Prostate Cancer—PET Imaging Update
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Biochemical Recurrence of Prostate Cancer
1.2. Earlier Generation Gamma-Emitting Planar/SPECT Agents
1.3. Prostate Targeted and Non-Targeted PET/CT Agents for Imaging
1.4. C-11 and F-18 Choline
1.5. Fluorine-18 Fluciclovine
1.6. Gallium-68 Prostate-Specific-Membrane-Antigen-11
1.7. Fluorine-18 DCFPyL
- -
- Fluorine-18 is cyclotron-produced and is more cost-effective for mass production.
- -
- Gallium-68′s physical half-life is shorter than Fluorine-18′s (68 min versus 110 min), limiting off-site transportation and the ability to perform delayed imaging [17].
- -
- The positron yield of Gallium-68 is lower than Fluorine-18 (89.14% vs. 96.86%).
- -
- Gallium-68 has higher positron energy resulting in lower spatial resolution.
1.8. PET Imaging Protocols
- -
- Avoid exercise for one day before the study.
- -
- NPO for >4 h before the study.
- -
- No voiding within 1 h of the scan (voiding can lead to early radiotracer excretion into the bladder [33]).
- -
- Position the patient supine with arms to the side and inject 370 MBq/10 mCi, preferably into right-sided intravenous access to avoid a false positive Virchow’s node on the left.
- -
- Flush with 0.9% normal saline.
- -
- Reposition patients with arms above their head and scan with low-dose CT for anatomic correlation.
- -
- Start the PET scan 3–5 min after the injection.
- -
- At our institution, the reconstruction of fused maximum intensity projections (MIPs) are obtained in the coronal and sagittal planes, both of the entire body (vertex to knees) and of a narrow field-of-view of the pelvis.
- -
- The patient has no specific activity or NPO status requirements; however they are encouraged to drink fluids (approximately 500 mL) 2 h before the scan, to improve hydration status and the subsequent clearance of radiotracer. The patient should still void before the scan.
- -
- After radiotracer administration, the PET scan is started for 50–100 min for Ga-68 PSMA-11, and 60–120 min for F-18 DCFPyL (60 min at our institution).
1.9. Image Interpretation
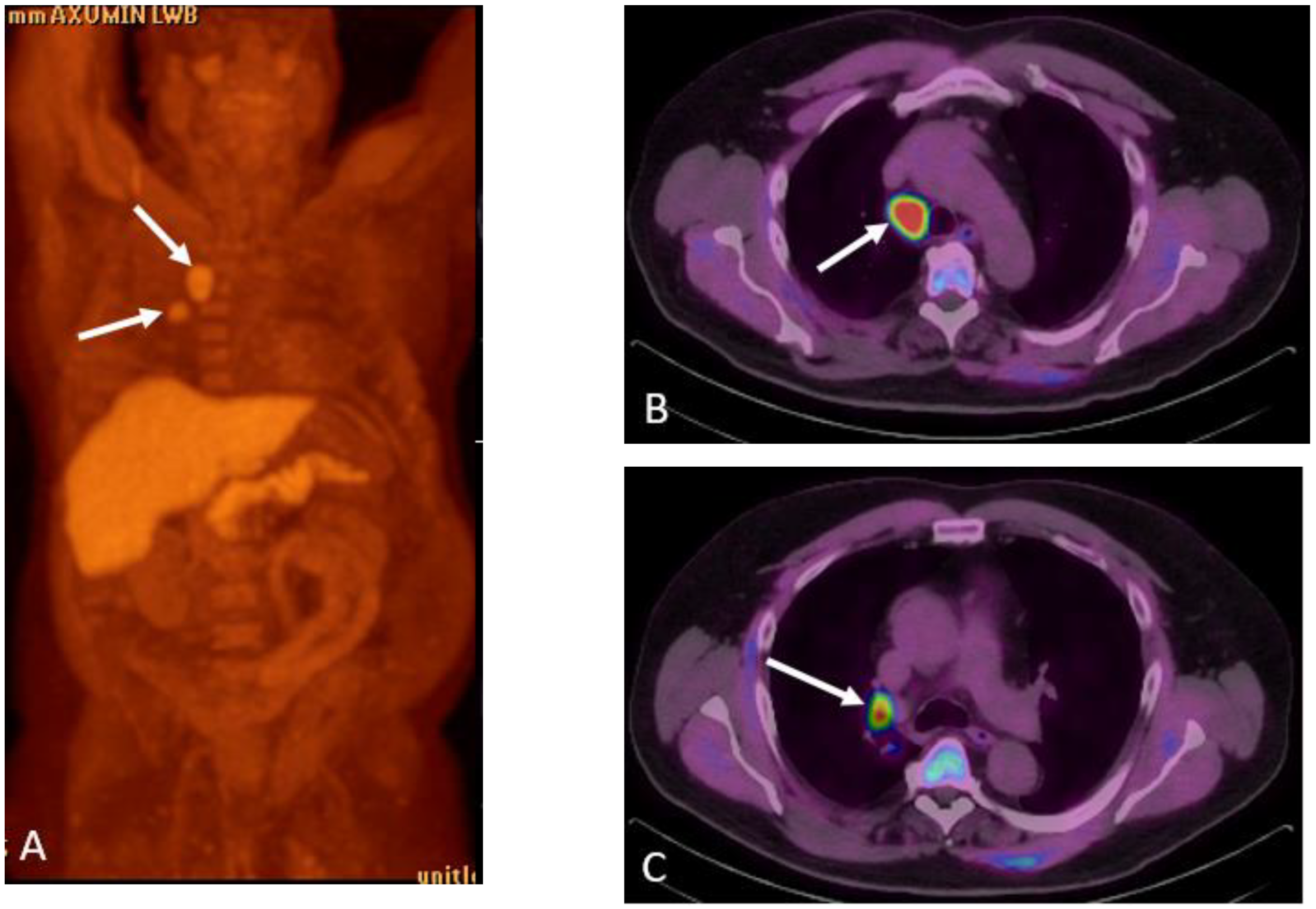
