Three-Dimensional Amide Proton Transfer-Weighted Imaging for Differentiating between Glioblastoma, IDH-Wildtype and Primary Central Nervous System Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Protocol and Image Analysis
2.3. Histopathology
2.4. DNA Isolation and Comparative Genomic Hybridization and PCR-Based Sequencing of the IDH1 or IDH2 Genes
2.5. Methylation-Specific PCR of the MGMT Gene
2.6. Statistical Analyses
3. Results
3.1. The Clinical Characteristics of PCNSL and Glioblastoma, IDH-Wildtype
3.2. APTw Imaging Was Useful to Distinguish PCNSL from Glioblastoma, IDH-Wildtype
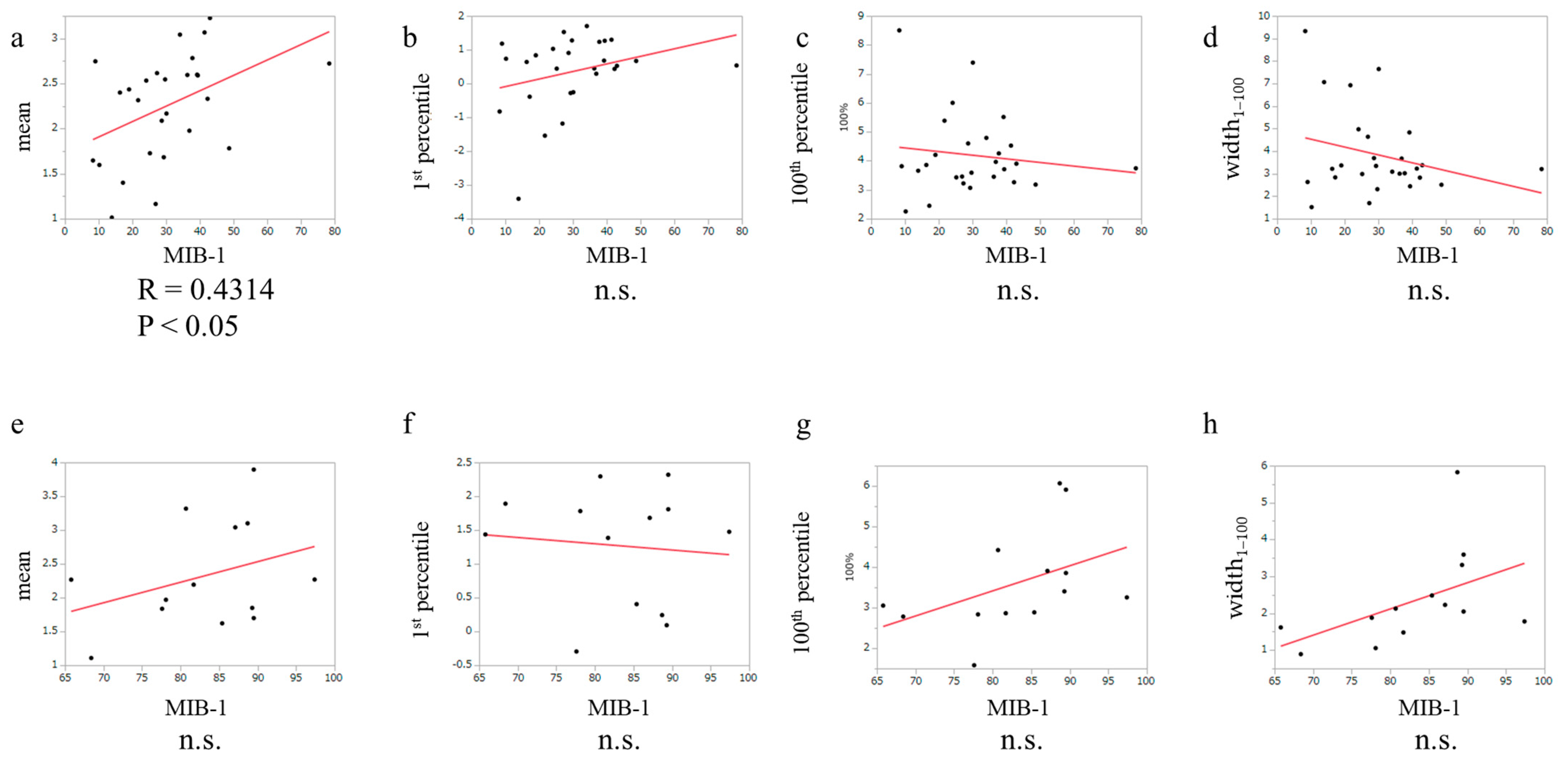
3.3. The Relationship between MIB-1 Index and APTw in Glioblastoma, IDH-Wildtype and PCNSL Cases
3.4. The Correlation between Molecular Markers and APTw Signals in PCNLS
3.5. The Correlation between Molecular Markers and APTw in Glioblastoma, IDH-Wildtype Cases
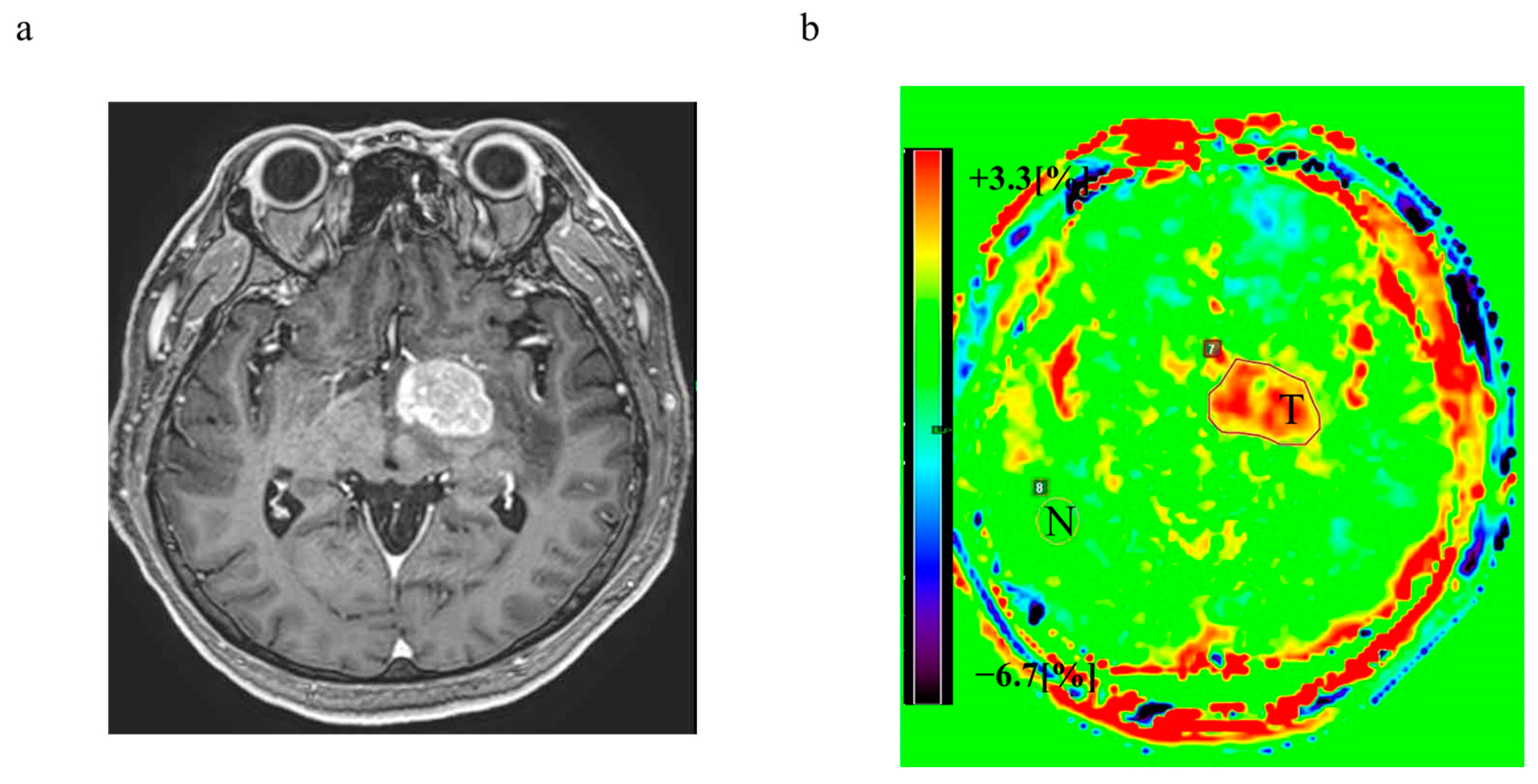
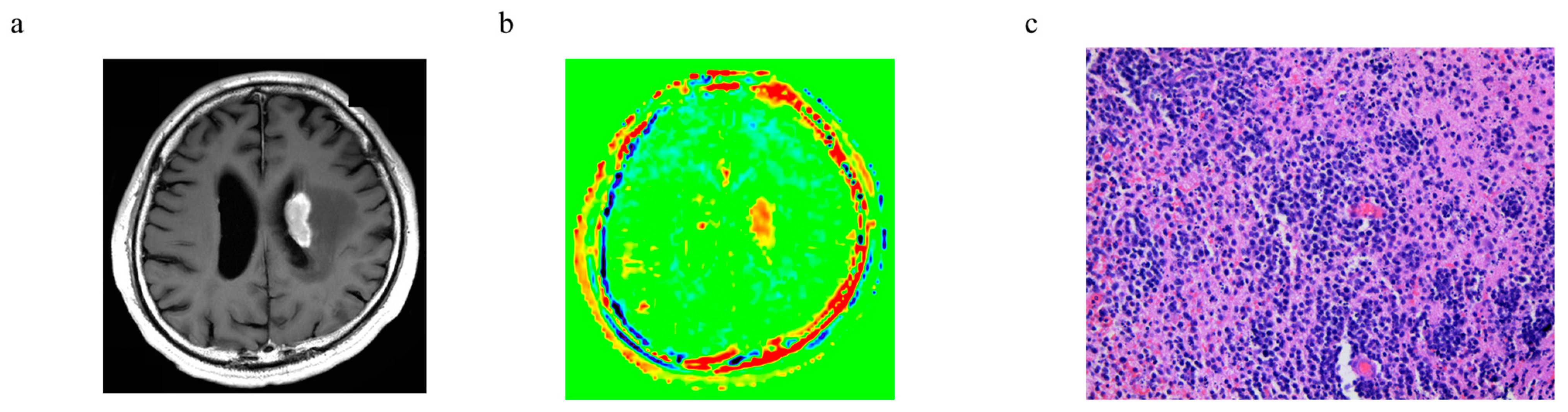
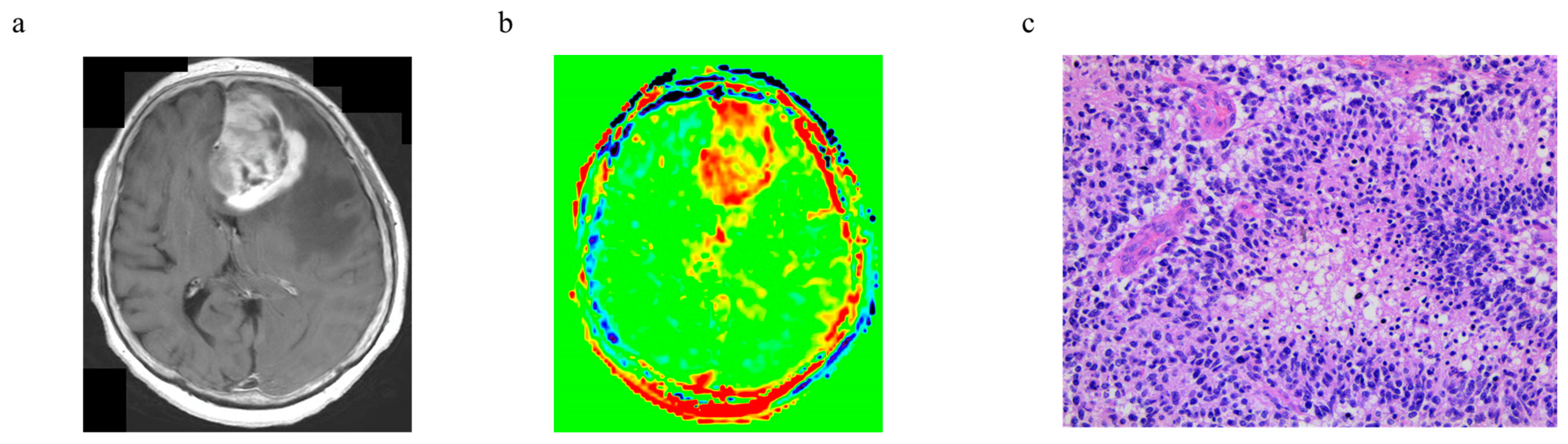
3.6. Representative Cases
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Ohba, S.; Hirose, Y. Biological Significance of Mutant Isocitrate Dehydrogenase 1 and 2 in Gliomagenesis. Neurol. Med. Chir. 2016, 56, 170–179. [Google Scholar] [CrossRef]
- Ohba, S.; Kuwahara, K.; Yamada, S.; Abe, M.; Hirose, Y. Correlation between IDH, ATRX, and TERT promoter mutations in glioma. Brain Tumor Pathol. 2020, 37, 33–40. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ohba, S.; Hirose, Y. Current and Future Drug Treatments for Glioblastomas. Curr. Med. Chem. 2016, 23, 4309–4316. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Tripathi, S.; Vivas-Buitrago, T.; Domingo, R.A.; Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sherman, W.; Gupta, V.; Middlebrooks, E.H.; et al. IDH-wild-type glioblastoma cell density and infiltration distribution influence on supramarginal resection and its impact on overall survival: A mathematical model. J. Neurosurg. 2021, 136, 1567–1575. [Google Scholar] [CrossRef]
- Vivas-Buitrago, T.; Domingo, R.A.; Tripathi, S.; De Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sabsevitz, D.S.; Bendok, B.R.; Sherman, W.; et al. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J. Neurosurg. 2021, 136, 1–8. [Google Scholar] [CrossRef]
- Citterio, G.; Reni, M.; Gatta, G.; Ferreri, A.J.M. Primary central nervous system lymphoma. Crit. Rev. Oncol. Hematol. 2017, 113, 97–110. [Google Scholar] [CrossRef]
- Ouyang, T.; Wang, L.; Zhang, N.; Zhang, Z.; Xiong, Y.; Li, M.; Hong, T. Clinical Characteristics, Surgical Outcomes, and Prognostic Factors of Intracranial Primary Central Nervous System Lymphoma. World Neurosurg. 2020, 139, e508–e516. [Google Scholar] [CrossRef]
- Asari, S.; Makabe, T.; Katayama, S.; Itoh, T.; Tsuchida, S.; Ohmoto, T. Assessment of the pathological grade of astrocytic gliomas using an MRI score. Neuroradiology 1994, 36, 308–310. [Google Scholar] [CrossRef]
- Guha, A.; Goda, J.S.; Dasgupta, A.; Mahajan, A.; Halder, S.; Gawde, J.; Talole, S. Classifying primary central nervous system lymphoma from glioblastoma using deep learning and radiomics based machine learning approach—A systematic review and meta-analysis. Front. Oncol. 2022, 12, 884173. [Google Scholar] [CrossRef]
