Alarming Upward Trend in Multidrug-Resistant Bacteria in a Large Cohort of Immunocompromised Children: A Four-Year Comparative Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Risk Stratification of Immunocompromised Children
2.3. Supportive Care
2.4. Data Collection and Definitions
2.5. Empirical Intravenous Antibiotic Therapy Protocols
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
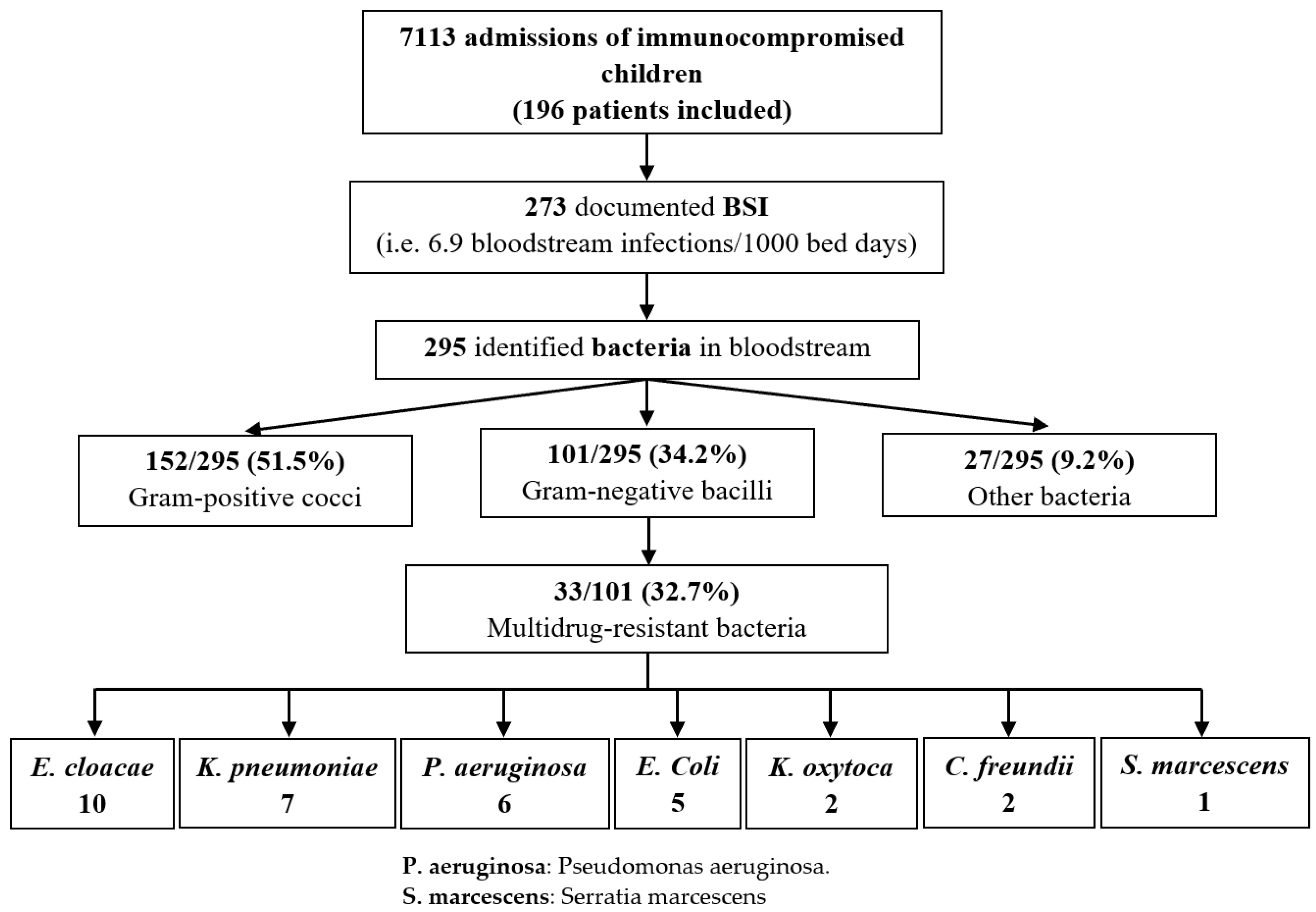
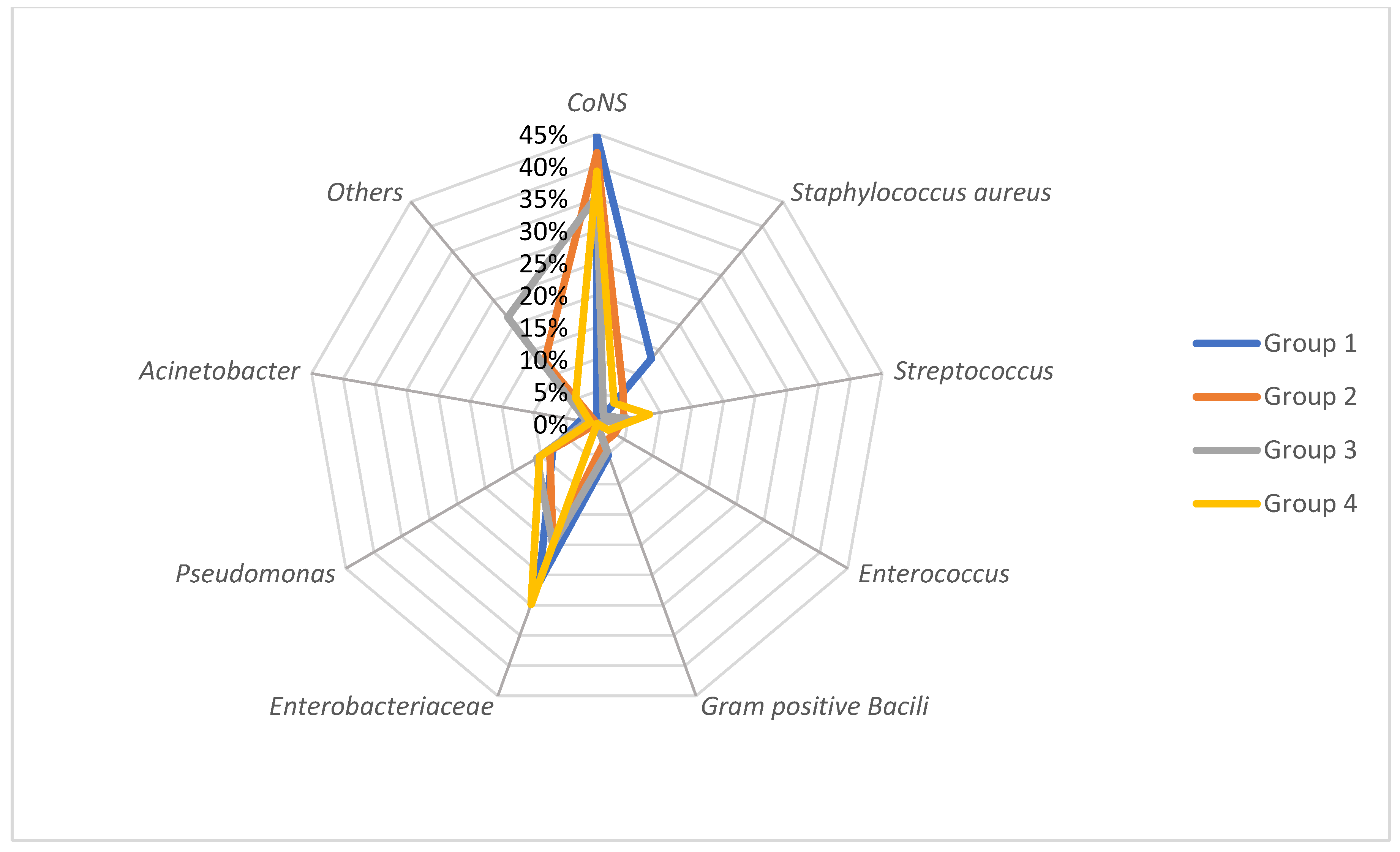
3.2. Bacteria Spectrum and Risk Group Distribution
3.3. Polymicrobial Infections
3.4. Incidence
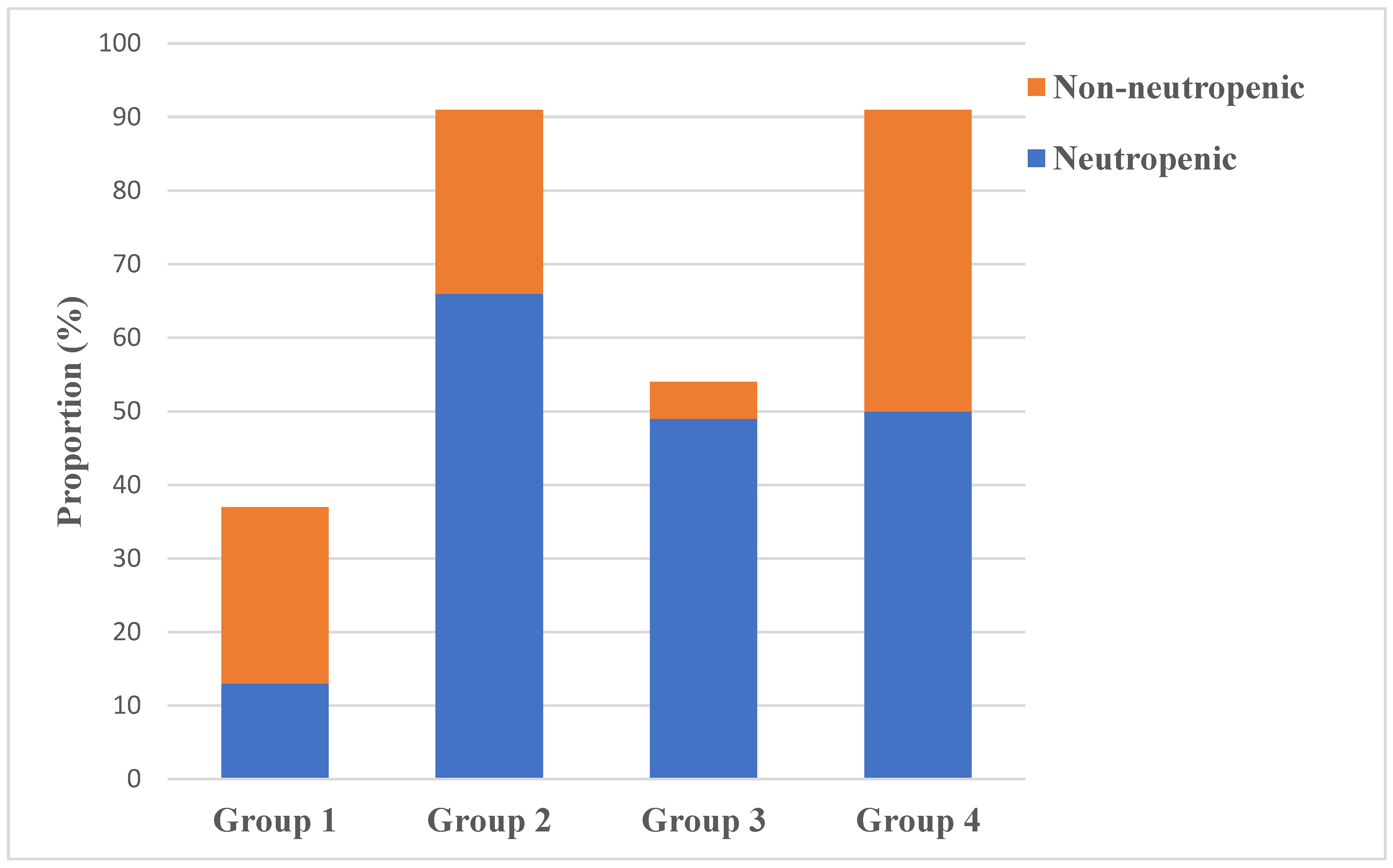
3.5. Neutropenic versus Non-Neutropenic Patients with BSIs
3.6. Evolution of BSI and Sepsis
3.7. Reinfections
3.8. Gum/Gut Colonisation and Probable Translocation
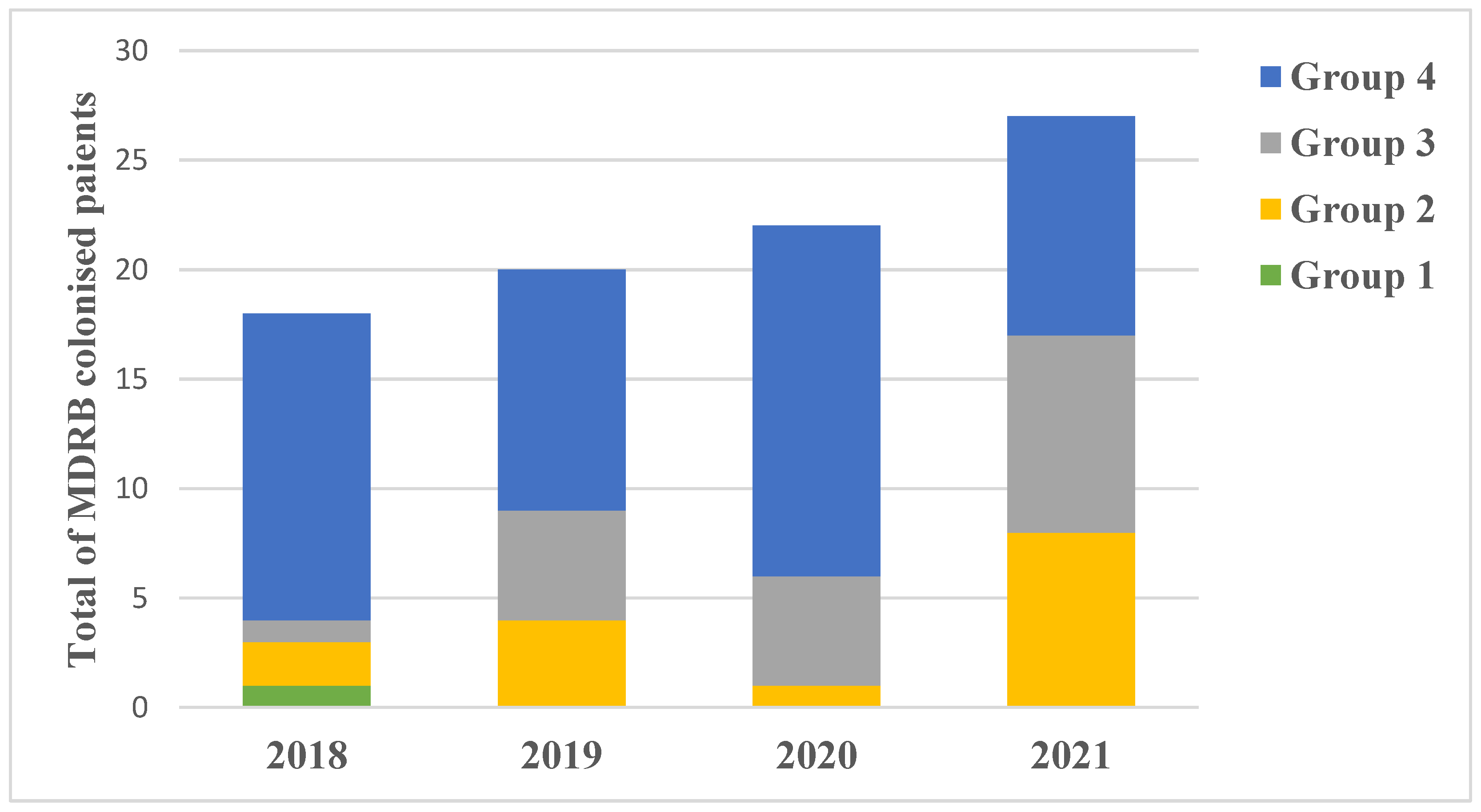
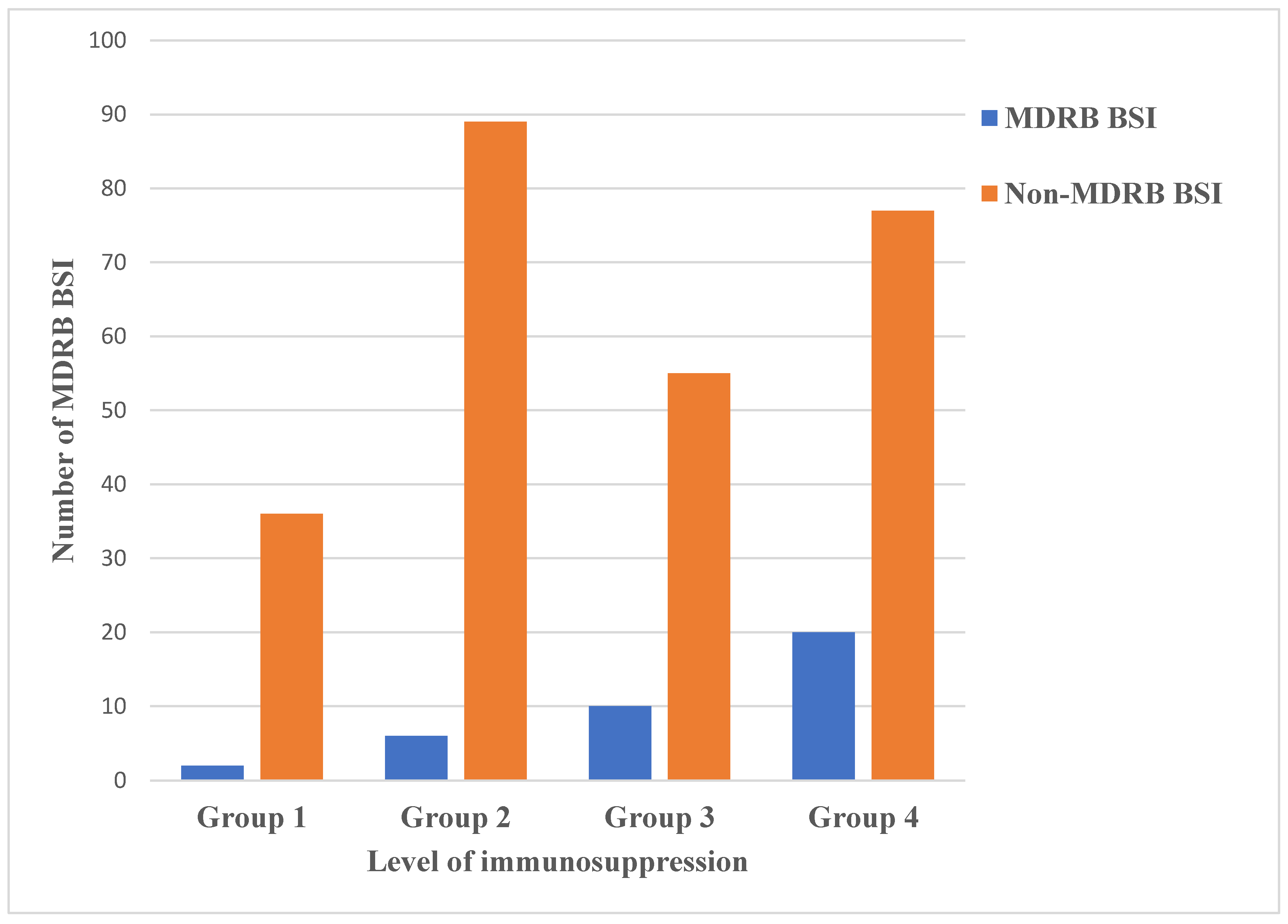
3.9. Multidrug Resistant Bacteria
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bochennek, K.; Luckowitsch, M.; Lehrnbecher, T. Recent advances and future directions in the management of the immunocompromised host. Semin. Oncol. 2020, 47, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Alexander, B.D. Editorial: Infections of the immunocompromised host: A musing on the changing times. Curr. Opin. Infect. Dis. 2020, 33, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, F.J.S.; Gennery, A.R.; Emonts, M. Biomarkers for Diagnosing Febrile Illness in Immunocompromised Children: A Systematic Review of the Literature. Front. Pediatr. 2022, 10, 2296–2360. [Google Scholar] [CrossRef] [PubMed]