1.9.1. Axumin PET:
Normal Physiologic Distribution
Detection of Loco-Regional Disease and Distant Metastases
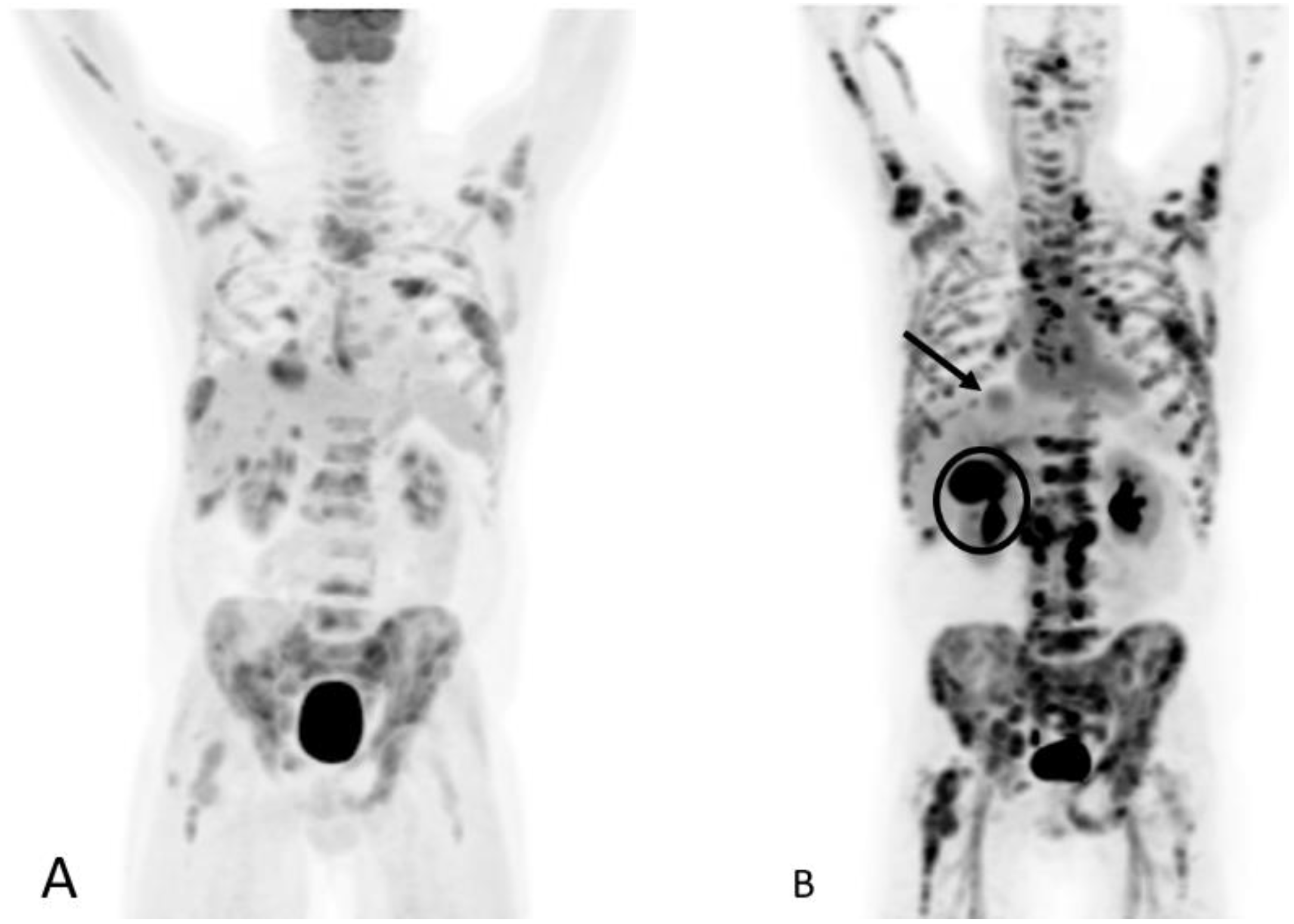
1.9.2. PSMA PET
Normal Physiologic Distribution
Detection of Loco-Regional Disease and Distant Metastases
1.9.3. Pearls and Pitfalls
1.9.4. Future PET Radiotracers
16β-18F—5α-dihydrotestosterone (18-F-FDHT)
1.9.5. Miscellaneous Radiotracers
2. Discussion
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Macias, V.; Tangella, K.; Kajdacsy-Balla, A.; Popescu, G. Prediction of prostate cancer recurrence using quantitative phase imaging. Sci. Rep. 2015, 5, 9976. [Google Scholar] [CrossRef] [PubMed]
- Un, S.; Turk, H.; Koca, O.; Divrik, R.T.; Zorlu, F. Factors determining biochemical recurrence in low-risk prostate cancer patients who underwent radical prostatectomy. Turk. J. Urol. 2015, 41, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- EUSA Pharma (USA), Inc. ProstaScint® Kit (Capromab Pendetide) [Package Insert]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103608s5043lbl.pdf (accessed on 25 September 2022).
