EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
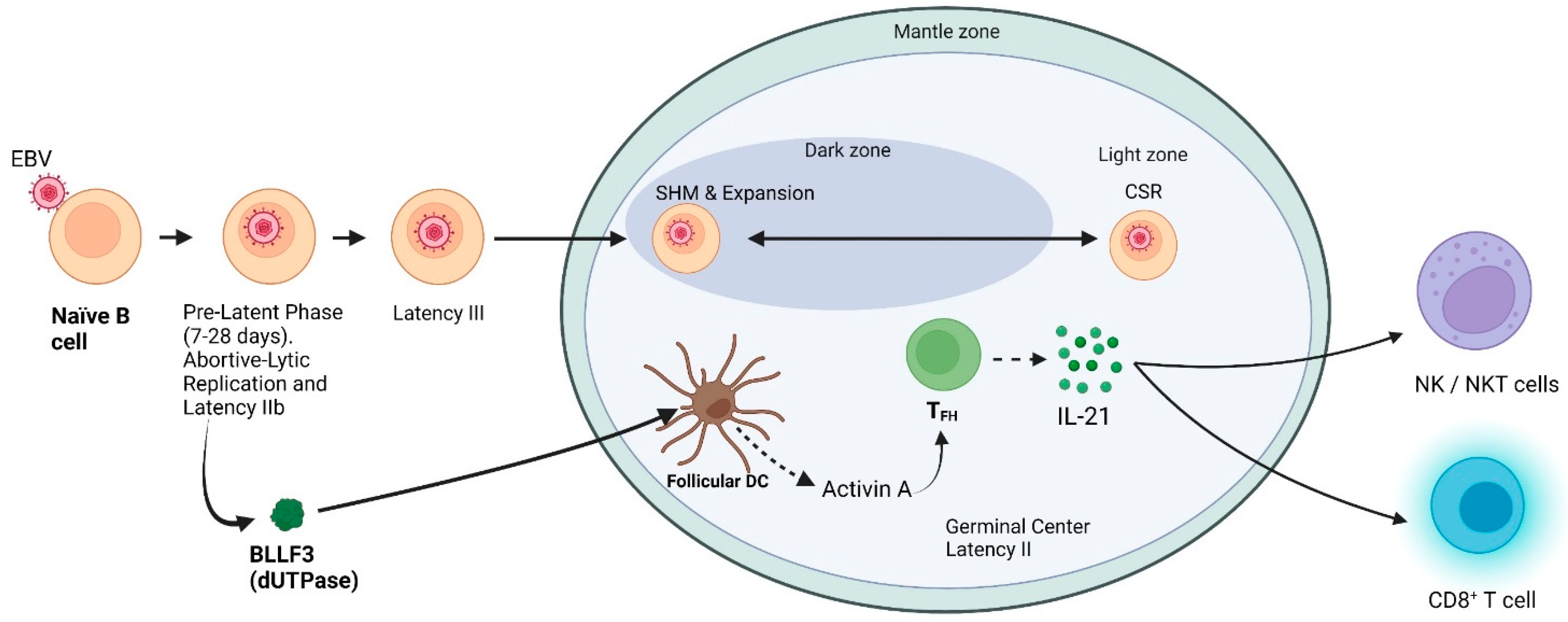
2. Establishment of Latency
2.1. Pre-Latent Phase
2.2. What Is the Relationship if Any between IL-21 and EBV?
3. EBV-Associated Germinal Center Malignancies
3.1. EBV-Positive Diffuse Large B-Cell Lymphoma (EBV+-DLBCL)
3.2. Classical Hodgkin Lymphoma (cHL)
3.3. Burkitt Lymphoma (BL)
3.4. TFH Derived Lymphomas
4. Modulation of the Tumor Microenvironment (TME)
4.1. By EBV dUTPase
4.2. Exosomes
4.3. Inflammation: Cytokines
4.4. Checkpoint Molecules
5. Other EBV-Associated Malignancies
5.1. Nasopharyngeal Carcinoma (NPC)
5.2. Gastric Carcinoma
5.3. Extranodal NK/T-Cell Lymphoma, Nasal Type (ENKT-NT)
6. Autoimmune Disease and Lymphoma
7. Summary and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kutok, J.L.; Wang, F. Spectrum of Epstein-Barr Virus–Associated Diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A.; Hawkins, J.B.; Tracy, S.I.; Shapiro, M. The pathogenesis of Epstein-Barr virus persistent infection. Curr. Opin. Virol. 2013, 3, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Long, H.M.; Brooks, J.M.; Rickinson, A.B.; Hislop, A.D. The immunology of Epstein-Barr virus-induced disease. Annu. Rev. Immunol. 2015, 33, 787–821. [Google Scholar] [CrossRef] [PubMed]
- Hislop, A.D. Early virological and immunological events in Epstein-Barr virus infection. Curr. Opin. Virol. 2015, 15, 75–79. [Google Scholar] [CrossRef]
- Rickinson, A.B. Co-infections, inflammation and oncogenesis: Future directions for EBV research. Semin. Cancer Biol. 2014, 26, 99–115. [Google Scholar] [CrossRef]
- Burkitt, D. A sarcoma involving the jaws in African children. Br. J. Surg. 1958, 46, 218–223. [Google Scholar] [CrossRef]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein-Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef]
- Laichalk, L.L.; Thorley-Lawson, D.A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 2005, 79, 1296–1307. [Google Scholar] [CrossRef]
- Al Tabaa, Y.; Tuaillon, E.; Bollore, K.; Foulongne, V.; Petitjean, G.; Seigneurin, J.M.; Duperray, C.; Desgranges, C.; Vendrell, J.P. Functional Epstein-Barr virus reservoir in plasma cells derived from infected peripheral blood memory B cells. Blood 2009, 113, 604–611. [Google Scholar] [CrossRef]
- Al Tabaa, Y.; Tuaillon, E.; Jeziorski, E.; Ouedraogo, D.E.; Bolloré, K.; Rubbo, P.A.; Foulongne, V.; Rodière, M.; Vendrell, J.P. B-cell polyclonal activation and Epstein-Barr viral abortive lytic cycle are two key features in acute infectious mononucleosis. J. Clin. Virol. 2011, 52, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Murata, T.; Sato, Y.; Muramatsu, H.; Ito, Y.; Watanabe, T.; Okuno, T.; Murakami, N.; Yoshida, K.; Sawada, A.; et al. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat. Microbiol. 2019, 4, 404–413. [Google Scholar] [CrossRef]
- Inagaki, T.; Sato, Y.; Ito, J.; Takaki, M.; Okuno, Y.; Yaguchi, M.; Masud, H.; Watanabe, T.; Sato, K.; Iwami, S.; et al. Direct Evidence of Abortive Lytic Infection-Mediated Establishment of Epstein-Barr Virus Latency During B-Cell Infection. Front. Microbiol. 2020, 11, 575255. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, A.; Fuentes-Panana, E.M. The Immunomodulatory Capacity of an Epstein-Barr Virus Abortive Lytic Cycle: Potential Contribution to Viral Tumorigenesis. Cancers 2018, 10, 98. [Google Scholar] [CrossRef]
- Manners, O.; Murphy, J.C.; Coleman, A.; Hughes, D.J.; Whitehouse, A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr. Opin. Virol. 2018, 32, 60–70. [Google Scholar] [CrossRef]
- Münz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef]
- Murata, T.; Okuno, Y.; Sato, Y.; Watanabe, T.; Kimura, H. Oncogenesis of CAEBV revealed: Intragenic deletions in the viral genome and leaky expression of lytic genes. Rev. Med. Virol. 2020, 30, e2095. [Google Scholar] [CrossRef]
- Rosemarie, Q.; Sugden, B. Epstein-Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms 2020, 8, 1824. [Google Scholar] [CrossRef]
- Münz, C. Tumor Microenvironment Conditioning by Abortive Lytic Replication of Oncogenic γ-Herpesviruses. Adv. Exp. Med. Biol. 2020, 1225, 127–135. [Google Scholar] [CrossRef]
- Frappier, L. Epstein-Barr virus: Current questions and challenges. Tumour Virus Res. 2021, 12, 200218. [Google Scholar] [CrossRef]
- Münz, C. The Role of Lytic Infection for Lymphomagenesis of Human γ-Herpesviruses. Front. Cell. Infect. Microbiol. 2021, 11, 605258. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Münz, C. Roles of Lytic Viral Replication and Co-Infections in the Oncogenesis and Immune Control of the Epstein-Barr Virus. Cancers 2021, 13, 2275. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A. Epstein-Barr virus: Exploiting the immune system. Nat. Rev. Immunol. 2001, 1, 75–82. [Google Scholar] [CrossRef]