- Miedema, K.G.E.; Winter, R.H.L.J.; Ammann, R.A.; Lodewijk Spanjaard, S.D.; De Bont, E.S.J.M.; Kamps, W.A.; Van de Wetering, M.D.; Tissing, W.J.E. Bacteria causing bacteremia in pediatric cancer patients presenting with febrile neutropenia- species distribution and susceptibility patterns. Support. Care Cancer 2013, 21, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Delebarre, M.; Tiphaine, A.; Martinot, A.; Dubos, F. Risk stratification management of febrile neutropenia in pediatric hematology oncology patients: Results of a French nationwide survey. Pediatr. Blood Cancer 2016, 63, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Entz-Werle, N.; Suciu, S.; Van der Werff ten Bosch, J.; Vilmer, E.; Bertrand, Y.; Benoit, Y.; Margueritte, G.; Plouvier, E.; Boutard, P.; Vandecruys, E.; et al. Results of 58,872 and 58,921 trials in acute myeloblastic leukemia and relative value of chemotherapy versus allogenic bone marrow transplantation in first complete remission: The EORTC Children Leukemia Group Report. Leukemia 2005, 19, 2072–2081. [Google Scholar] [CrossRef]
- Glausera, M.; Boogaerts, M.; Cordonnier, C.; Palmblad, J.; Martino, P. Empiric therapy of bacterial infections in severe neutropenia. Clin. Microbiol. Infect. 1997, 3, 77–86. [Google Scholar] [CrossRef]
- Trecarichi, E.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: An Italian multicentre prospective survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef]
- Raad, C.; Behdenna, A.; Fuhrmann, C.; Conter, C.; Cuzzubbo, D.; Rasigade, J.P.; Bertrand, Y.; Domenech, C. Trends in bacterial bloodstream infections and resistance in immuno-compromised patients with febrile neutropenia: A retrospective study. Eur. J. Pediatr. 2021, 180, 2921–2930. [Google Scholar] [CrossRef]