- Barren, R.J., III; Holmes, E.H.; Boynton, A.L.; Misrock, S.L.; Murphy, G.P. Monoclonal antibody 7E11.C5 staining of viable LNCaP cells. Prostate 1997, 30, 65–68. [Google Scholar] [CrossRef]
- Belhocine, T.; Rachinsky, I.; Akincioglu, C.; Gambhir, S.; Wilcox, B.; Vezina, W.; Stitt, L.; Driedger, A.; Urbain, J.-L. How Useful is an Integrated SPECT/CT in Clinical Setting and Research?: Evaluation of a Low Radiation Dose 4 Slice System. Open Med. Imaging J. 2008, 2, 80–108. [Google Scholar] [CrossRef]
- Wong, T.Z.; Turkington, T.G.; Polascik, T.J.; Coleman, R.E. ProstaScint (capromab pendetide) imaging using hybrid gamma camera-CT technology. Am. J. Roentgenol. 2005, 184, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sodee, D.B.; Malguria, N.; Faulhaber, P.; Resnick, M.I.; Albert, J.; Bakale, G. Multicenter ProstaScint imaging findings in 2154 patients with prostate cancer. The ProstaScint Imaging Centers. Urology 2000, 56, 988–993. [Google Scholar] [CrossRef]
- Raj, G.V.; Partin, A.W.; Polascik, T.J. Clinical utility of indium 111-capromab pendetide immunoscintigraphy in the detection of early, recurrent prostate carcinoma after radical prostatectomy. Cancer 2002, 94, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hovels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wallitt, K.L.; Khan, S.R.; Dubash, S.; Tam, H.H.; Khan, S.; Barwick, T.D. Clinical PET Imaging in Prostate Cancer. Radiographics 2017, 37, 1512–1536. [Google Scholar] [CrossRef] [PubMed]
- Pernthaler, B.; Kulnik, R.; Gstettner, C.; Salamon, S.; Aigner, R.M.; Kvaternik, H. A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin. Nucl. Med. 2019, 44, e566–e573. [Google Scholar] [CrossRef]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Kesch, C.; Kratochwil, C.; Mier, W.; Kopka, K.; Giesel, F.L. 68Ga or 18F for Prostate Cancer Imaging? J. Nucl. Med. 2017, 58, 687–688. [Google Scholar] [CrossRef]
- Piron, S.; Verhoeven, J.; Vanhove, C.; De Vos, F. Recent advancements in 18F-labeled PSMA targeting PET radiopharmaceuticals. Nucl. Med. Biol. 2022, 106–107, 29–51. [Google Scholar] [CrossRef]
- Welle, C.L.; Cullen, E.L.; Peller, P.J.; Lowe, V.J.; Murphy, R.C.; Johnson, G.B.; Binkovitz, L.A. (1)(1)C-Choline PET/CT in Recurrent Prostate Cancer and Nonprostatic Neoplastic Processes. Radiographics 2016, 36, 279–292. [Google Scholar] [CrossRef]
- Murphy, R.C.; Kawashima, A.; Peller, P.J. The utility of 11C-choline PET/CT for imaging prostate cancer: A pictorial guide. Am. J. Roentgenol. 2011, 196, 1390–1398. [Google Scholar] [CrossRef]
- Mayo Clinic PET Radiochemistry Facility. Choline C 11 [Package Insert]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203155s000lbl.pdf (accessed on 22 September 2022).
- Bouchelouche, K.; Tagawa, S.T.; Goldsmith, S.J.; Turkbey, B.; Capala, J.; Choyke, P. PET/CT Imaging and Radioimmunotherapy of Prostate Cancer. Semin. Nucl. Med. 2011, 41, 29–44. [Google Scholar] [CrossRef]
- Middendorp, M.; Maute, L.; Sauter, B.; Vogl, T.J.; Grunwald, F. Initial experience with 18F-fluoroethylcholine PET/CT in staging and monitoring therapy response of advanced renal cell carcinoma. Ann. Nucl. Med. 2010, 24, 441–446. [Google Scholar] [CrossRef]
- Fallanca, F.; Picchio, M.; Spinapolice, E.G.; Ugolini, C.; Proietti, A.; Messa, C. Imaging of a thymoma incidentally detected by C-11 choline PET/CT. Clin. Nucl. Med. 2011, 36, 134–135. [Google Scholar] [CrossRef]
- Garzon, J.G.; Bassa, P.; Moragas, M.; Soler, M.; Riera, E. Incidental diagnosis of diffuse large B-cell lymphoma by 11C-choline PET/CT in a patient with biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2014, 39, 742–743. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Castellucci, P.; Balduzzi, S.; Herrmann, K.; Krause, B.J.; Oyen, W.; Chiti, A. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 55–69. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer With Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef]
- Gusman, M.; Aminsharifi, J.A.; Peacock, J.G.; Anderson, S.B.; Clemenshaw, M.N.; Banks, K.P. Review of 18F-Fluciclovine PET for Detection of Recurrent Prostate Cancer. Radiographics 2019, 39, 822–841. [Google Scholar] [CrossRef]
- Blue Earth Diagnostics Ltd. Fluciclovine F 18 (Axumin) [Package Insert]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208054s034lbl.pdf (accessed on 22 September 2022).