- Lolli, V.; Tampieri, D.; Melançon, D.; Cortes, M.D. Imaging in primary central nervous system lymphoma. Neuroradiol. J. 2010, 23, 680–689. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, X.W.; Deng, L.J.; She, H.L. Differentiation between glioma recurrence and treatment effects using amide proton transfer imaging: A mini-Bayesian bivariate meta-analysis. Front. Oncol. 2022, 12, 852076. [Google Scholar] [CrossRef]
- Heo, H.Y.; Tee, Y.K.; Harston, G.; Leigh, R.; Chappell, M.A. Amide proton transfer imaging in stroke. NMR Biomed. 2022, Early View, e4734. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, J.; Wang, M.; Ren, Y.; Yang, Q.; Qian, L.; Luo, H.; Feng, S.; He, C.; Liu, X.; et al. Breast Amide Proton Transfer Imaging at 3 T: Diagnostic Performance and Association With Pathologic Characteristics. J. Magn. Reson. Imaging, 2022; in press. [Google Scholar] [CrossRef]
- Momosaka, D.; Togao, O.; Kikuchi, K.; Kikuchi, Y.; Wakisaka, Y.; Hiwatashi, A. Correlations of amide proton transfer-weighted MRI of cerebral infarction with clinico-radiological findings. PLoS ONE 2020, 15, e0237358. [Google Scholar] [CrossRef]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro Oncol. 2014, 16, 441–448. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, H.; Wang, X.; Lu, S.; Li, Y.; Feng, L.; Zhang, Y.; Heo, H.Y.; Lee, D.H.; Zhou, J.; et al. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur. Radiol. 2016, 26, 64–71. [Google Scholar] [CrossRef]
- Koike, H.; Morikawa, M.; Ishimaru, H.; Ideguchi, R.; Uetani, M.; Hiu, T.; Matsuo, T.; Miyoshi, M. Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results. Int. J. Mol. Sci. 2022, 23, 10187. [Google Scholar] [CrossRef]