- Thurman, C.; Abbott, M.; Liu, J.; Larson, E. Risk for health care-associated bloodstream infections in pediatric oncology patients with various malignancies. J. Pediatr. Oncol. Nurs. 2017, 34, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Domenech, C.; Leick-Courtois, C.; Bienvenu, A.; Pracros, J.; Picot, S. Improvement in the outcome of invasive aspergillosis in a pediatric hematology department: A 10-year review. J. Pediatr. Hematol. 2015, 37, 560–565. [Google Scholar] [CrossRef]
- Brunet, A.; Ploton, C.; Galambrun, C.; Pondarre, C.; Panges, M.; Bleyzac, M.; Freydiere, A.; Barbe, G.; Bertrand, Y. Low incidence of sepsis due to viridans streptococci in a ten-year retrospective study of pediatric acute myeloid leukemia. Pediatr. Blood Cancer 2006, 47, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, 2019. Glossary of Terms Related to Antibiotic Resistance. Available online: https://www.cdc.gov/narms/resources/glossary.html (accessed on 15 August 2022).
- Lamprecht, G.; Heininger, A. Current aspects of sepsis caused by bacterial translocation. Zent. Chir. 2012, 137, 274–278. [Google Scholar] [CrossRef]
- Wong, M.; Barqasho, B.; Ohrmalm, L.; Tolfvenstam, T.; Novak, P. Microbial Translocation Contribute to Febrile Episodes in Adults with Chemotherapy-Induced Neutropenia. PLoS ONE 2013, 8, e68056. [Google Scholar] [CrossRef]
- AlAzmi, A.; Jastaniah, W.; AlDabbagh, M.; Elimam, N. A clinical approach to non-neutropenic fever in children with cancer. J. Oncol. Pharm. Pract. 2021, 27, 560–569. [Google Scholar] [CrossRef]