- Evans, J.C.; Malhotra, M.; Cryan, J.F.; O’Driscoll, C.M. The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br. J. Pharmacol. 2016, 173, 3041–3079. [Google Scholar] [CrossRef]
- Hofman, M.S. Prostate-Specific Membrane Antigen: The Target of the Decade, from Biochemical Recurrence to Widespread Adoption. J. Nucl. Med. 2020, 61, 246S–247S. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Tade, F.I.; Sajdak, R.A.; Gabriel, M.; Wagner, R.H.; Savir-Baruch, B. Best Practices for 18F-Fluciclovine PET/CT Imaging of Recurrent Prostate Cancer: A Guide for Technologists. J. Nucl. Med. Technol. 2019, 47, 282–287. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sorensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.M.; Barentsz, J.O.; Hambrock, T.; Yakar, D.; Somford, D.M.; Heijmink, S.W.; Scheenen, T.W.; Vos, P.C.; Huisman, H.; van Oort, I.M.; et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology 2011, 261, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Akin-Akintayo, O.O.; Jani, A.B.; Odewole, O.; Tade, F.I.; Nieh, P.T.; Master, V.A.; Bellamy, L.M.; Halkar, R.K.; Zhang, C.; Chen, Z.; et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin. Nucl. Med. 2017, 42, e22–e28. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Bagni, O.; Crisafulli, C.; Cerio, I.; Brunotti, G.; Chiaravalloti, A.; Schillaci, O.; Dore, F. Detection Rate and Clinical Impact of PET/CT with 18F-FACBC in Patients with Biochemical Recurrence of Prostate Cancer: A Retrospective Bicentric Study. Biomedicines 2022, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Abiodun-Ojo, O.A.; Jani, A.B.; Schuster, D.M. Clinical utility of 18F-Fluciclovine PET/CT in recurrent prostate cancer with very low (≤0.3 ng/mL) prostate-specific antigen levels. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 406–414. [Google Scholar] [PubMed]
- Songmen, S.; Nepal, P.; Olsavsky, T.; Sapire, J. Axumin Positron Emission Tomography: Novel Agent for Prostate Cancer Biochemical Recurrence. J. Clin. Imaging Sci. 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Mena, E.; Shih, J.; Pinto, P.A.; Merino, M.J.; Lindenberg, M.L.; Bernardo, M.; McKinney, Y.L.; Adler, S.; Owenius, R.; et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology 2014, 270, 849–856. [Google Scholar] [CrossRef]
- Mena, E.; Black, P.C.; Rais-Bahrami, S.; Gorin, M.; Allaf, M.; Choyke, P. Novel PET imaging methods for prostate cancer. World J. Urol. 2021, 39, 687–699. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Halkar, R.K.; Master, V.A.; Nye, J.A.; Odewole, O.A.; Osunkoya, A.O.; et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Queiroz, M.A.; Nunes, R.F.; Viana, P.C.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Revisiting Prostate Cancer Recurrence with PSMA PET: Atlas of Typical and Atypical Patterns of Spread. Radiographics 2019, 39, 186–212. [Google Scholar] [CrossRef]
- Giesel, F.L.; Sterzing, F.; Schlemmer, H.P.; Holland-Letz, T.; Mier, W.; Rius, M.; Afshar-Oromieh, A.; Kopka, K.; Debus, J.; Haberkorn, U.; et al. Intra-individual comparison of 68Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Sprute, K.; Kramer, V.; Koerber, S.A.; Meneses, M.; Fernandez, R.; Soza-Ried, C.; Eiber, M.; Weber, W.A.; Rauscher, I.; Rahbar, K.; et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J. Nucl. Med. 2021, 62, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Saule, L.; Radzina, M.; Liepa, M.; Roznere, L.; Kalnina, M.; Lioznovs, A.; Mamis, E.; Mikelsone, M.; Biederer, J.; Vjaters, E. Diagnostic scope of 18F-PSMA-1007 PET/CT: Comparison with multiparametric MRI and bone scintigraphy for the assessment of early prostate cancer recurrence. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 395–405. [Google Scholar] [PubMed]
