| V5 brain SRT1 | 2.84 | 0.53–6.35 | 0.35 | - | - | - |
| V5 brain SRT2 | 3.81 | 3.58–11.14 | 0.03 | 1.74 | 1.01–4.23 | 0.04 |
| V10 brain SRT1 | 2.63 | 0.18–5.25 | 0.38 | - | - | - |
| V10 brain SRT2 | 2.79 | 0.46–9.36 | 0.24 | - | - | - |
| V15 brain SRT1 | 2.52 | 0.04–8.20 | 0.56 | - | - | - |
| V15 brain SRT2 | 2.68 | 0.27–9.75 | 0.28 | - | - | - |
| V20 brain SRT1 | 2.49 | 0.006–38.91 | 0.73 | - | - | - |
| V20 brain SRT2 | 2.61 | 0.16–8.30 | 0.34 | - | - | - |
| V25 brain SRT1 | 3.84 | 0.003–5463.85 | 0.73 | - | - | - |
| V25 brain SRT2 | 2.60 | 0.10–6.54 | 0.46 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touati, R.; Bourbonne, V.; Dissaux, G.; Goasduff, G.; Pradier, O.; Peltier, C.; Seizeur, R.; Schick, U.; Lucia, F. Re-Irradiation by Stereotactic Radiotherapy of Brain Metastases in the Case of Local Recurrence. Cancers 2023, 15, 996. https://doi.org/10.3390/cancers15030996
Touati R, Bourbonne V, Dissaux G, Goasduff G, Pradier O, Peltier C, Seizeur R, Schick U, Lucia F. Re-Irradiation by Stereotactic Radiotherapy of Brain Metastases in the Case of Local Recurrence. Cancers. 2023; 15(3):996. https://doi.org/10.3390/cancers15030996
Chicago/Turabian StyleTouati, Ruben, Vincent Bourbonne, Gurvan Dissaux, Gaëlle Goasduff, Olivier Pradier, Charles Peltier, Romuald Seizeur, Ulrike Schick, and François Lucia. 2023. "Re-Irradiation by Stereotactic Radiotherapy of Brain Metastases in the Case of Local Recurrence" Cancers 15, no. 3: 996. https://doi.org/10.3390/cancers15030996
APA StyleTouati, R., Bourbonne, V., Dissaux, G., Goasduff, G., Pradier, O., Peltier, C., Seizeur, R., Schick, U., & Lucia, F. (2023). Re-Irradiation by Stereotactic Radiotherapy of Brain Metastases in the Case of Local Recurrence. Cancers, 15(3), 996. https://doi.org/10.3390/cancers15030996