- CDC Device Associated Module BSI. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line-Associated Bloodstream Infection). 2016. Available online: http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf (accessed on 15 August 2022).
- Schöning, S.; Barnbrock, A.; Bochennek, K.; Gordon, K.; Groll, A.H.; Lehrnbecher, T. Infections during Non-Neutropenic Episodes in Pediatric Cancer Patients—Results from a Prospective Study in Two Major Large European Cancer Centers. Antibiotics 2022, 11, 900. [Google Scholar] [CrossRef]
- Beauchemin, M.; Marshall, A.F.; Ricci, A.M.; Lopez, I.D.; Yao, Y.; Lee, A.; Jin, Z.; Sulis, M.L. Bacteremia in Febrile, Non-neutropenic, and Well-appearing Children with Cancer. J. Pediatr. Hematol. 2022, 44, 194–198. [Google Scholar] [CrossRef]
- Mojtahedi, S.Y.; Rahbarimanesh, A.; Khedmat, L.; Izadi, A. The Prevalence of Risk Factors for the Development of Bacteraemia in Children. Open Access Maced. J. Med Sci. 2018, 6, 2023–2029. [Google Scholar] [CrossRef]
- Celkan, T.; Ozkan, A.; Apak, H.; Diren, S.; Can, G.; Yuksel, L.; Yildiz, I. Bacteremia in childhood cancer. J. Trop. Pediatr. 2002, 48, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Cecinati, V.; Brescia, L.; Tagliaferri, L.; Giordano, P.; Esposito, S. Catheter-related infections in pediatric patients with cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Picardi, M.; Pagliuca, S.; Chiurazzi, F.; Iula, D.; Catania, M.; Rossano, F.; Pane, F. Early ultrasonographic finding of septic thrombophlebitis is the main indicator of central venous catheter removal to reduce infection-related mortality in neutropenic patients with bloodstream infection. Ann. Oncol. 