- Guler, O.C.; Engels, B.; Onal, C.; Everaert, H.; Van den Begin, R.; Gevaert, T.; de Ridder, M. The feasibility of prostate-specific membrane antigen positron emission tomography(PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin. Transl. Oncol. 2018, 20, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Demirci, E.; Sahin, O.E.; Ocak, M.; Akovali, B.; Nematyazar, J.; Kabasakal, L. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl. Med. Commun. 2016, 37, 1169–1179. [Google Scholar] [CrossRef]
- Chen, Y.; Sawyers, C.L.; Scher, H.I. Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 2008, 8, 440–448. [Google Scholar] [CrossRef]
- Beattie, B.J.; Smith-Jones, P.M.; Jhanwar, Y.S.; Schoder, H.; Schmidtlein, C.R.; Morris, M.J.; Zanzonico, P.; Squire, O.; Meirelles, G.S.; Finn, R.; et al. Pharmacokinetic assessment of the uptake of 16β-18F-fluoro-5α-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J. Nucl. Med. 2010, 51, 183–192. [Google Scholar] [CrossRef]
- Larson, S.M.; Morris, M.; Gunther, I.; Beattie, B.; Humm, J.L.; Akhurst, T.A.; Finn, R.D.; Erdi, Y.; Pentlow, K.; Dyke, J.; et al. Tumor localization of 16β-18F-fluoro-5α-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J. Nucl. Med. 2004, 45, 366–373. [Google Scholar]
- Rathkopf, D.E.; Morris, M.J.; Fox, J.J.; Danila, D.C.; Slovin, S.F.; Hager, J.H.; Rix, P.J.; Chow Maneval, E.; Chen, I.; Gonen, M.; et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2013, 31, 3525–3530. [Google Scholar] [CrossRef]
- Fox, J.J.; Gavane, S.C.; Blanc-Autran, E.; Nehmeh, S.; Gonen, M.; Beattie, B.; Vargas, H.A.; Schoder, H.; Humm, J.L.; Fine, S.W.; et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgen Receptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Minamimoto, R.; Macke, H.; Iagaru, A.H. Bombesin-Targeted PET of Prostate Cancer. J. Nucl. Med. 2016, 57, 67S–72S. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef]
- Kahkonen, E.; Jambor, I.; Kemppainen, J.; Lehtio, K.; Gronroos, T.J.; Kuisma, A.; Luoto, P.; Sipila, H.J.; Tolvanen, T.; Alanen, K.; et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef]
- Almasi, C.E.; Brasso, K.; Iversen, P.; Pappot, H.; Hoyer-Hansen, G.; Dano, K.; Christensen, I.J. Prognostic and predictive value of intact and cleaved forms of the urokinase plasminogen activator receptor in metastatic prostate cancer. Prostate 2011, 71, 899–907. [Google Scholar] [CrossRef]
- Persson, M.; Skovgaard, D.; Brandt-Larsen, M.; Christensen, C.; Madsen, J.; Nielsen, C.H.; Thurison, T.; Klausen, T.L.; Holm, S.; Loft, A.; et al. First-in-human uPAR PET: Imaging of Cancer Aggressiveness. Theranostics 2015, 5, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Doran, M.G.; Watson, P.A.; Cheal, S.M.; Spratt, D.E.; Wongvipat, J.; Steckler, J.M.; Carrasquillo, J.A.; Evans, M.J.; Lewis, J.S. Annotating STEAP1 regulation in prostate cancer with 89Zr immuno-PET. J. Nucl. Med. 2014, 55, 2045–2049. [Google Scholar] [CrossRef]