- Buschle, A.; Hammerschmidt, W. Epigenetic lifestyle of Epstein-Barr virus. Semin. Immunopathol. 2020, 42, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Sugimoto, A.; Inagaki, T.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H. Molecular Basis of Epstein-Barr Virus Latency Establishment and Lytic Reactivation. Viruses 2021, 13, 2344. [Google Scholar] [CrossRef] [PubMed]
- Pich, D.; Mrozek-Gorska, P.; Bouvet, M.; Sugimoto, A.; Akidil, E.; Grundhoff, A.; Hamperl, S.; Ling, P.D.; Hammerschmidt, W. First Days in the Life of Naive Human B Lymphocytes Infected with Epstein-Barr Virus. mBio 2019, 10, e01723-19. [Google Scholar] [CrossRef]
- Mrozek-Gorska, P.; Buschle, A.; Pich, D.; Schwarzmayr, T.; Fechtner, R.; Scialdone, A.; Hammerschmidt, W. Epstein-Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc. Natl. Acad. Sci. USA 2019, 116, 16046–16055. [Google Scholar] [CrossRef]
- Zeidler, R.; Eissner, G.; Meissner, P.; Uebel, S.; Tampé, R.; Lazis, S.; Hammerschmidt, W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood 1997, 90, 2390–2397. [Google Scholar] [CrossRef]
- Halder, S.; Murakami, M.; Verma, S.C.; Kumar, P.; Yi, F.; Robertson, E.S. Early events associated with infection of Epstein-Barr virus infection of primary B-cells. PLoS ONE 2009, 4, e7214. [Google Scholar] [CrossRef]
- Altmann, M.; Hammerschmidt, W. Epstein-Barr virus provides a new paradigm: A requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005, 3, e404. [Google Scholar] [CrossRef]
- Wen, W.; Iwakiri, D.; Yamamoto, K.; Maruo, S.; Kanda, T.; Takada, K. Epstein-Barr virus BZLF1 gene, a switch from latency to lytic infection, is expressed as an immediate-early gene after primary infection of B lymphocytes. J. Virol. 2007, 81, 1037–1042. [Google Scholar] [CrossRef]
- Bouvet, M.; Voigt, S.; Tagawa, T.; Albanese, M.; Chen, Y.A.; Chen, Y.; Fachko, D.N.; Pich, D.; Göbel, C.; Skalsky, R.L.; et al. Multiple Viral microRNAs Regulate Interferon Release and Signaling Early during Infection with Epstein-Barr Virus. mBio 2021, 12, e03440-20. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Luftig, M.A. Dynamic Epstein-Barr virus gene expression on the path to B-cell transformation. Adv. Virus Res. 2014, 88, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Luftig, M.A. To be or not IIb: A multi-step process for Epstein-Barr virus latency establishment and consequences for B cell tumorigenesis. PLoS Pathog. 2015, 11, e1004656. [Google Scholar] [CrossRef] [PubMed]
- Kalla, M.; Hammerschmidt, W. Human B cells on their route to latent infection--early but transient expression of lytic genes of Epstein-Barr virus. Eur. J. Cell. Biol. 2012, 91, 65–69. [Google Scholar] [CrossRef]
- Jochum, S.; Ruiss, R.; Moosmann, A.; Hammerschmidt, W.; Zeidler, R. RNAs in Epstein-Barr virions control early steps of infection. Proc. Natl. Acad. Sci. USA 2012, 109, E1396–E1404. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Zhang, L.; Jiang, S.; Liang, J.; Narita, Y.; Hou, I.; Zhong, Q.; Zheng, Z.; Xiao, H.; et al. RNA Sequencing Analyses of Gene Expression during Epstein-Barr Virus Infection of Primary B Lymphocytes. J. Virol. 2019, 93, e00226-19. [Google Scholar] [CrossRef]
- Padgett, D.A.; Hotchkiss, A.K.; Pyter, L.M.; Nelson, R.J.; Yang, E.; Yeh, P.E.; Litsky, M.; Williams, M.; Glaser, R. Epstein-Barr virus-encoded dUTPase modulates immune function and induces sickness behavior in mice. J. Med. Virol. 2004, 74, 442–448. [Google Scholar] [CrossRef]
- Glaser, R.; Litsky, M.L.; Padgett, D.A.; Baiocchi, R.A.; Yang, E.V.; Chen, M.; Yeh, P.E.; Green-Church, K.B.; Caligiuri, M.A.; Williams, M.V. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology 2006, 346, 205–218. [Google Scholar] [CrossRef]
- Waldman, W.J.; Williams, M.V., Jr.; Lemeshow, S.; Binkley, P.; Guttridge, D.; Kiecolt-Glaser, J.K.; Knight, D.A.; Ladner, K.J.; Glaser, R. Epstein-Barr virus-encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: Evidence for depression-induced atherosclerotic risk. Brain Behav. Immun. 2008, 22, 215–223. [Google Scholar] [CrossRef]
- Ariza, M.E.; Glaser, R.; Kaumaya, P.T.; Jones, C.; Williams, M.V. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 2009, 182, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E.; Rivailler, P.; Glaser, R.; Chen, M.; Williams, M.V. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS ONE 2013, 8, e69827. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E.; Williams, M.V. EBV-dUTPase modulates host immune responses potentially altering the tumor microenvironment in EBV-associated malignancies. J. Curr. Res. HIV/AIDS 2016, 2016, 1–9. [Google Scholar]