2012, 23, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Picardi, M.; Della Pepa, R.; Cerchione, C.; Pugliese, N.; Mortaruolo, C.; Trastulli, F.; Giordano, C.; Grimaldi, F.; Zacheo, I.; Raimondo, M.; et al. A Frontline Approach with Peripherally Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin. Lymphoma Myeloma Leuk. 2019, 19, e184–e194. [Google Scholar] [CrossRef]
- Noailly Charny, P.A.; Bleyzac, N.; Ohannessian, R.; Aubert, E.; Bertrand, Y.; Renard, C. Increased Risk of Thrombosis Associated with Peripherally Inserted Central Catheters Compared with Conventional Central Venous Catheters in Children with Leukemia. J. Pediatr. 2018, 198, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.; Xiao, W.; Tayeb, M.; Khan, S. Epidemiology and prognostic factors of pediatric brain tumor survival in the US: Evidence from four decades of population data. Cancer Epidemiol. 2021, 72, 101942. [Google Scholar] [CrossRef]
- Wook Lee, J.; Cho, B. Prognostic factors and treatement of pediatric acute lymphoblastic leukemia. Korean J. Pediatr. 2017, 60, 129–137. [Google Scholar] [CrossRef]
- Butler, E.; Ludwig, K.; Pacenta, H.L.; Klesse, L.J.; Watt, T.C.; Laetsch, T.W. Recent progress in the treatment of cancer in children. CA Cancer J. Clin. 2021, 71, 315–332. [Google Scholar] [CrossRef]