- Lingl, J.P.; Wunderlich, A.; Goerke, S.; Paech, D.; Ladd, M.E.; Liebig, P.; Pala, A.; Kim, S.Y.; Braun, M.; Schmitz, B.L.; et al. The Value of APTw CEST MRI in Routine Clinical Assessment of Human Brain Tumor Patients at 3T. Diagnostics 2022, 12, 490. [Google Scholar] [CrossRef]
- Joo, B.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J.; Lee, S.K. Amide proton transfer imaging might predict survival and IDH mutation status in high-grade glioma. Eur. Radiol. 2019, 29, 6643–6652. [Google Scholar] [CrossRef]
- Yao, J.; Hagiwara, A.; Raymond, C.; Shabani, S.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; Liau, L.M.; et al. Human IDH mutant 1p/19q co-deleted gliomas have low tumor acidity as evidenced by molecular MRI and PET: A retrospective study. Sci. Rep. 2020, 10, 11922. [Google Scholar] [CrossRef]
- Hou, H.; Diao, Y.; Yu, J.; Xu, M.; Wang, L.; Li, Z.; Song, T.; Liu, Y.; Yuan, Z. Differentiation of true progression from treatment response in high-grade glioma treated with chemoradiation: A comparison study of 3D-APTW and 3D-PcASL imaging and DWI. NMR Biomed. 2022, 36, e4821. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Chen, Y.; Lv, X.; Lv, Y.; Zhou, J.; Xi, S.; Dou, W.; Qian, L.; Zheng, H.; et al. Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J. Magn. Reson. Imaging 2020, 51, 1154–1161. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Hattori, N.; Hirose, Y.; Sasaki, H.; Nakae, S.; Hayashi, S.; Ohba, S.; Adachi, K.; Hayashi, T.; Nishiyama, Y.; Hasegawa, M.; et al. World Health Organization grade IIIII astrocytomas consist of genetically distinct tumor lineages. Cancer Sci. 2016, 107, 1159–1164. [Google Scholar] [CrossRef]