- Williams, M.; Ariza, M.E. EBV Positive Diffuse Large B Cell Lymphoma and Chronic Lymphocytic Leukemia Patients Exhibit Increased Anti-dUTPase Antibodies. Cancers 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Cox, B.; Lafuse, D.W.; Ariza, M.E. Epstein-Barr Virus dUTPase Induces Neuroinflammatory Mediators: Implications for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Clin. Ther. 2019, 41, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.S.; Alharshawi, K.; Mena-Palomo, I.; Lafuse, W.P.; Ariza, M.E. EBV/HHV-6A dUTPases contribute to myalgic encephalomyelitis/chronic fatigue syndrome pathophysiology by enhancing TFH cell differentiation and extrafollicular activities. JCI Insight 2022, 7, e158193. [Google Scholar] [CrossRef]
- Bergbauer, M.; Kalla, M.; Schmeinck, A.; Göbel, C.; Rothbauer, U.; Eck, S.; Benet-Pagès, A.; Strom, T.M.; Hammerschmidt, W. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog. 2010, 6, e1001114. [Google Scholar] [CrossRef]
- Bernaudat, F.; Gustems, M.; Günther, J.; Oliva, M.F.; Buschle, A.; Göbel, C.; Pagniez, P.; Lupo, J.; Signor, L.; Müller, C.W.; et al. Structural basis of DNA methylation-dependent site selectivity of the Epstein-Barr virus lytic switch protein ZEBRA/Zta/BZLF1. Nucleic Acids Res. 2022, 50, 490–511. [Google Scholar] [CrossRef]
- Chiu, Y.F.; Sugden, B. Epstein-Barr Virus: The Path from Latent to Productive Infection. Annu. Rev. Virol. 2016, 3, 359–372. [Google Scholar] [CrossRef]
- Hong, G.K.; Kumar, P.; Wang, L.; Damania, B.; Gulley, M.L.; Delecluse, H.J.; Polverini, P.J.; Kenney, S.C. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 2005, 79, 13984–13992. [Google Scholar] [CrossRef]
- Ma, S.D.; Yu, X.; Mertz, J.E.; Gumperz, J.E.; Reinheim, E.; Zhou, Y.; Tang, W.; Burlingham, W.J.; Gulley, M.L.; Kenney, S.C. An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J. Virol. 2012, 86, 7976–7987. [Google Scholar] [CrossRef]
- Bristol, J.A.; Djavadian, R.; Albright, E.R.; Coleman, C.B.; Ohashi, M.; Hayes, M.; Romero-Masters, J.C.; Barlow, E.A.; Farrell, P.J.; Rochford, R.; et al. A cancer-associated Epstein-Barr virus BZLF1 promoter variant enhances lytic infection. PLoS Pathog. 2018, 14, e1007179. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanyan, S.; Kanhere, A.; Osborn, K.; Flower, K.; Jenner, R.G.; Sinclair, A.J. Genome-wide analyses of Zta binding to the Epstein-Barr virus genome reveals interactions in both early and late lytic cycles and an epigenetic switch leading to an altered binding profile. J. Virol. 2012, 86, 12494–12502. [Google Scholar] [CrossRef] [PubMed]
- Locci, M.; Wu, J.E.; Arumemi, F.; Mikulski, Z.; Dahlberg, C.; Miller, A.T.; Crotty, S. Activin A programs the differentiation of human TFH cells. Nat. Immunol. 2016, 17, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Loomans, H.A.; Andl, C.D. Intertwining of Activin A and TGFβ Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion. Cancers 2014, 7, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Portale, F.; Cricrì, G.; Bresolin, S.; Lupi, M.; Gaspari, S.; Silvestri, D.; Russo, B.; Marino, N.; Ubezio, P.; Pagni, F.; et al. ActivinA: A new leukemia-promoting factor conferring migratory advantage to B-cell precursor-acute lymphoblastic leukemic cells. Haematologica 2019, 104, 533–545. [Google Scholar] [CrossRef]
- Leonard, W.J.; Spolski, R. Interleukin-21: A modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 2005, 5, 688–698. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008, 26, 57–79. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Davis, M.R.; Zhu, Z.; Hansen, D.M.; Bai, Q.; Fang, Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015, 358, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chabab, G.; Bonnefoy, N.; Lafont, V. IL-21 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.H.; Van Veldhuizen, P.J. Interleukin-21: Updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert. Opin. Biol. Ther. 2010, 10, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Sarosiek, K.A.; Lossos, I.S. Interleukin 21-its potential role in the therapy of B-cell lymphomas. Leuk. Lymphoma 2017, 58, 17–29. [Google Scholar] [CrossRef]
