Evaluation of Exposure Doses of Elective Nodal Irradiation in Chemoradiotherapy for Advanced Esophageal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiotherapeutic Strategy
2.3. Evaluation of Toxicities
2.4. Statistical Analysis
3. Results
3.1. Survival
3.2. Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.; Kumar, N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur. J. Cancer Prev. 2017, 26, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Galais, M.-P.; Raoul, J.-L.; Bouché, O.; Gourgou-Bourgade, S.; Douillard, J.-Y.; Etienne, P.-L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Van De Voorde, L.; Larue, R.T.; Pijls, M.; Buijsen, J.; Troost, E.G.; Berbée, M.; Sosef, M.; van Elmpt, W.; Schraepen, M.-C.; Vanneste, B.; et al. A qualitative synthesis of the evidence behind elective lymph node irradiation in oesophageal cancer. Radiother. Oncol. 2014, 113, 166–174. [Google Scholar] [CrossRef]
- Akutsu, Y.; Kato, K.; Igaki, H.; Ito, Y.; Nozaki, I.; Daiko, H.; Yano, M.; Udagawa, H.; Nakagawa, S.; Takagi, M.; et al. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the results of JCOG0502, a prospective multicenter study. Ann. Surg. 2016, 264, 1009–1015. [Google Scholar] [CrossRef]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef]
- Okada, M.; Murakami, T.; Kumano, S.; Kuwabara, M.; Shimono, T.; Hosono, M.; Shiozaki, H. Integrated FDG-PET/CT compared with intravenous contrast-enhanced CT for evaluation of metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. Ann. Nucl. Med. 2009, 23, 73–80. [Google Scholar] [CrossRef]
- Gao, X.-S.; Qiao, X.; Wu, F.; Cao, L.; Meng, X.; Dong, Z.; Wang, X.; Gao, G.; Wu, T.-T.; Komaki, R.; et al. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 389–396. [Google Scholar] [CrossRef]
- Rice, T.W.; Zuccaro, G.; Adelstein, D.J.; Rybicki, L.A.; Blackstone, E.H.; Goldblum, J.R. Esophageal Carcinoma: Depth of Tumor Invasion Is Predictive of Regional Lymph Node Status. Ann. Thorac. Surg. 1998, 65, 787–792. [Google Scholar] [CrossRef]
- Fujita, H.; Sueyoshi, S.; Tanaka, T.; Fujii, T.; Toh, U.; Mine, T.; Sasahara, H.; Sudo, T.; Matono, S.; Yamana, H.; et al. Optimal Lymphadenectomy for Squamous Cell Carcinoma in the Thoracic Esophagus: Comparing the Short- and Long-term Outcome among the Four Types of Lymphadenectomy. World J. Surg. 2003, 27, 571–579. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Li, S.; Deng, W. Elective nodal irradiation provides a superior therapeutic modality for lymph node positivity esophageal squamous cell carcinoma patients receiving definitive radiotherapy versus involved-field irradiation. Medicine 2019, 98, e14080. [Google Scholar] [CrossRef] [PubMed]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hasatani, K.; Tamamura, H.; Yamamoto, K.; Aoyagi, H.; Miyanaga, T.; Kaizaki, Y.; Sawada, T. Efficacy of Endoscopic Evaluation of Acute Radiation Esophagitis during Chemoradiotherapy with Proton Beam Therapy Boost for Esophageal Cancer. Digestion 2020, 101, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.-W.; Situ, D.-R.; Ma, Q.-L.; Long, H.; Zhang, L.-J.; Lin, P.; Rong, T.-H. Three-field vs two-field lymph node dissection for esophageal cancer: A meta-analysis. World J. Gastroenterol. 2014, 20, 18022–18030. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Maruyama, K.; Sato, Y.; Usami, S.; Nakatsu, T.; Saito, H.; Minamiya, Y.; Ogawa, J.-I. Status of Involved Lymph Nodes and Direction of Metastatic Lymphatic Flow Between Submucosal and T2-4 Thoracic Squamous Cell Esophageal Cancers. World J. Surg. 2009, 33, 512–517. [Google Scholar] [CrossRef]
- Zhao, K.-L.; Ma, J.-B.; Liu, G.; Wu, K.-L.; Shi, X.-H.; Jiang, G.-L. Three-Dimensional Conformal Radiation Therapy for Esophageal Squamous Cell Carcinoma: Is Elective Nodal Irradiation Necessary? Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 446–451. [Google Scholar] [CrossRef]
- Yamashita, H.; Okuma, K.; Wakui, R.; Kobayashi-Shibata, S.; Ohtomo, K.; Nakagawa, K. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma–A retrospective analysis. Radiother. Oncol. 2011, 98, 255–260. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.L.; Mao, Q.F.; Liu, Y.H.; Kong, L.; Li, M. Elective Nodal Irradiation or Involved-Field Irradiation in Definitive Chemoradiotherapy for Esophageal Squamous Cell Cancer: A Retrospective Analysis in Clinical N0 Patients. Curr. Oncol. 2018, 25, 423–429. [Google Scholar] [CrossRef]
- Zhu, S.C.; Li, Q.F.; Zhang, X.Y.; Deng, W.Z.; Song, C.Y.; Wang, X.; Yan, K. Clinical outcomes of different irradiation ranges in definitive intensity-modulated radiotherapy for esophageal cancer. Zhonghua Zhong Liu Za Zhi 2020, 42, 1040–1047. [Google Scholar]
