Three Months’ PSA and Toxicity from a Prospective Trial Investigating STereotactic sAlvage Radiotherapy for Macroscopic Prostate Bed Recurrence after Prostatectomy—STARR (NCT05455736)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
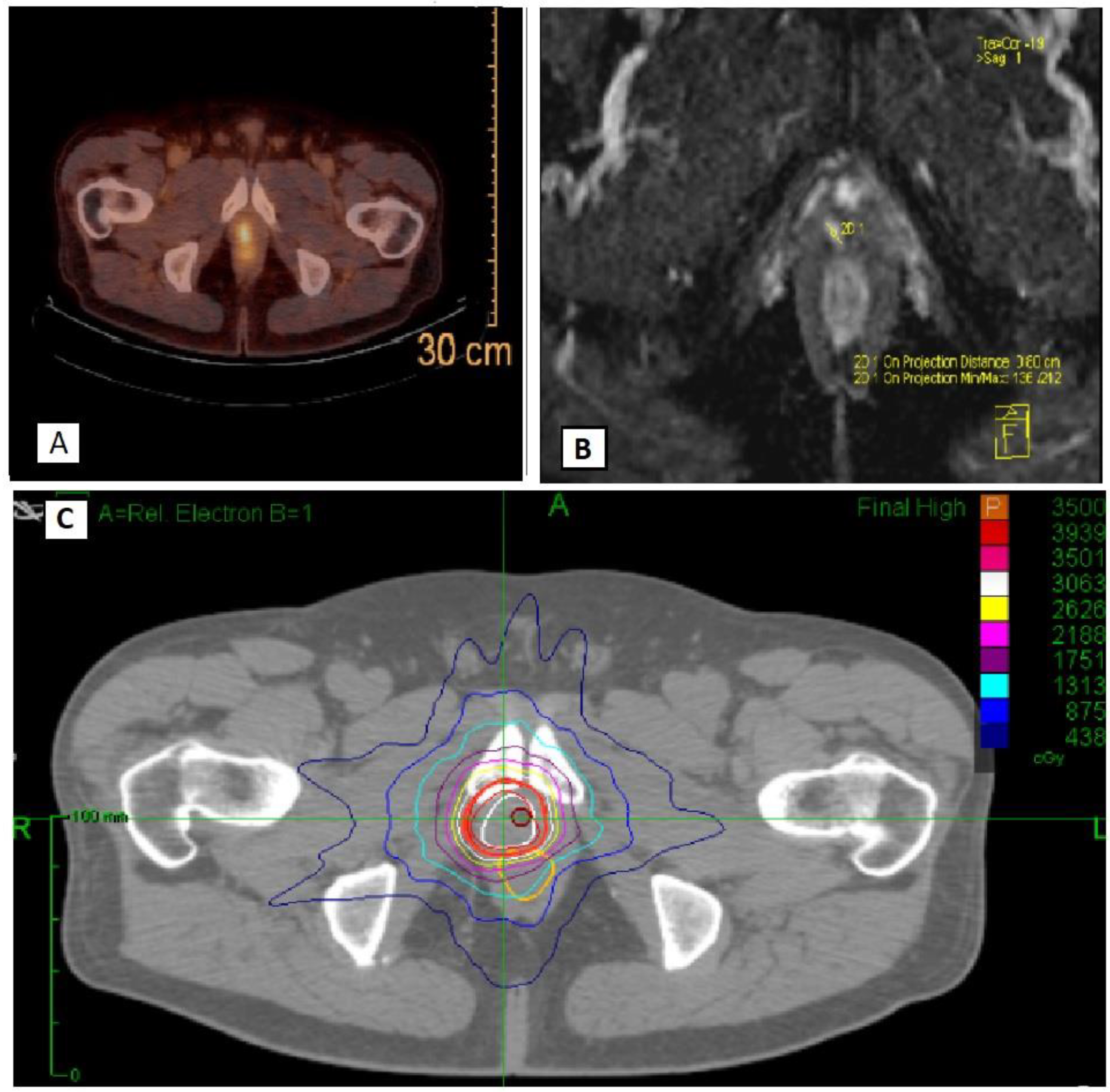
2.2. Study Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Outcomes
4.2. ADT Administration
4.3. Current Status of PET-Directed sRT
4.4. Dose-Escalated Radiotherapy in Salvage Setting and Comparison with Other SSRT Series
4.5. Potential Advantages of SSRT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stensland, K.D.; Caram, M.V.; Burns, J.A.; Sparks, J.B.; Shin, C.; Zaslavsky, A.; Hollenbeck, B.K.; Tsodikov, A.; Skolarus, T.A. Recurrence, metastasis, and survival after radical prostatectomy in the era of advanced treatments. J. Clin. Oncol. 2022, 40, 5090. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; van Leeuwen, P.J.; Nandurkar, R.; Scheltema, M.J.; Cusick, T.; Hruby, G.; Kneebone, A.; Eade, T.; Fogarty, G.; Jagavkar, R. Treatment outcomesfrom 68Ga-PSMA PET/CT–informed salvage radiation treatment in menwith rising PSA after radical prostatectomy: Prognostic value of a negativePSMA PET. J. Nucl. Med. 2017, 58, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Shelan, M.; Odermatt, S.; Bojaxhiu, B.; Nguyen, D.P.; Thalmann, G.N.; Aebersold, D.M.; Pra, A.D. Disease Control with Delayed Salvage Radiotherapy for Macroscopic Local Recurrence Following Radical Prostatectomy. Front. Oncol. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Tamihardja, J.; Zehner, L.; Hartrampf, P.E.; Cirsi, S.; Wegener, S.; Buck, A.K.; Flentje, M.; Polat, B. Dose-Escalated Salvage Radiotherapy for Macroscopic Local Recurrence of Prostate Cancer in the Prostate-Specific Membrane Antigen Positron Emission Tomography Era. Cancers 2022, 14, 4956. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Schröder, C.; Tang, H.; Windisch, P.; Zwahlen, D.R.; Buchali, A.; Vu, E.; Bostel, T.; Sprave, T.; Zilli, T.; Murthy, V.; et al. Stereotactic Radiotherapy after Radical Prostatectomy in Patients with Prostate Cancer in the Adjuvant or Salvage Setting: A Systematic Review. Cancers 2022, 14, 696. [Google Scholar] [CrossRef]
- Francolini, G.; Jereczek-Fossa, B.A.; Di Cataldo, V.; Simontacchi, G.; Marvaso, G.; Gandini, S.; Corso, F.; Ciccone, L.P.; Zerella, M.A.; Gentile, P.; et al. Stereotactic or conventional radiotherapy for macroscopic prostate bed recurrence: A propensity score analysis. La Radiol. Medica 2022, 127, 449–457. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services NIH, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-0614_QuickReference_5x7.pdf (accessed on 21 December 2022).
- Francolini, G.; Jereczek-Fossa, B.A.; Di Cataldo, V.; Simontacchi, G.; Marvaso, G.; Zerella, M.A.; Gentile, P.; Bianciardi, F.; Allegretta, S.; Detti, B.; et al. Stereotactic radiotherapy for prostate bed recurrence after prostatectomy, a multicentric series. BJU Int. 2020, 125, 417–425. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.-L.; Supiot, S.; Belkacemi, Y.; Peiffert, D.; Allouache, N.; et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.-P.; Zietman, A.L.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N. Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef]