- Shen, S.; Sckisel, G.; Sahoo, A.; Lalani, A.; Otter, D.D.; Pearson, J.; DeVoss, J.; Cheng, J.; Casey, S.C.; Case, R.; et al. Engineered IL-21 Cytokine Muteins Fused to Anti-PD-1 Antibodies Can Improve CD8+ T Cell Function and Anti-tumor Immunity. Front. Immunol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Wu, S.; Sun, R.; Tan, B.; Chen, B.; Zhou, W.; Gao, D.S.; Zhong, J.; Huang, H.; Jiang, J.; Lu, B. The Half-Life-Extended IL21 can Be Combined With Multiple Checkpoint Inhibitors for Tumor Immunotherapy. Front. Cell. Dev. Biol. 2021, 9, 779865. [Google Scholar] [CrossRef]
- Jin, H.; Carrio, R.; Yu, A.; Malek, T.R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 2004, 173, 657–665. [Google Scholar] [CrossRef]
- de Totero, D.; Meazza, R.; Zupo, S.; Cutrona, G.; Matis, S.; Colombo, M.; Balleari, E.; Pierri, I.; Fabbi, M.; Capaia, M.; et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood 2006, 107, 3708–3715. [Google Scholar] [CrossRef]
- Akamatsu, N.; Yamada, Y.; Hasegawa, H.; Makabe, K.; Asano, R.; Kumagai, I.; Murata, K.; Imaizumi, Y.; Tsukasaki, K.; Tsuruda, K.; et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Lett. 2007, 256, 196–206. [Google Scholar] [CrossRef]
- Dien Bard, J.; Gelebart, P.; Anand, M.; Zak, Z.; Hegazy, S.A.; Amin, H.M.; Lai, R. IL-21 contributes to JAK3/STAT3 activation and promotes cell growth in ALK-positive anaplastic large cell lymphoma. Am. J. Pathol. 2009, 175, 825–834. [Google Scholar] [CrossRef]
- Gelebart, P.; Zak, Z.; Anand, M.; Dien-Bard, J.; Amin, H.M.; Lai, R. Interleukin-21 effectively induces apoptosis in mantle cell lymphoma through a STAT1-dependent mechanism. Leukemia 2009, 23, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.A.; Malumbres, R.; Nechushtan, H.; Gentles, A.J.; Avisar, E.; Lossos, I.S. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood 2010, 115, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.; Rigo, V.; Ferrini, S. IL-21: A pleiotropic cytokine with potential applications in oncology. J. Immunol. Res. 2015, 2015, 696578. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lacarte, M.; Grijalba, S.C.; Melchor, J.; Arnaiz-Leché, A.; Roa, S. The PD-1/PD-L1 Checkpoint in Normal Germinal Centers and Diffuse Large B-Cell Lymphomas. Cancers 2021, 13, 4683. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Foster, D.C.; Holly, R.D.; Clegg, C.H. Interleukin-21 and the IL-21 receptor: Novel effectors of NK and T cell responses. J. Leukoc. Biol. 2002, 72, 856–863. [Google Scholar] [CrossRef]
- Moroz, A.; Eppolito, C.; Li, Q.; Tao, J.; Clegg, C.H.; Shrikant, P.A. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: Comparative evaluation of IL-2, IL-15, and IL-21. J. Immunol. 2004, 173, 900–909. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Finkelstein, S.E.; Oh, S.; Kovanen, P.E.; Hinrichs, C.S.; Pise-Masison, C.A.; Radonovich, M.F.; Brady, J.N.; Restifo, N.P.; et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005, 201, 139–148. [Google Scholar] [CrossRef]
- Konforte, D.; Paige, C.J. Interleukin-21 regulates expression of the immediate-early lytic cycle genes and proteins in Epstein-Barr Virus infected B cells. Virus Res. 2009, 144, 339–343. [Google Scholar] [CrossRef]
- Konforte, D.; Paige, C.J. Identification of cellular intermediates and molecular pathways induced by IL-21 in human B cells. J. Immunol. 2006, 177, 8381–8392. [Google Scholar] [CrossRef]
- Konforte, D.; Simard, N.; Paige, C.J. Interleukin-21 regulates expression of key Epstein-Barr virus oncoproteins, EBNA2 and LMP1, in infected human B cells. Virology 2008, 374, 100–113. [Google Scholar] [CrossRef]
- Kis, L.L.; Salamon, D.; Persson, E.K.; Nagy, N.; Scheeren, F.A.; Spits, H.; Klein, G.; Klein, E. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc. Natl. Acad. Sci. USA 2010, 107, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, C.; Fontán, L.; Melnick, A. Germinal center-derived lymphomas: The darkest side of humoral immunity. Immunol. Rev. 2019, 288, 214–239. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Fuentes, R.; Maqueda-Alfaro, R.A.; Chacón-Salinas, R.; Flores-Romo, L.; Yam-Puc, J.C. Germinal Center Cells Turning to the Dark Side: Neoplasms of B Cells, Follicular Helper T Cells, and Follicular Dendritic Cells. Front. Oncol. 2020, 10, 587809. [Google Scholar] [CrossRef] [PubMed]
- Mackrides, N.; Chapman, J.; Larson, M.C.; Ramos, J.C.; Toomey, N.; Lin, P.; Maurer, M.J.; Rafaelle, M.; Tan, Y.; Ikpatt, O.; et al. Prevalence, clinical characteristics and prognosis of EBV-positive follicular lymphoma. Am. J. Hematol. 2019, 94, E62–E64. [Google Scholar] [CrossRef]