- Du, D.; Song, T.; Liang, X.; Fang, M.; Wu, S. Concurrent chemoradiotherapy with elective lymph node irradiation for esophageal cancer: A systemic review and pooled analysis of the literature. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef]
- Kato, K.; Muro, K.; Minashi, K.; Ohtsu, A.; Ishikura, S.; Boku, N.; Takiuchi, H.; Komatsu, Y.; Miyata, Y.; Fukuda, H. Phase II Study of Chemoradiotherapy With 5-Fluorouracil and Cisplatin for Stage II–III Esophageal Squamous Cell Carcinoma: JCOG Trial (JCOG 9906). Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 684–690. [Google Scholar] [CrossRef]
- Morota, M.; Gomi, K.; Kozuka, T.; Chin, K.; Matsuura, M.; Oguchi, M.; Ito, H.; Yamashita, T. Late Toxicity After Definitive Concurrent Chemoradiotherapy for Thoracic Esophageal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Beukema, J.C.; van Luijk, P.; Widder, J.; Langendijk, J.A.; Muijs, C.T. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother. Oncol. 2015, 114, 85–90. [Google Scholar] [CrossRef]
- Ling, T.C.; Slater, J.M.; Nookala, P.; Mifflin, R.; Grove, R.; Ly, A.M.; Patyal, B.; Yang, G.Y. Analysis of Intensity-Modulated Radiation Therapy (IMRT), Proton and 3D Conformal Radiotherapy (3D-CRT) for Reducing Perioperative Cardiopulmonary Complications in Esophageal Cancer Patients. Cancers 2014, 6, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Withers, H.; Peters, L.J.; Taylor, J.M. Dose-response relationship for radiation therapy of subclinical disease. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 353–359. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Winter, K.; Kocha, W.I.; Coia, L.R.; Herskovic, A.; Graham, M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): Final report. Cancer 2000, 88, 988–995. [Google Scholar] [CrossRef]
- Gignoux, M.; Roussel, A.; Paillot, B.; Gillet, M.; Schlag, P.; Favre, J.-P.; Dalesio, O.; Buyse, M.; Duez, N. The value of preoperative radiotherapy in esophageal cancer: Results of a study of the E.O.R.T.C. World J. Surg. 1987, 11, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.-F.; Gignoux, M.; Triboulet, J.-P.; Tiret, E.; Mantion, G.; Elias, D.; Lozach, P.; Ollier, J.-C.; Pavy, J.-J.; Mercier, M.; et al. Chemoradiotherapy Followed by Surgery Compared with Surgery Alone in Squamous-Cell Cancer of the Esophagus. N. Engl. J. Med. 1997, 337, 161–167. [Google Scholar] [CrossRef]