- Williams, A.M.; Liu, Q.; Bhakta, N.; Krull, K.R.; Hudson, M.M.; Robison, L.L.; Yasui, Y. Rethinking success in pediatric oncology: Beyond 5-year Survival. J. Clin. Oncol. 2021, 39, 2227–2231. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G.; Jezek, A., Jr.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Excalating challanges and paths forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef]
- Elhadi, M.; Khaled, A.; Msherghi, A. Infectious diseases as a cause of death among cancer patients: A trend analysis and population-based study of outcome in the United States based on the surveillance, epidemiology and end results database. Infect. Agents Cancer 2021, 16, 72. [Google Scholar] [CrossRef] [PubMed]

| 2018 | 2019 | 2020 | 2021 | 4 Years | ||
|---|---|---|---|---|---|---|
| Total admissions | 1809 | 2129 | 1583 | 1592 | 7113 | |
| Patient bed days | 10,349 | 10,657 | 8953 | 9549 | 39,508 | |
| BSI (%) | 71 (3.9%) | 57 (2.7%) | 78 (4.9%) | 67 (4.2%) | 273 (3.8%) | |
| BSIs/1000 patient bed days | 6.9 | 5.3 | 8.7 | 7 | 6.9 | |
| Group 1 | Total BSIs | 14 (19.7%) | 8 (14%) | 6 (7.7%) | 9 (13.4%) | 37 (13.5%) |
| Solid tumours | Age (years)(median-range) | 6.5 (0–22) | 14 (1–23) | 5 (1–22) | 7 (2–14) | 7.5 (0–23) |
| Hodgkin malignant lymphoma | CVAD removal | 3 (4.2%) | 3 (5.3%) | 2 (2.6%) | 2 (3%) | 10 (3.7%) |
| Neutropenia | 6 (8.5%) | 2 (3.5%) | 2 (2.6%) | 3 (4.5%) | 13 (4.8%) | |
| MDRB septicaemia | 0 | 0 | 0 | 2 (3%) | 2 (0.7%) | |
| Gum and gut MDRB colonisation | 1 (1.4%) | 0 | 0 | 0 | 1 (0.4%) | |
| Group 2 | Total BSIs | 23 (32.4%) | 16 (28.1%) | 30 (38.5%) | 22 (32.8%) | 91 (33.3%) |
| ALL: low and medium risk groups | Age (years)(median-range) | 5 (1–18) | 7.5 (1–12) | 13 (2–18) | 7 (1–20) | 9 (1–20) |
| Non-Hodgkin malignant lymphoma | CVAD removal | 4 (5.6%) | 5 (8.8%) | 8 (10.3%) | 9 (13.4%) | 26 (9.5%) |