- Granai, M.; Ambrosio, M.R.; Akarca, A.; Mundo, L.; Vergoni, F.; Santi, R.; Mancini, V.; di Stefano, G.; Amato, T.; Bellan, C.; et al. Role of Epstein-Barr virus in transformation of follicular lymphoma to diffuse large B-cell lymphoma: A case report and review of the literature. Haematologica 2019, 104, e269–e273. [Google Scholar] [CrossRef]
- Sigmund, A.M.; Kittai, A.S. Richter’s Transformation. Curr. Oncol. Rep. 2022, 24, 1081–1090. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveria Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M. The 5th edition of the World Health Organization classification of haematolymphoid tumors: Lymphoid neoplasms. Leukemia 2022, 36, 172–1748. [Google Scholar] [CrossRef]
- Chabay, P. Advances in the Pathogenesis of EBV-Associated Diffuse Large B Cell Lymphoma. Cancers 2021, 13, 2717. [Google Scholar] [CrossRef]
- Kato, H.; Karube, K.; Yamamoto, K.; Takizawa, J.; Tsuzuki, S.; Yatabe, Y.; Kanda, T.; Katayama, M.; Ozawa, Y.; Ishitsuka, K.; et al. Gene expression profiling of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly reveals alterations of characteristic oncogenetic pathways. Cancer Sci. 2014, 105, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Lin, W.; Duan, Y.; Lu, C.; Liu, W.; Su, W.; Yan, Y.; Liu, H.; Liu, L.; et al. Comprehensive Genomic Profiling of EBV-Positive Diffuse Large B-cell Lymphoma and the Expression and Clinicopathological Correlations of Some Related Genes. Front. Oncol. 2019, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, N.; Künstner, A.; Ketzer, J.; Witte, H.M.; Rausch, T.; Benes, V.; Zimmermann, J.; Gebauer, J.; Merz, H.; Bernard, V.; et al. Genomic insights into the pathogenesis of Epstein-Barr virus-associated diffuse large B-cell lymphoma by whole-genome and targeted amplicon sequencing. Blood Cancer J. 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ehlin-Henriksson, B.; Zhu, H.; Ernberg, I.; Klein, G. EBV counteracts IL-21-induced apoptosis in an EBV-positive diffuse large B-cell lymphoma cell line. Int. J. Cancer 2013, 133, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ehlin-Henriksson, B.; Zhou, X.; Zhu, H.; Ernberg, I.; Kis, L.L.; Klein, G. Epstein-Barr virus (EBV) provides survival factors to EBV(+) diffuse large B-cell lymphoma (DLBCL) lines and modulates cytokine induced specific chemotaxis in EBV(+) DLBCL. Immunology 2017, 152, 562–573. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Cai, X.; Mou, C.; Cui, X.; Zhang, Y.; Ge, F.; Dong, H.; Hao, Y.; Cai, L.; et al. IL-21 Stimulates the expression and activation of cell cycle regulators and promotes cell proliferation in EBV-positive diffuse large B cell lymphoma. Sci. Rep. 2020, 10, 12326. [Google Scholar] [CrossRef]
- Cohen, M.; Vistarop, A.G.; Huaman, F.; Narbaitz, M.; Metrebian, F.; De Matteo, E.; Preciado, M.V.; Chabay, P.A. Epstein-Barr virus lytic cycle involvement in diffuse large B cell lymphoma. Hematol. Oncol. 2018, 36, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bayda, N.; Tilloy, V.; Chaunavel, A.; Bahri, R.; Halabi, M.A.; Feuillard, J.; Jaccard, A.; Ranger-Rogez, S. Comprehensive Epstein-Barr Virus Transcriptome by RNA-Sequencing in Angioimmunoblastic T Cell Lymphoma (AITL) and Other Lymphomas. Cancers 2021, 13, 610. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef]
- Vrzalikova, K.; Pugh, M.; Mundo, L.; Murray, P. The contribution of ebv to the pathogenesis of classical hodgkin lymphoma. Ann. Lymphoma 2021, 5, 30. [Google Scholar] [CrossRef]
- Kelly, G.L.; Stylianou, J.; Rasaiyaah, J.; Wei, W.; Thomas, W.; Croom-Carter, D.; Kohler, C.; Spang, R.; Woodman, C.; Kellam, P.; et al. Different patterns of Epstein-Barr virus latency in endemic Burkitt lymphoma (BL) lead to distinct variants within the BL-associated gene expression signature. J. Virol. 2013, 87, 2882–2894. [Google Scholar] [CrossRef] [PubMed]
- Abate, F.; Ambrosio, M.R.; Mundo, L.; Laginestra, M.A.; Fuligni, F.; Rossi, M.; Zairis, S.; Gazaneo, S.; De Falco, G.; Lazzi, S.; et al. Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma. PLoS Pathog. 2015, 11, e1005158. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, H.; Lin, Z.; Wang, X.; Roberts, C.; Dong, Y.; Flemington, E. High-Throughput Sequence Analysis of Peripheral T-Cell Lymphomas Indicates Subtype-Specific Viral Gene Expression Patterns and Immune Cell Microenvironments. mSphere 2019, 4, e00248-19. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Menter, T.; Tzankov, A. Lymphomas and Their Microenvironment: A Multifaceted Relationship. Pathobiology 2019, 86, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Höpken, U.E.; Rehm, A. Targeting the Tumor Microenvironment of Leukemia and Lymphoma. Trends Cancer 2019, 5, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Tzankov, A.; Dirnhofer, S. The tumor microenvironment of lymphomas: Insights into the potential role and modes of actions of checkpoint inhibitors. Hematol. Oncol. 2021, 39, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shi, Y.; Feng, Z.; Zheng, Y.; Li, Z.; Zhao, Y.; Wang, Y. Oncogenic effects of exosomes in γ-herpesvirus-associated neoplasms. J. Cell. Physiol. 2019, 234, 19167–19179. [Google Scholar] [CrossRef]