- Suwinski, R.; Maciejewski, B.; Withers, H.R. Dose-response relationship for elective neck irradiation of head and neck cancer--facts and controversies. Neoplasma 1998, 45, 107–112. [Google Scholar]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
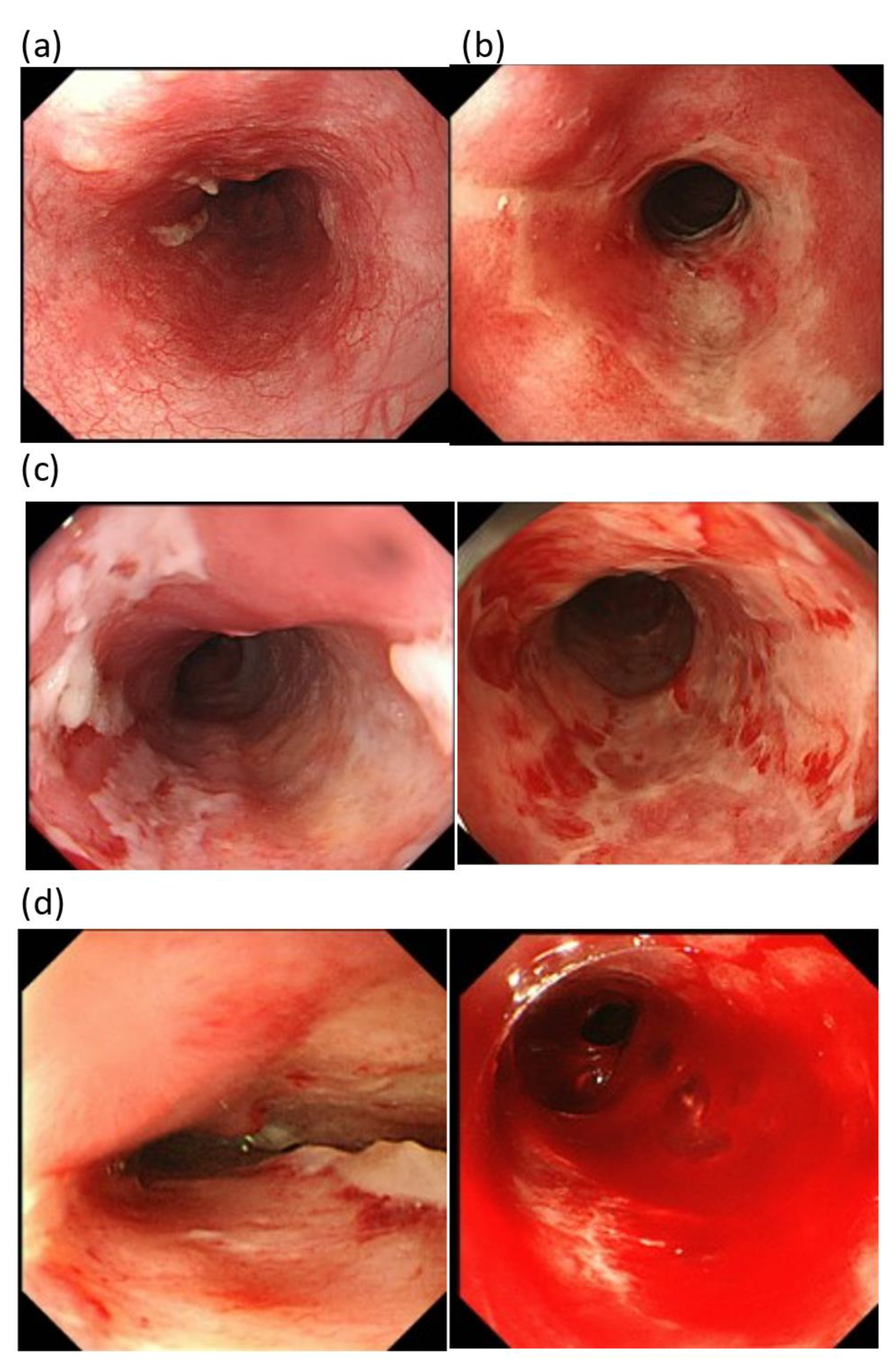
| Characteristics | Patients | HG | LG | p-Value |
|---|---|---|---|---|
| Eligible patients, n (%) | 79 | 38(48.1) | 41(51.9) | |
| Follow-up time, | 36.7 | 41.9 | 33 | 0.073 |
| median (range), months | (4.3–119.8) | (4.9–119.8) | (4.3–84.1) | |
| Age, median age (range), years | 67(49–80) | 68(49–80) | 66(52–80) | 0.632 |
| Gender, n (%) | 0.554 | |||
| Male | 65(82.3) | 31(81.6) | 34(82.9) | |
| Female | 14(17.7) | 7(18.4) | 7(17.1) | |
| Performance status, n (%) | 0.755 | |||
| 0 | 45(57.0) | 23(60.5) | 22(53.7) | |
| 1 | 26(32.9) | 12(31.6) | 14(34.1) | |
| 2 | 8(10.1) | 3(7.9) | 5(12.2) | |
| Operability, n (%) | 0.447 | |||
| operable | 36(45.6) | 19(50.0) | 17(41.5) | |
| inoperable | 43(54.4) | 19(50.0) | 24(58.5) | |
| Histology, n (%) | 0.771 | |||
| Adenocarcinoma | 6(7.6) | 3(7.9) | 3(7.3) | |
| Squamous cell carcinoma | 73(92.4) | 35(92.1) | 38(92.7) | |
| T category UICC 8th, n (%) | 0.564 | |||
| T1 | 25(31.6) | 14(36.8) | 11(26.8) | |
| T2 | 12(15.2) | 5(13.2) | 7(17.1) | |
| T3 | 42(53.2) | 19(50.0) | 23(56.1) | |
| N category UICC 8th, n (%) | 0.830 | |||
| N0 | 19(24.1) | 10(26.3) | 9(22.0) | |
| N1 | 28(35.4) | 12(31.6) | 16(39.0) | |
| N2 | 29(36.7) | 14(36.8) | 15(36.6) | |
| N3 | 3(3.8) | 2(5.3) | 1(2.4) | |
| Stage UICC 8th, n (%) | 0.438 | |||
| I | 19(24.1) | 12(31.6) | 7(17.1) | |
| II | 16(20.3) | 6(15.8) | 10(24.4) | |
| III | 33(41.7) | 16(42.1) | 17(41.4) | |
| IV | 11(13.9) | 4(10.5) | 7(17.1) | |
| Tumor location, n (%) | 0.735 | |||
| Cervical | 12(15.2) | 7(18.4) | 5(12.2) | |
| Thoracic | 63(79.7) | 29(76.3) | 34(82.9) | |
| Upper/Median/Lower, n | 10/30/23 | 5/14/10 | 5/16/13 | |
| Abdominal | 4(5.1) | 2(5.3) | 2(4.9) |
| Characteristics | Patients | HG | LG | p-Value |
|---|---|---|---|---|
| Elective nodal irradiation (ENI) | ||||
| Median (range), Gy | 39.6 | 40 | 36 | <0.001 |
| (30.6–48.0) | (40.0–48.0) | (30.6–39.6) | ||
| The single exposure dose, median (range), Gy | 2.0(1.8–2.0) | 2.0(1.8–2.0) | 1.8(1.8–2.0) | <0.001 |
| Irradiation fractions, | 20(17–23) | 20(20–23) | 20(17–20) | 0.175 |
| Median (range) | ||||
| Irradiation period, (days) median (range) | 28(22–36) | 28(23–36) | 28(22–34) | 0.907 |
| Large-area irradiation | 68(86.1) | 32(84.2) | 36(87.8) | 0.645 |