| Metastatic neuroblastoma | Neutropenia | 17 (23.9%) | 10 (17.5%) | 22 (28.2%) | 17 (25.4%) | 66 (24.2%) |
| MDRB septicaemia | 0 | 0 | 2 (2.6%) | 4 (6%) | 6 (2.2%) | |
| Gum and gut MDRB colonisation | 2 (2.8%) | 4 (7%) | 1 (1.3%) | 8 (11.9%) | 15 (5.5%) | |
| Group 3 | Total BSIs | 9 (12.7%) | 14 (24.6%) | 15 (19.2%) | 16 (23.9%) | 54 (19.7%) |
| ALL: high risk group | Age (years)(median-range) | 11 (3–18) | 5 (1–19) | 10 (3–18) | 8 (1–19) | 8 (1–19) |
| High risk Burkitt lymphoma/leukaemia | CVAD removal | 4 (5.6%) | 3 (5.3%) | 3 (3.8%) | 9 (13.4%) | 19 (7%) |
| Non-Hodgkin malignant lymphoma high risk group/relapse | Neutropenia | 9 (12.7%) | 11 (19.3%) | 15 (19.2%) | 14 (20.9%) | 49 (17.9%) |
| MDRB septicaemia | 2 (2.8%) | 2 (3.5%) | 1 (1.3%) | 4 (6%) | 9 (3.3%) | |
| Gum and gut MDRB colonisation | 1 (1.4%) | 5 (8.8%) | 5 (6.4%) | 9 (13.4%) | 20 (7.3%) | |
| Group 4 | Total BSIs | 25 (35.2%) | 19 (33.3%) | 27 (34.6%) | 20 (29.9%) | 91 (33.3%) |
| AML | Age (years)(median-range) | 7 (0–19) | 3 (0–15) | 7 (0–19) | 8 (0–19) | 5 (0–19) |
| Acute leukaemia relapse | HSCT | 16 (22.5%) | 13 (22.8%) | 19 (24.4%) | 12 (17.9%) | 60 (22%) |
| Medullary aplasia | CVAD removal | 8 (11.3%) | 8 (14%) | 9 (11.5%) | 7 (10.4%) | 32 (11.7%) |
| Severe combined immunodeficiency syndrome (SCID) | Neutropenia | 15 (21.1%) | 10 (17.5%) | 13 (16.7%) | 12 (17.9%) | 50 (18.3%) |
| HSCT (allogenic, autologous *) | MDRB septicaemia | 4 (5.6%) | 5 (8.8%) | 5 (6.4%) | 4 (6%) | 18 (6.6%) |
| Gum and gut MDRB colonisation | 14 (19.7%) | 11 (19.3%) | 16 (20.5%) | 10 (14.9%) | 51 (18.7%) | |