- Iwakiri, D.; Zhou, L.; Samanta, M.; Matsumoto, M.; Ebihara, T.; Seya, T.; Imai, S.; Fujieda, M.; Kawa, K.; Takada, K. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 2009, 206, 2091–2099. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Shair, K.H.; Marquitz, A.R.; Kung, C.P.; Edwards, R.H.; Raab-Traub, N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Kawanishi, E.; Yoshida, R.; Yoshiyama, H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 2013, 87, 10334–10347. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, R.; McCormack, M.; Swanson-Mungerson, M. Epstein-Barr virus LMP2A increases IL-10 production in mitogen-stimulated primary B-cells and B-cell lymphomas. J. Gen. Virol. 2013, 94, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Sueur, C.; Lupo, J.; Mas, P.; Morand, P.; Boyer, V. Difference in cytokine production and cell cycle progression induced by Epstein-Barr virus Lmp1 deletion variants in Kmh2, a Hodgkin lymphoma cell line. Virol. J. 2014, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Philip, P.S.; Tariq, S.; Khan, G. Epstein-Barr virus-encoded small RNAs (EBERs) are present in fractions related to exosomes released by EBV-transformed cells. PLoS ONE 2014, 9, e99163. [Google Scholar] [CrossRef]
- Burassakarn, A.; Srisathaporn, S.; Pientong, C.; Wongjampa, W.; Vatanasapt, P.; Patarapadungkit, N.; Ekalaksananan, T. Exosomes-carrying Epstein-Barr virus-encoded small RNA-1 induces indoleamine 2,3-dioxygenase expression in tumor-infiltrating macrophages of oral squamous-cell carcinomas and suppresses T-cell activity by activating RIG-I/IL-6/TNF-α pathway. Oral Oncol. 2021, 117, 105279. [Google Scholar] [CrossRef]
- Vallhov, H.; Gutzeit, C.; Johansson, S.M.; Nagy, N.; Paul, M.; Li, Q.; Friend, S.; George, T.C.; Klein, E.; Scheynius, A.; et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J. Immunol. 2011, 186, 73–82. [Google Scholar] [CrossRef]
- Sato, Y.; Yaguchi, M.; Okuno, Y.; Ishimaru, H.; Sagou, K.; Ozaki, S.; Suzuki, T.; Inagaki, T.; Umeda, M.; Watanabe, T.; et al. Epstein-Barr virus tegument protein BGLF2 in exosomes released from virus-producing cells facilitates de novo infection. Cell. Commun. Signal 2022, 20, 95. [Google Scholar] [CrossRef]
- Ito, M.; Kudo, K.; Higuchi, H.; Otsuka, H.; Tanaka, M.; Fukunishi, N.; Araki, T.; Takamatsu, M.; Ino, Y.; Kimura, Y.; et al. Proteomic and phospholipidomic characterization of extracellular vesicles inducing tumor microenvironment in Epstein-Barr virus-associated lymphomas. FASEB J. 2021, 35, e21505. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Malpica, L.; Marques-Piubelli, M.L.; Beltran, B.E.; Chavez, J.C.; Miranda, R.N.; Castillo, J.J. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2022 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2022, 97, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Bao, C.; Zhou, D.; Zhu, L.; Qian, W.; Ye, X. Increased serum level of interleukin-6 correlates with negative prognostic factors in extranodal NK/T-cell lymphoma. Transl. Cancer Res. 2020, 9, 2378–2389. [Google Scholar] [CrossRef]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef]
- Calip, G.S.; Patel, P.R.; Adimadhyam, S.; Xing, S.; Wu, Z.; Sweiss, K.; Schumock, G.T.; Lee, T.A.; Chiu, B.C. Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int. J. Cancer 2018, 143, 1062–1071. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Ogden, C.A.; Pound, J.D.; Batth, B.K.; Owens, S.; Johannessen, I.; Wood, K.; Gregory, C.D. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: Implications for Burkitt’s lymphoma. J. Immunol. 2005, 174, 3015–3023. [Google Scholar] [CrossRef]