- Tree, A.C.; Ostler, P.; van der Voet, H.; Chu, W.; Loblaw, A.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; Staffurth, J.; et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022, 23, 1308–1320. [Google Scholar] [CrossRef]
- Spratt, D.E. Evidence-based Risk Stratification to Guide Hormone Therapy Use with Salvage Radiation Therapy for Prostate Cancer. Int. J. Radiat. Oncol. 2018, 102, 556–560. [Google Scholar] [CrossRef]
- Jani, A.B.; Schreibmann, E.; Goyal, S.; Halkar, R.; Hershatter, B.; Rossi, P.J.; Shelton, J.W.; Patel, P.R.; Xu, K.M.; Goodman, M.; et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomised controlled trial. Lancet 2021, 397, 1895–1904. [Google Scholar] [CrossRef]
- Bruni, A.; Ingrosso, G.; Trippa, F.; Di Staso, M.; Lanfranchi, B.; Rubino, L.; Parente, S.; Frassinelli, L.; Maranzano, E.; Santoni, R.; et al. Macroscopic locoregional relapse from prostate cancer: Which role for salvage radiotherapy? Clin. Transl. Oncol. 2019, 21, 1532–1537. [Google Scholar] [CrossRef]
- Zaine, H.; Vandendorpe, B.; Bataille, B.; Lacornerie, T.; Wallet, J.; Mirabel, X.; Lartigau, E.; Pasquier, D. Salvage Radiotherapy for Macroscopic Local Recurrence Following Radical Prostatectomy. Front. Oncol. 2021, 11, 669261. [Google Scholar] [CrossRef]
- Zilli, T.; Jorcano, S.; Peguret, N.; Caparrotti, F.; Hidalgo, A.; Khan, H.G.; Vees, H.; Miralbell, R. Results of Dose-adapted Salvage Radiotherapy After Radical Prostatectomy Based on an Endorectal MRI Target Definition Model. Am. J. Clin. Oncol. 2017, 40, 194–199. [Google Scholar] [CrossRef]
- Lee, S.U.; Cho, K.H.; Kim, J.H.; Kim, Y.S.; Nam, T.-K.; Kim, J.-S.; Cho, J.; Choi, S.H.; Shim, S.J.; Chang, A.R. Clinical Outcome of Salvage Radiotherapy for Locoregional Clinical Recurrence After Radical Prostatectomy. Technol. Cancer Res. Treat. 2021, 20, 15330338211041212. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.S.; Stief, C.; Kim, T.H.; Eze, C.; Kirste, S.; Strouthos, I.; Li, M.; Schultze-Seemann, W.; Ilhan, H.; Fendler, W.P.; et al. Outcome after PSMA PET/CT based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: A bi-institutional retrospective analysis. J. Nucl. Med. 2019, 60, 227–233. [Google Scholar] [CrossRef]
- Ballas, L.K.; Luo, C.; Chung, E.; Kishan, A.U.; Shuryak, I.; Quinn, D.I.; Dorff, T.; Jhimlee, S.; Chiu, R.; Abreu, A.; et al. Phase 1 Trial of SBRT to the Prostate Fossa After Prostatectomy. Int. J. Radiat. Oncol. 2018, 104, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Frankel, P.; del Vecchio, B.; Ruel, N.; Yuh, B.; Liu, A.; Tsai, T.; Wong, J. Stereotactic Body Radiation Therapy to the Prostate Bed: Results of a Phase 1 Dose-Escalation Trial. Int. J. Radiat. Oncol. 2019, 106, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Benziane-Ouaritini, N.; Zilli, T.; Ingrosso, G.; di Staso, M.; Trippa, F.; Francolini, G.; Meyer, E.; Achard, V.; Schick, U.; Cosset, J.; et al. Salvage Radiotherapy Guided by Functional Imaging for Macroscopic Local Recurrence Following Radical Prostatectomy: A Multicentric Retrospective Study. Int. J. Radiat. Oncol. 2022, 114, S131–S132. [Google Scholar] [CrossRef]