- Ezaki, T.; Sasaki, H.; Hirose, Y.; Miwa, T.; Yoshida, K.; Kawase, T. Molecular characteristics of pediatric non-ependymal, non-pilocytic gliomas associated with resistance to temozolomide. Mol. Med. Rep. 2011, 4, 1101–1105. [Google Scholar]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reason. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Mancini, L.; Casagranda, S.; Gautier, G.; Peter, P.; Lopez, B.; Thorne, L.; McEvoy, A.; Miserocchi, A.; Samandouras, G.; Kitchen, N.; et al. CEST MRI provides amide/amine surrogate biomarkers for treatment-naïve glioma sub-typing. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2377–2391. [Google Scholar] [CrossRef]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro Oncol. 2018, 20, 1661–1671. [Google Scholar] [CrossRef]
- Cho, N.S.; Hagiwara, A.; Yao, J.; Nathanson, D.A.; Prins, R.M.; Wang, C.; Raymond, C.; Desousa, B.R.; Divakaruni, A.; Morrow, D.H.; et al. Amine-weighted chemical exchange saturation transfer magnetic resonance imaging in brain tumors. NMR Biomed. 2022, 15, e4785. [Google Scholar] [CrossRef]
- Sotirios, B.; Demetriou, E.; Topriceanu, C.C.; Zakrzewska, Z. The role of APT imaging in gliomas grading: A systematic review and meta-analysis. Eur. J. Radiol. 2020, 133, 109353. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, S.; Xie, H.; Li, W. An evidence-based approach to evaluate the accuracy of amide proton transfer-weighted MRI in characterization of gliomas. Medicine 2019, 98, e14768. [Google Scholar] [CrossRef]