- Lo, A.K.; Dawson, C.W.; Lung, H.L.; Wong, K.L.; Young, L.S. The Role of EBV-Encoded LMP1 in the NPC Tumor Microenvironment: From Function to Therapy. Front. Oncol. 2021, 11, 640207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, Y.; Li, K.; Luo, R.; Cai, M.; Yun, J. Immunosuppressive Tumor Microenvironment and Immunotherapy of Epstein-Barr Virus-Associated Malignancies. Viruses 2022, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Hassenrück, F.; Hallek, M. Relevant Cytokines in the B Cell Lymphoma Micro-Environment. Cancers 2020, 12, 2525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W. Expression of PD-L1 in EBV-associated malignancies. Int. Immunopharmacol. 2021, 95, 107553. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Santarelli, R.; Falcinelli, L.; Gonnella, R.; Granato, M.; Di Renzo, L.; Cuomo, L.; Vitillo, M.; Faggioni, A.; Cirone, M. EBV up-regulates PD-L1 on the surface of primary monocytes by increasing ROS and activating TLR signaling and STAT3. J. Leukoc. Biol. 2018, 104, 821–832. [Google Scholar] [CrossRef]
- Cristino, A.S.; Nourse, J.; West, R.A.; Sabdia, M.B.; Law, S.C.; Gunawardana, J.; Vari, F.; Mujaj, S.; Thillaiyampalam, G.; Snell, C.; et al. EBV microRNA-BHRF1-2-5p targets the 3’UTR of immune checkpoint ligands PD-L1 and PD-L2. Blood 2019, 134, 2261–2270. [Google Scholar] [CrossRef]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2017, 8, 1932. [Google Scholar] [CrossRef]
- Ramayanti, O.; Juwana, H.; Verkuijlen, S.A.; Adham, M.; Pegtel, M.D.; Greijer, A.E.; Middeldorp, J.M. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer 2017, 140, 149–162. [Google Scholar] [CrossRef]
- Martel-Renoir, D.; Grunewald, V.; Touitou, R.; Schwaab, G.; Joab, I. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J. Gen. Virol. 1995, 76 (Pt 6), 1401–1408. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Li, F.; Xu, Y.; Wang, H.; Cheng, W.; Tang, J.; Zhou, G.; Chen, H.; Ng, M.; et al. Intermittent abortive reactivation of Epstein-Barr virus during the progression of nasopharyngeal cancer as indicated by elevated antibody levels. Oral Oncol. 2019, 93, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Caves, E.A.; Cook, S.A.; Lee, N.; Stoltz, D.; Watkins, S.; Shair, K.H.Y. Air-Liquid Interface Method To Study Epstein-Barr Virus Pathogenesis in Nasopharyngeal Epithelial Cells. mSphere 2018, 3, e00152-18. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, Z.; Wu, Y.; Dong, J.; Zhao, B.; Cheng, Y.; Huang, P.; Xu, L.; Xia, T.; Xiong, D.; et al. Comprehensive profiling of EBV gene expression in nasopharyngeal carcinoma through paired-end transcriptome sequencing. Front. Med. 2016, 10, 61–75. [Google Scholar] [CrossRef]
- Re, V.; Brisotto, G.; Repetto, O.; De Zorzi, M.; Caggiari, L.; Zanussi, S.; Alessandrini, L.; Canzonieri, V.; Miolo, G.; Puglisi, F.; et al. Overview of Epstein-Barr-Virus-Associated Gastric Cancer Correlated with Prognostic Classification and Development of Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 9400. [Google Scholar] [CrossRef]
- Luo, B.; Wang, Y.; Wang, X.F.; Liang, H.; Yan, L.P.; Huang, B.H.; Zhao, P. Expression of Epstein-Barr virus genes in EBV-associated gastric carcinomas. World J. Gastroenterol. 2005, 11, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Morgan, D.R.; Meyers, M.O.; Dominguez, R.L.; Martinez, E.; Kakudo, K.; Kuan, P.F.; Banet, N.; Muallem, H.; Woodward, K.; et al. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agent. Cancer 2012, 7, 21. [Google Scholar] [CrossRef]
- Strong, M.J.; Xu, G.; Coco, J.; Baribault, C.; Vinay, D.S.; Lacey, M.R.; Strong, A.L.; Lehman, T.A.; Seddon, M.B.; Lin, Z.; et al. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: Implications for possible immune adjuvant therapy. PLoS Pathog. 2013, 9, e1003341. [Google Scholar] [CrossRef]
- Borozan, I.; Zapatka, M.; Frappier, L.; Ferretti, V. Analysis of Epstein-Barr Virus Genomes and Expression Profiles in Gastric Adenocarcinoma. J. Virol. 2018, 92, e01239-17. [Google Scholar] [CrossRef]
- Song, L.; Song, M.; Camargo, M.C.; Van Duine, J.; Williams, S.; Chung, Y.; Kim, K.M.; Lissowska, J.; Sivins, A.; Gao, W.; et al. Identification of anti-Epstein-Barr virus (EBV) antibody signature in EBV-associated gastric carcinoma. Gastric. Cancer 2021, 24, 858–867. [Google Scholar] [CrossRef]
- Harabuchi, Y.; Takahara, M.; Kishibe, K.; Nagato, T.; Kumai, T. Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Basic Science and Clinical Progress. Front. Pediatr. 2019, 7, 141. [Google Scholar] [CrossRef]
- Montes-Mojarro, I.A.; Fend, F.; Quintanilla-Martinez, L. EBV and the Pathogenesis of NK/T Cell Lymphoma. Cancers 2021, 13, 1414. [Google Scholar] [CrossRef]
- Xiong, J.; Cui, B.W.; Wang, N.; Dai, Y.T.; Zhang, H.; Wang, C.F.; Zhong, H.J.; Cheng, S.; Ou-Yang, B.S.; Hu, Y.; et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell 2020, 37, 403–419.e406. [Google Scholar] [CrossRef]