| (Long T type + long I type), n (%) | ||||
| 3DCRT irradiation method, n (%) | 76(96.2) | 37(97.4) | 39(95.1) | 0.602 |
| Total dose, median (range), Gy | 66 | 60 | 66 | 0.003 |
| (59.6–73.4) | (60.0–70.0) | (59.6–73.4) | ||
| Irradiation combination | ||||
| (ENI + Boost Therapy) | ||||
| XT + PT, n (%) | 53(67.1) | 18(47.4) | 35(85.4) | <0.001 |
| XT + XT, n (%) | 26(32.9) | 20(52.6) | 6(14.6) | |
| Chemotherapy | ||||
| Cisplatin and 5-fluorouracil, n (%) | 79(100.0) | 38(100.0) | 41(100.0) | 1.000 |
| Ineligible patients | 52 | |||
| Reasons for non-eligibility | ||||
| Over 81 years | 19 | |||
| T4 | 12 | |||
| PS3-4 | 12 | |||
| ENI using PT | 9 |
| Changes in Patient Status | HG | LG | p-Value |
|---|---|---|---|
| Lymph node recurrence | |||
| In the ENI field, n (%) | 0(0.0) | 0(0.0) | 1.000 |
| Near the ENI field, n (%) | 4(10.5) | 2(4.9) | 0.344 |
| Patient weight status | |||
| Weight before treatment, kg | 56.2 | 56.5 | 0.513 |
| Weight change after ENI, kg (weight change, kg) | 53.1(−3.1) | 52.6(−3.9) | 0.537 |
| Weight after treatment, kg (weight change, kg) | 52.3(−3.9) | 53.6(−2.9) | 0.437 |
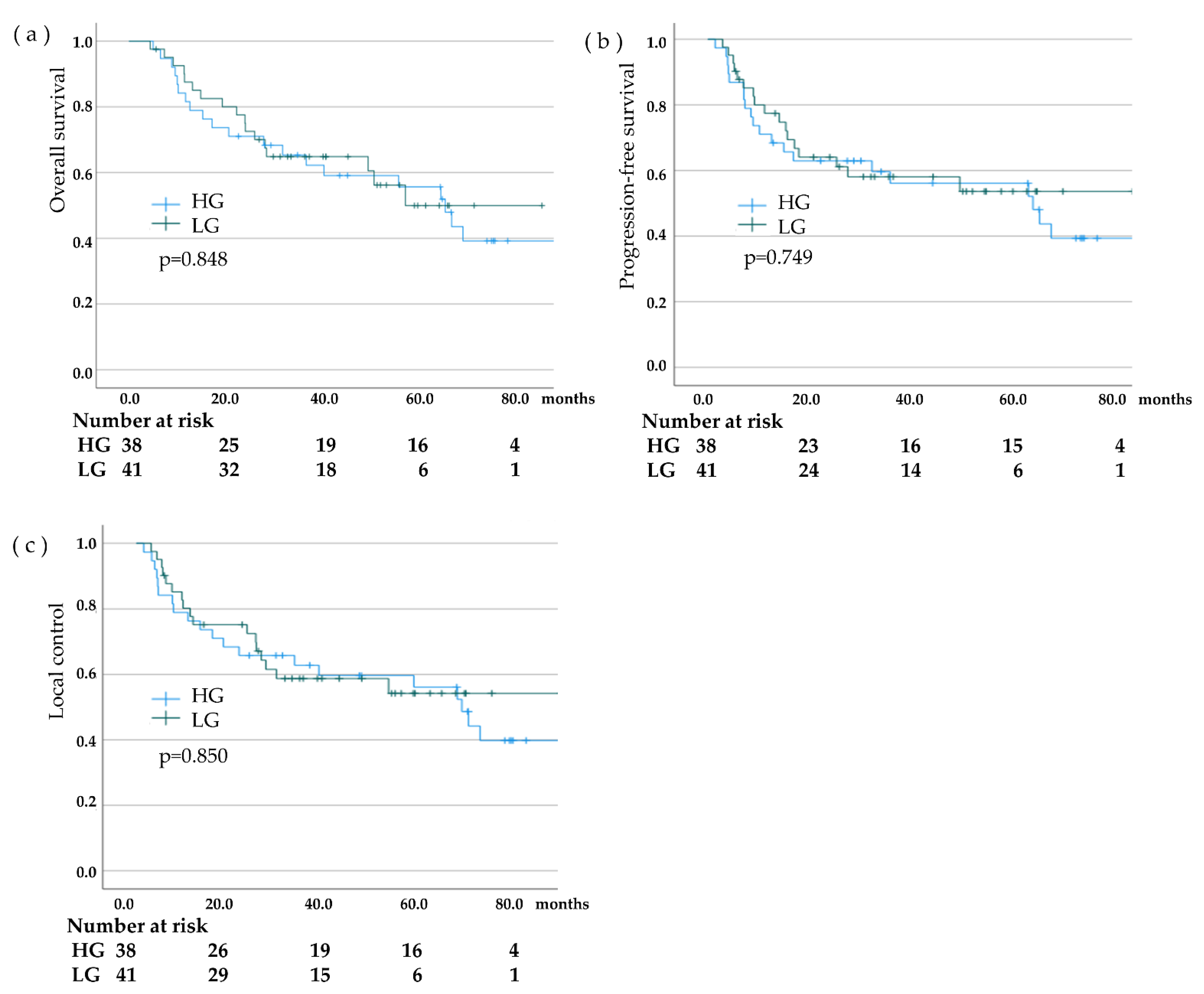
| The LC rate at 3 years, % | 62.8 | 58.8 | 0.850 |
| The PFS rate at 3 years, % | 59.6 | 58.2 | 0.749 |
| The OS rate at 3 years, % | 65.3 | 64.9 | 0.848 |
| HG (n = 38) | LG (n = 41) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ||||||||
| CTCAE Grade | 0 + 1 | 2 | 3 | 4 | 0 + 1 | 2 | 3 | 4 | p-Value |
| Leukopenia | |||||||||
| Before treatment | 38(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 39(95.1) | 2(4.9) | 0(0.0) | 0(0.0) | 0.616 |
| After ENI | 24(63.2) | 10(26.3) | 4(10.5) | 0(0.0) | 19(46.3) | 17(41.5) | 5(12.2) | 0(0.0) | 0.552 |
| After treatment | 26(68.4) | 9(23.7) | 2(5.3) | 1(2.6) | 25(61.0) | 14(34.1) | 2(4.9) | 0(0.0) | 0.703 |
| Anemia | |||||||||
| Before treatment | 37(97.4) | 0(0.0) | 1(2.6) | 0(0.0) | 41(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0.694 |
| After ENI | 35(92.1) | 2(5.3) | 1(2.6) | 0(0.0) | 41(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0.433 |
| After treatment | 31(81.6) | 5(13.1) | 2(5.3) | 0(0.0) | 34(82.9) | 3(7.3) | 4(9.8) | 0(0.0) | 0.289 |
| Thrombocytopenia | |||||||||
| Before treatment | 38(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 41(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0.671 |