- Buyyounouski, M.; Pugh, S.; Chen, R.; Mann, M.; Kudchadker, R.; Konski, A.; Mian, O.; Michalski, J.; Vigneault, E.; Valicenti, R.; et al. Primary Endpoint Analysis of a Randomized Phase III Trial of Hypofractionated vs. Conventional Post-Prostatectomy Radiotherapy: NRG Oncology GU003. Int. J. Radiat. Oncol. 2021, 111, S2–S3. [Google Scholar] [CrossRef]
- Francolini, G.; Stocchi, G.; Detti, B.; Di Cataldo, V.; Bruni, A.; Triggiani, L.; Guerini, A.E.; Mazzola, R.; Cuccia, F.; Mariotti, M.; et al. Dose-escalated pelvic radiotherapy for prostate cancer in definitive or postoperative setting. La Radiol. Medica 2021, 127, 206–213. [Google Scholar] [CrossRef]
- Nicosia, L.; Mazzola, R.; Vitale, C.; Cuccia, F.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Ruggeri, R.; Cavalleri, S.; et al. Postoperative moderately hypofractionated radiotherapy in prostate cancer: A mono-institutional propensity-score-matching analysis between adjuvant and early-salvage radiotherapy. La Radiol. Medica 2022, 127, 560–570. [Google Scholar] [CrossRef]
- Francolini, G.; Detti, B.; Di Cataldo, V.; Garlatti, P.; Aquilano, M.; Allegra, A.; Lucidi, S.; Cerbai, C.; Ciccone, L.P.; Salvestrini, V.; et al. Study protocol and preliminary results from a mono-centric cohort within a trial testing stereotactic body radiotherapy and abiraterone (ARTO-NCT03449719). La Radiol. Medica 2022, 127, 912–918. [Google Scholar] [CrossRef]
- D’Angelillo, R.M.; Fiore, M.; Trodella, L.E.; Sciuto, R.; Ippolito, E.; Carnevale, A.; Iurato, A.; Miele, M.; Trecca, P.; Trodella, L.; et al. 18F-choline PET/CT driven salvage radiotherapy in prostate cancer patients: Up-date analysis with 5-year median follow-up. La Radiol. Medica 2020, 125, 668–673. [Google Scholar] [CrossRef]
- Barra, S.; Guarnieri, A.; di Monale EBastia, M.B.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2021, 126, 142–146. [Google Scholar] [CrossRef]
- Fersino, S.; Borghesi, S.; Jereczek-Fossa, B.A.; Arcangeli, S.; Mortellaro, G.; Magrini, S.M.; Alongi, F. Uro-Oncology study group of Italian association of Radiotherapy and Clinical Oncology (AIRO) PROACTA: A survey on the actual attitude of the Italian radiation oncologists in the management and prescription of hormonal therapy in prostate cancer patients. La Radiol. Medica 2020, 126, 460–465. [Google Scholar] [CrossRef]
- Meattini, I.; Palumbo, I.; Becherini, C.; Borghesi, S.; Cucciarelli, F.; Dicuonzo, S.; Fiorentino, A.; Spoto, R.; Poortmans, P.; Aristei, C.; et al. The Italian Association for Radiotherapy and Clinical Oncology (AIRO) position statements for postoperative breast cancer radiation therapy volume, dose, and fractionation. La Radiol. Medica 2022, 127, 1407–1411. [Google Scholar] [CrossRef]