- Murayama, K.; Nishiyama, Y.; Hirose, Y.; Abe, M.; Ohyu, S.; Ninomiya, A.; Fukuba, T.; Katada, K.; Toyama, H. Differentiating between Central Nervous System Lymphoma and High-grade Glioma Using Dynamic Susceptibility Contrast and Dynamic Contrast-enhanced MR Imaging with Histogram Analysis. Magn. Reson. Med. Sci. 2018, 17, 42–49. [Google Scholar] [CrossRef]
- Ohba, S.; Murayama, K.; Abe, M.; Hasegawa, M.; Hirose, Y. Magnetic Resonance Imaging and Proton Magnetic Resonance Spectroscopy for Differentiating Between Enhanced Gliomas and Malignant Lymphomas. World Neurosurg. 2019, 127, e779–e787. [Google Scholar] [CrossRef]
- Uchinomura, S.; Mitamura, K.; Norikane, T.; Yamamoto, Y.; Oishi, A.; Hatakeyama, T.; Miyake, K.; Nishiyama, Y. Distinguishing between primary central nervous system lymphoma and glioblastoma using [18F]fluoromisonidazole and [18F]FDG PET. Nucl. Med. Commun. 2022, 43, 270–274. [Google Scholar] [CrossRef]
- Murayama, K.; Ohno, Y.; Yui, M.; Yamamoto, K.; Meng, M.I.; Ohba, S.; Hanamatsu, S.; Iwase, A.; Ikeda, H.; Hirose, Y.; et al. 3D Gradient-Echo Based Amide Proton Transfer-Weighted (APTw) Imaging of Brain Tumors: Comparison with 2D Spin-Echo Based APTw Imaging. J. Comput. Assist. Tomogr, 2023; in press. [Google Scholar]
- Zhao, X.; Wen, Z.; Zhang, G.; Huang, F.; Lu, S.; Wang, X.; Hu, S.; Chen, M.; Zhou, J. Three-dimensional turbo-spin-echo amide proton transfer MR imaging at 3-Tesla and its application to high-grade human brain tumors. Mol. Imaging Biol. 2013, 15, 114–122. [Google Scholar] [CrossRef]
- Jiang, S.; Rui, Q.; Wang, Y.; Heo, H.Y.; Zou, T.; Yu, H.; Zhang, Y.; Wang, X.; Du, Y.; Wen, X.; et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur. Radiol. 2018, 28, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