| After ENI | 37(97.4) | 1(2.6) | 0(0.0) | 0(0.0) | 41(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0.328 |
| After treatment | 31(81.6) | 7(18.4) | 0(0.0) | 0(0.0) | 37(90.3) | 3(7.3) | 1(2.4) | 0(0.0) | 0.334 |
| Pneumonitis | 37(97.4) | 1(2.6) | 0(0.0) | 0(0.0) | 39(95.1) | 2(4.9) | 0(0.0) | 0(0.0) | 0.602 |
| Pericardial effusion | 38(100.0) | - | 0(0.0) | 0(0.0) | 40(97.6) | - | 1(2.4) | 0(0.0) | 0.333 |
| Pleural effusion, | 37(97.4) | 0(0.0) | 1(2.6) | 0(0.0) | 39(95.1) | 2(4.9) | 0(0.0) | 0(0.0) | 0.602 |
| Esophagitis | 19(50.0) | 17(44.7) | 2(5.3) | 0(0.0) | 13(31.7) | 21(51.2) | 7(17.1) | 0(0.0) | 0.098 |
| HG (n = 18) | LG (n = 35) | ||||
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Grade | 0–1 | 2–4 [2, 3, 4] | 0–1 | 2–4 [2, 3, 4] | p-Value |
| The CTCAE grade | 12(66.7) | 6 [6,0,0 (33.3,0.0,0.0)] | 11 (31.4) | 24 [18,6,0 (51.4,17.2,0.0)] | 0.014 |
| The FARE grade | 14(77.7) | 4 [3,1,0 (16.7,5.6,0.0)] | 26 (74.2) | 9 [8,0,1 (22.9,0.0,2.9)] | 0.780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamamura, H.; Hasatani, K.; Matsumoto, S.; Asahi, S.; Tatebe, H.; Sato, Y.; Matsusita, K.; Tameshige, Y.; Maeda, Y.; Sasaki, M.; et al. Evaluation of Exposure Doses of Elective Nodal Irradiation in Chemoradiotherapy for Advanced Esophageal Cancer. Cancers 2023, 15, 860. https://doi.org/10.3390/cancers15030860
Tamamura H, Hasatani K, Matsumoto S, Asahi S, Tatebe H, Sato Y, Matsusita K, Tameshige Y, Maeda Y, Sasaki M, et al. Evaluation of Exposure Doses of Elective Nodal Irradiation in Chemoradiotherapy for Advanced Esophageal Cancer. Cancers. 2023; 15(3):860. https://doi.org/10.3390/cancers15030860
Chicago/Turabian StyleTamamura, Hiroyasu, Kenkei Hasatani, Sae Matsumoto, Satoko Asahi, Hitoshi Tatebe, Yoshitaka Sato, Keiichiro Matsusita, Yuji Tameshige, Yoshikazu Maeda, Makoto Sasaki, and et al. 2023. "Evaluation of Exposure Doses of Elective Nodal Irradiation in Chemoradiotherapy for Advanced Esophageal Cancer" Cancers 15, no. 3: 860. https://doi.org/10.3390/cancers15030860
APA StyleTamamura, H., Hasatani, K., Matsumoto, S., Asahi, S., Tatebe, H., Sato, Y., Matsusita, K., Tameshige, Y., Maeda, Y., Sasaki, M., Takamatsu, S., & Yamamoto, K. (2023). Evaluation of Exposure Doses of Elective Nodal Irradiation in Chemoradiotherapy for Advanced Esophageal Cancer. Cancers, 15(3), 860. https://doi.org/10.3390/cancers15030860