- Peng, R.J.; Han, B.W.; Cai, Q.Q.; Zuo, X.Y.; Xia, T.; Chen, J.R.; Feng, L.N.; Lim, J.Q.; Chen, S.W.; Zeng, M.S.; et al. Genomic and transcriptomic landscapes of Epstein-Barr virus in extranodal natural killer T-cell lymphoma. Leukemia 2019, 33, 1451–1462. [Google Scholar] [CrossRef]
- Giat, E.; Ehrenfeld, M.; Shoenfeld, Y. Cancer and autoimmune diseases. Autoimmun. Rev. 2017, 16, 1049–1057. [Google Scholar] [CrossRef]
- Agrawal, M.; Shah, S.; Patel, A.; Pinotti, R.; Colombel, J.F.; Burisch, J. Changing epidemiology of immune-mediated inflammatory diseases in immigrants: A systematic review of population-based studies. J. Autoimmun. 2019, 105, 102303. [Google Scholar] [CrossRef]
- He, M.M.; Lo, C.H.; Wang, K.; Polychronidis, G.; Wang, L.; Zhong, R.; Knudsen, M.D.; Fang, Z.; Song, M. Immune-Mediated Diseases Associated With Cancer Risks. JAMA Oncol. 2022, 8, 209–219. [Google Scholar] [CrossRef]
- Kleinstern, G.; Maurer, M.J.; Liebow, M.; Habermann, T.M.; Koff, J.L.; Allmer, C.; Witzig, T.E.; Nowakowski, G.S.; Micallef, I.N.; Johnston, P.B.; et al. History of autoimmune conditions and lymphoma prognosis. Blood Cancer J. 2018, 8, 73. [Google Scholar] [CrossRef]
- Miller, E. Autoimmunity and lymphoma: A brief review. J. Rheum. Dis. Treat. 2018, 4, 62. [Google Scholar]
- Balandraud, N.; Roudier, J. Epstein-Barr virus and rheumatoid arthritis. Jt. Bone Spine 2018, 85, 165–170. [Google Scholar] [CrossRef]
- Houen, G.; Trier, N.H. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front. Immunol. 2020, 11, 587380. [Google Scholar] [CrossRef]
- Barcelos, F.; Martins, C.; Monteiro, R.; Cardigos, J.; Prussiani, T.; Sítima, M.; Alves, N.; Vaz-Patto, J.; Cunha-Branco, J.; Borrego, L.M. Association between EBV serological patterns and lymphocytic profile of SjS patients support a virally triggered autoimmune epithelitis. Sci. Rep. 2021, 11, 4082. [Google Scholar] [CrossRef]
- Jog, N.R.; James, J.A. Epstein Barr Virus and Autoimmune Responses in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 623944. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Williams, M.V.; Cox, B.; Ariza, M.E. Herpesviruses dUTPases: A new family of pathogen-associated molecular pattern (PAMP) proteins with implications for human disease. Pathogens 2016, 6, 2. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Tengvall, K.; Huang, J.; Hellström, C.; Kammer, P.; Biström, M.; Ayoglu, B.; Lima Bomfim, I.; Stridh, P.; Butt, J.; Brenner, N.; et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. USA 2019, 116, 16955–16960. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Robinson, W.H.; Steinman, L. Epstein-Barr virus and multiple sclerosis. Science 2022, 375, 264–265. [Google Scholar] [CrossRef]
- Munroe, M.E.; Anderson, J.R.; Gross, T.F.; Stunz, L.L.; Bishop, G.A.; James, J.A. Epstein-Barr Functional Mimicry: Pathogenicity of Oncogenic Latent Membrane Protein-1 in Systemic Lupus Erythematosus and Autoimmunity. Front. Immunol. 2020, 11, 606936. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yang, J.Q.; Kim, P.J.; Singh, R.R. Homeostatic regulation of marginal zone B cells by invariant natural killer T cells. PLoS ONE 2011, 6, e26536. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P. Regulation of B-cell responses by Toll-like receptors. Immunology 2012, 136, 370–379. [Google Scholar] [CrossRef] [PubMed]



| Gene | Function | Phase | References |
|---|---|---|---|
| BZLF1 | Transcriptional Activator | IE | [28,31,33] |
| BRLF1 | Transcriptional Activator | IE | [33] |
| BMLF1 | Transcriptional Activator | E | [13,35] |
| BCRF1 | Immune Evasion | L | [28,33] |
| BNLF2a | Immune Evasion | E | [33] |
| BHRF1 | BCL2 homolog | E | [13,30,33] |
| BALF1 | BCL2 homolog | E | [30,33] |
| BLLF3 | TFH | E | [37] |
| BILF1 | Immune evasion | L | [37] |
| BDLF3 | Immune Evasion | L | [37] |
| BOLF1 | Immune Evasion | L | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, M.V.; Mena-Palomo, I.; Cox, B.; Ariza, M.E. EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies. Cancers 2023, 15, 855. https://doi.org/10.3390/cancers15030855
Williams MV, Mena-Palomo I, Cox B, Ariza ME. EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies. Cancers. 2023; 15(3):855. https://doi.org/10.3390/cancers15030855
Chicago/Turabian StyleWilliams, Marshall V., Irene Mena-Palomo, Brandon Cox, and Maria Eugenia Ariza. 2023. "EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies" Cancers 15, no. 3: 855. https://doi.org/10.3390/cancers15030855
APA StyleWilliams, M. V., Mena-Palomo, I., Cox, B., & Ariza, M. E. (2023). EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies. Cancers, 15(3), 855. https://doi.org/10.3390/cancers15030855