| PCNCL | Glioblastoma, IDH-Wildtype | p-Value | ||
|---|---|---|---|---|
| Number | 14 | 27 | ||
| Age (mean ± standard deviation) | 66.3 ± 11.1 | 65.0 ± 17.9 | n.s. | |
| Gender | M | 8 | 21 | n.s. |
| F | 6 | 6 | ||
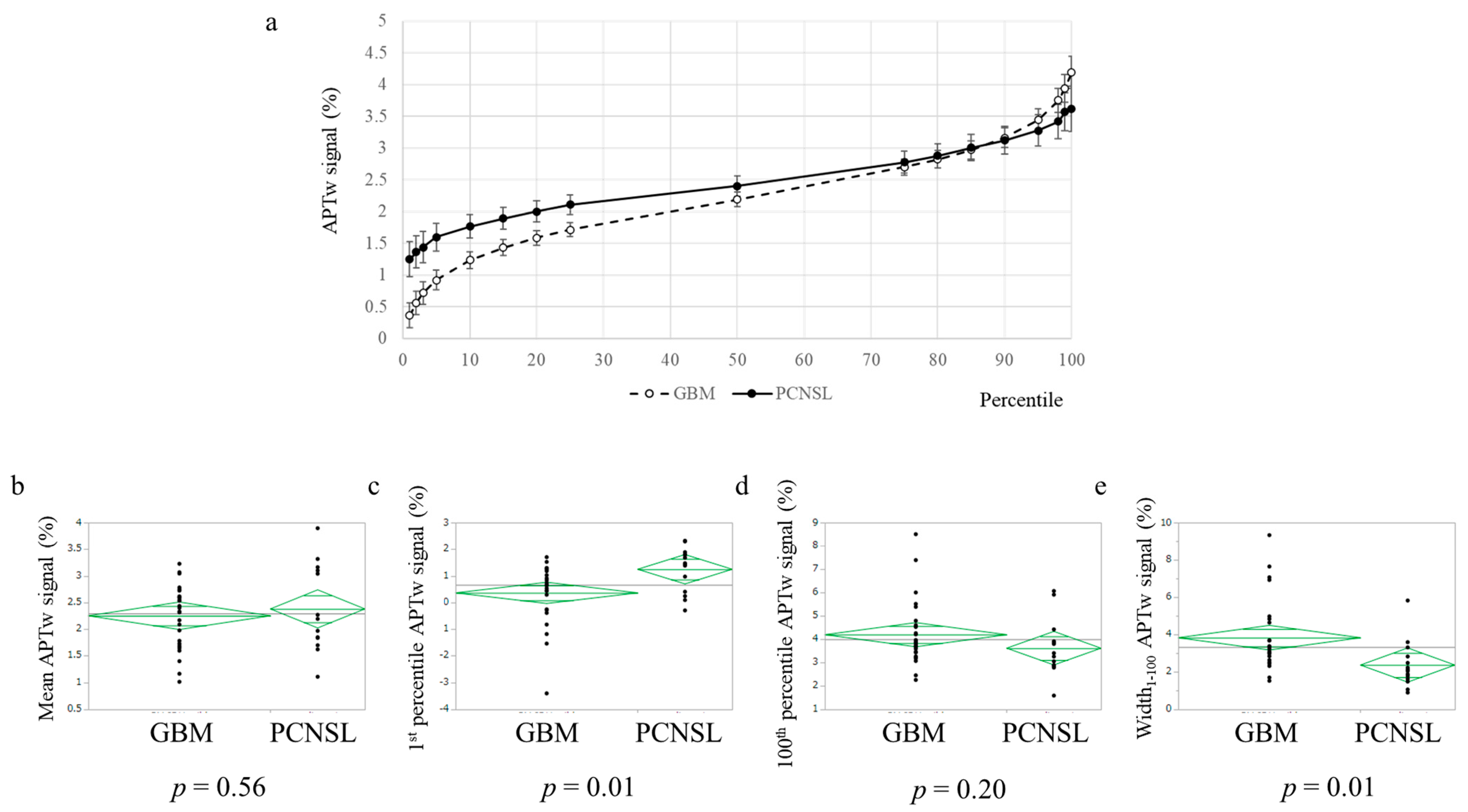
| Mean APTw signal (mean ± standard deviation) | 2.38 ± 0.79 | 2.25 ± 0.58 | n.s. | |
| Percentile | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 10 | 15 | 20 | 25 | 50 | 75 | 80 | 85 | 90 | 95 | 98 | 99 | 100 | Width1–100 | Mean | ||
| APTw signal (%) | PCNSL | 1.25 | 1.37 | 1.44 | 1.60 | 1.77 | 1.89 | 2.00 | 2.11 | 2.40 | 2.78 | 2.88 | 3.01 | 3.12 | 3.28 | 3.42 | 3.57 | 3.62 | 2.37 | 2.38 |
| GBM | 0.36 | 0.56 | 0.72 | 0.92 | 1.24 | 1.43 | 1.59 | 1.72 | 2.19 | 2.70 | 2.83 | 2.97 | 3.16 | 3.45 | 3.75 | 3.94 | 4.19 | 3.83 | 2.25 | |
| p-value | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.05 | 0.05 | 0.29 | 0.73 | 0.81 | 0.88 | 0.88 | 0.58 | 0.33 | 0.32 | 0.20 | 0.01 | 0.56 | |
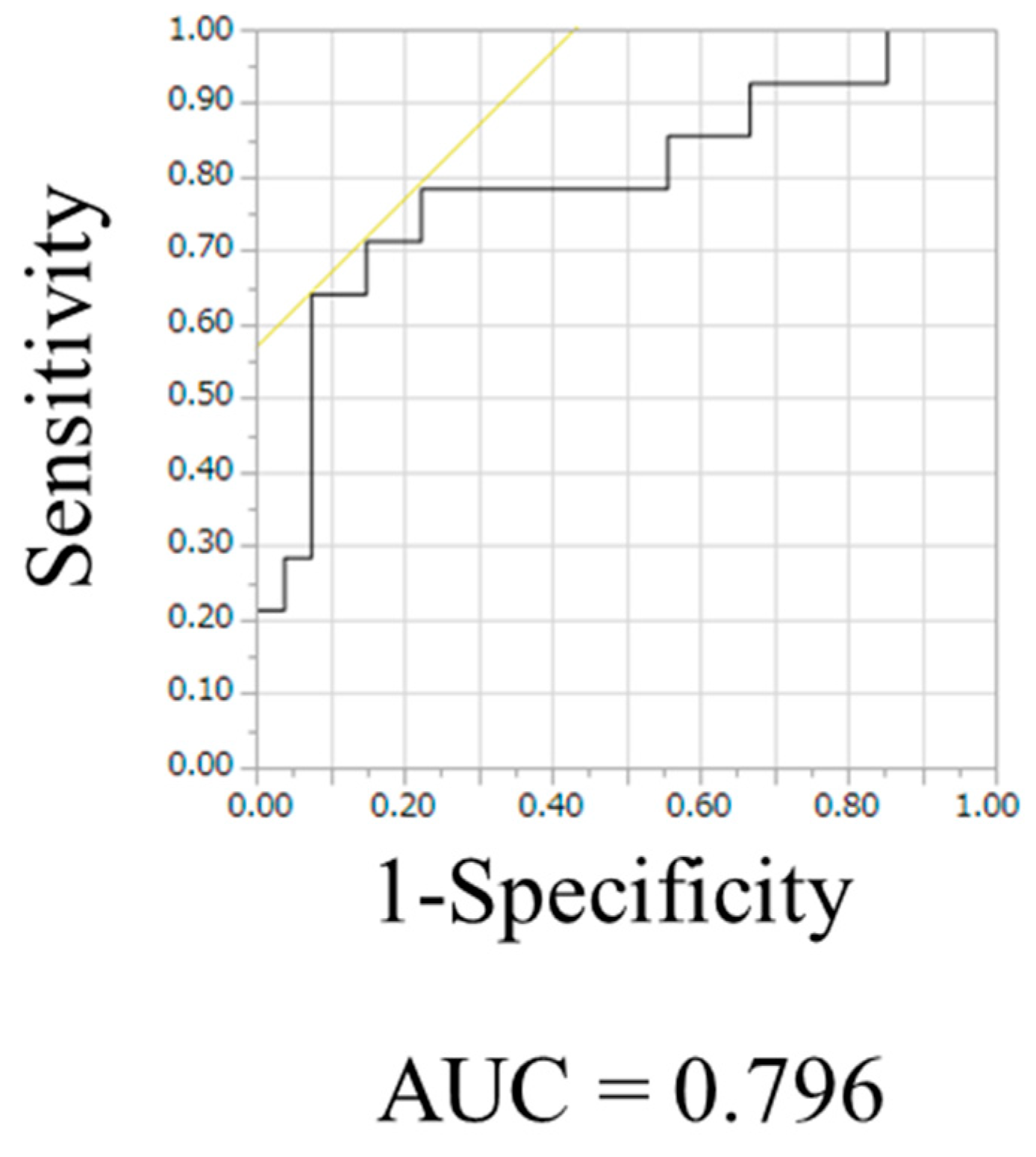
| Cutoff value | 1.39 | 1.47 | 1.51 | 1.57 | 1.74 | 1.79 | 1.86 | 2.23 | ||||||||||||
| AUC | 0.75 | 0.76 | 0.76 | 0.78 | 0.70 | 0.70 | 0.67 | 0.80 | ||||||||||||
| Predictable | Unpredictable | p-Value | |
|---|---|---|---|
| Number | 9 | 5 | |
| GCB | 3 | 2 | 1.00 |
| Non-GCB | 5 | 3 | |
| MIB-1 index (mean ± standard deviation) | 80.7 ± 9.9 | 88.2 ± 1.9 | 0.17 |
| Predictable | Unpredictable | p-Value | ||
|---|---|---|---|---|
| Number | 24 | 3 | ||
| p53 | Positive | 16 | 2 | 1.00 |
| Negative | 8 | 1 | ||
| MGMT promoter | Methylated | 5 | 2 | 0.18 |