- Gregucci, F.; Fozza, A.; Falivene, S.; Smaniotto, D.; Morra, A.; Daidone, A.; Barbara, R.; Ciabattoni, A. Present clinical practice of breast cancer radiotherapy in Italy: A nationwide survey by the Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group. La Radiol. Medica 2020, 125, 674–682. [Google Scholar] [CrossRef]
| Organ at Risk | Dose Constraint | Aim |
|---|---|---|
| Rectum | V18.1 Gy V29 Gy V36 Gy | <50% <20% <1 cc |
| Bladder | V18.1 Gy V37 Gy | <40% <10 cc |
| Urethra | V42 Gy | <50% (not mandatory) |
| Femoral heads | V14.5 Gy | <5% |
| Penile bulb | V29.5 Gy | <50% |
| Bowel | V18.1 Gy V30 Gy | <5 cc <1 cc |
| Age (Median Value, IQR) | 74 (IQR 69–80) |
|---|---|
| Baseline T stage (%) | T2b-c: 8 (42%) T3a-b: 11 (58%) |
| Baseline N stage | N0: 12 (63%) N1: 0 (0) Nx: 7 (37%) |
| Margin status | R0: 7(37%) R1: 12 (63%) |
| Baseline ISUP pattern | ≤3: 14 (74%) Gleason 3 + 3:1 Gleason 3 + 4: 8 Gleason 4 + 3: 5 >3: 5 (26%) Gleason 4 + 4:4 Gleason 4 + 5:1 |
| Baseline PSA (median, IQR) | 1.13 ng/mL (IQR 0.4–2.3) |
| Baseline NCCN risk category | Low: 0 (0) Intermediate: 6 (32%) High: 13 (68%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francolini, G.; Garlatti, P.; Di Cataldo, V.; Detti, B.; Loi, M.; Greto, D.; Simontacchi, G.; Morelli, I.; Burchini, L.; Allegra, A.G.; et al. Three Months’ PSA and Toxicity from a Prospective Trial Investigating STereotactic sAlvage Radiotherapy for Macroscopic Prostate Bed Recurrence after Prostatectomy—STARR (NCT05455736). Cancers 2023, 15, 992. https://doi.org/10.3390/cancers15030992
Francolini G, Garlatti P, Di Cataldo V, Detti B, Loi M, Greto D, Simontacchi G, Morelli I, Burchini L, Allegra AG, et al. Three Months’ PSA and Toxicity from a Prospective Trial Investigating STereotactic sAlvage Radiotherapy for Macroscopic Prostate Bed Recurrence after Prostatectomy—STARR (NCT05455736). Cancers. 2023; 15(3):992. https://doi.org/10.3390/cancers15030992
Chicago/Turabian StyleFrancolini, Giulio, Pietro Garlatti, Vanessa Di Cataldo, Beatrice Detti, Mauro Loi, Daniela Greto, Gabriele Simontacchi, Ilaria Morelli, Luca Burchini, Andrea Gaetano Allegra, and et al. 2023. "Three Months’ PSA and Toxicity from a Prospective Trial Investigating STereotactic sAlvage Radiotherapy for Macroscopic Prostate Bed Recurrence after Prostatectomy—STARR (NCT05455736)" Cancers 15, no. 3: 992. https://doi.org/10.3390/cancers15030992
APA StyleFrancolini, G., Garlatti, P., Di Cataldo, V., Detti, B., Loi, M., Greto, D., Simontacchi, G., Morelli, I., Burchini, L., Allegra, A. G., Frosini, G., Ganovelli, M., Salvestrini, V., Olmetto, E., Visani, L., Becherini, C., Valzano, M., Carnevale, M. G., Roghi, M., ... Livi, L. (2023). Three Months’ PSA and Toxicity from a Prospective Trial Investigating STereotactic sAlvage Radiotherapy for Macroscopic Prostate Bed Recurrence after Prostatectomy—STARR (NCT05455736). Cancers, 15(3), 992. https://doi.org/10.3390/cancers15030992