| ALL acute lymphoblastic leukaemia | ||||||
| AML acute myeloid leukaemia | ||||||
| HSCT haematopoietic stem cell transplantation | ||||||
| MDR multi-drug resistant bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalcea, A.-R.; Garnier, N.; Faure-Conter, C.; Rama, N.; Renard, C.; Benezech, S.; Bertrand, Y.; Fuhrmann, C.; Domenech, C. Alarming Upward Trend in Multidrug-Resistant Bacteria in a Large Cohort of Immunocompromised Children: A Four-Year Comparative Study. Cancers 2023, 15, 938. https://doi.org/10.3390/cancers15030938
Mihalcea A-R, Garnier N, Faure-Conter C, Rama N, Renard C, Benezech S, Bertrand Y, Fuhrmann C, Domenech C. Alarming Upward Trend in Multidrug-Resistant Bacteria in a Large Cohort of Immunocompromised Children: A Four-Year Comparative Study. Cancers. 2023; 15(3):938. https://doi.org/10.3390/cancers15030938
Chicago/Turabian StyleMihalcea, Ana-Raluca, Nathalie Garnier, Cécile Faure-Conter, Nicolas Rama, Cécile Renard, Sarah Benezech, Yves Bertrand, Christine Fuhrmann, and Carine Domenech. 2023. "Alarming Upward Trend in Multidrug-Resistant Bacteria in a Large Cohort of Immunocompromised Children: A Four-Year Comparative Study" Cancers 15, no. 3: 938. https://doi.org/10.3390/cancers15030938
APA StyleMihalcea, A.-R., Garnier, N., Faure-Conter, C., Rama, N., Renard, C., Benezech, S., Bertrand, Y., Fuhrmann, C., & Domenech, C. (2023). Alarming Upward Trend in Multidrug-Resistant Bacteria in a Large Cohort of Immunocompromised Children: A Four-Year Comparative Study. Cancers, 15(3), 938. https://doi.org/10.3390/cancers15030938