| Unmethylated | 9 | 0 | ||
| MIB-1 index (mean ± standard deviation) | 29.1 ± 11.2 | 38.6 ± 35.5 | 0.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohba, S.; Murayama, K.; Teranishi, T.; Kumon, M.; Nakae, S.; Yui, M.; Yamamoto, K.; Yamada, S.; Abe, M.; Hasegawa, M.; et al. Three-Dimensional Amide Proton Transfer-Weighted Imaging for Differentiating between Glioblastoma, IDH-Wildtype and Primary Central Nervous System Lymphoma. Cancers 2023, 15, 952. https://doi.org/10.3390/cancers15030952
Ohba S, Murayama K, Teranishi T, Kumon M, Nakae S, Yui M, Yamamoto K, Yamada S, Abe M, Hasegawa M, et al. Three-Dimensional Amide Proton Transfer-Weighted Imaging for Differentiating between Glioblastoma, IDH-Wildtype and Primary Central Nervous System Lymphoma. Cancers. 2023; 15(3):952. https://doi.org/10.3390/cancers15030952
Chicago/Turabian StyleOhba, Shigeo, Kazuhiro Murayama, Takao Teranishi, Masanobu Kumon, Shunsuke Nakae, Masao Yui, Kaori Yamamoto, Seiji Yamada, Masato Abe, Mitsuhiro Hasegawa, and et al. 2023. "Three-Dimensional Amide Proton Transfer-Weighted Imaging for Differentiating between Glioblastoma, IDH-Wildtype and Primary Central Nervous System Lymphoma" Cancers 15, no. 3: 952. https://doi.org/10.3390/cancers15030952
APA StyleOhba, S., Murayama, K., Teranishi, T., Kumon, M., Nakae, S., Yui, M., Yamamoto, K., Yamada, S., Abe, M., Hasegawa, M., & Hirose, Y. (2023). Three-Dimensional Amide Proton Transfer-Weighted Imaging for Differentiating between Glioblastoma, IDH-Wildtype and Primary Central Nervous System Lymphoma. Cancers, 15(3), 952. https://doi.org/10.3390/cancers15030952