- Hofmann, M.R.; Hussain, M.; Dehm, S.M.; Beltran, H.; Wyatt, A.W.; Halabi, S.; Sweeney, C.; Scher, H.I.; Ryan, C.J.; Feng, F.Y.; et al. Prostate Cancer Foundation Hormone-Sensitive Prostate Cancer Biomarker Working Group Meeting Summary. Urology 2020, 155, 165–171. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Emmett, L.; Papa, N.; Buteau, J.; Ho, B.; Liu, V.; Roberts, M.; Thompson, J.; Moon, D.; Sheehan-Dare, G.; Alghazo, O.; et al. The PRIMARY Score: Using Intraprostatic 68Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar] [CrossRef]
- Hagens, M.J.; van Leeuwen, P.J. A Future Prebiopsy Imaging-guided Pathway to Safely Omit Systematic Biopsies and Prevent Diagnosis of Indolent Prostate Cancer. Eur. Urol. 2021, 80, 690–692. [Google Scholar] [CrossRef]
- Yadav, D.; Hwang, H.; Qiao, W.; Upadhyay, R.; Chapin, B.F.; Tang, C.; Aparicio, A.; Lopez-Olivo, M.A.; Kang, S.K.; Macapinlac, H.A.; et al. (18)F-Fluciclovine versus PSMA PET Imaging in Primary Tumor Detection during Initial Staging of High-Risk Prostate Cancer: A Systematic Review and Meta-Analysis. Radiol. Imaging Cancer 2022, 4, e210091. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Frank, S.; Meirovitz, A.; Nechushtan, H.; Orevi, M. PET/CT With 68Ga-DOTA-TATE for Diagnosis of Neuroendocrine: Differentiation in Patients With Castrate-Resistant Prostate Cancer. Clin. Nucl. Med. 2017, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef]
- Huang, M.M.; Patel, H.D.; Wainger, J.J.; Su, Z.T.; Becker, R.E.N.; Han, M.; Pierorazio, P.M.; Allaf, M.E. Comparison of Perioperative and Pathologic Outcomes Between Single-port and Standard Robot-assisted Radical Prostatectomy: An Analysis of a High-volume Center and the Pooled World Experience. Urology 2021, 147, 223–229. [Google Scholar] [CrossRef]
- Malaspina, S.; Ettala, O.; Tolvanen, T.; Rajander, J.; Eskola, O.; Bostrom, P.J.; Kemppainen, J. Flare on [18F]PSMA-1007 PET/CT after short-term androgen deprivation therapy and its correlation to FDG uptake: Possible marker of tumor aggressiveness in treatment-naive metastatic prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]





















| PET Agents | Fluorine-18 Fluciclovine | Gallium-68 PSMA-11 | 18F DCFPyL |
|---|---|---|---|
| FDA Approval Date | 27 May 2016 | 1 December 2020 | 27 May 2021 |
| Physical Half-Life | 110 min | 68 min | 110 min |
| Mechanism of Action | Amino acid transport | PSMA binding | PSMA binding |
| Patient Preparation | Avoid exercise for one day prior to the study. NPO for at least 4 h prior to the study. | Gentle hydration and void prior to imaging. | Gentle hydration and void prior to imaging. |
| Administered Activity MBq (mSv) | 370 (10) | 111–259 (3–7) | 333 (9) |
| Effective dose (mSv) | 8 | 1.9–4.4 | 4.3 |
| Uptake period | 3–5 min | 50–100 min | 60–120 min (*package insert suggests >90 min although literature has demonstrated increased lesion detection at delayed time point). |
| Acquisition | -Thighs to vertex. -Preferred injection is the right upper extremity to avoid a false positive Virchow’s node on the left. -Start PET scan 3–5 min after the injection. | -Thighs to vertex. -Preferred injection is the right upper extremity to avoid a false positive Virchow’s node on the left. -Start PET scan 50–100 min after the injection. | -Thighs to vertex. -Preferred injection is the right upper extremity to avoid a false positive Virchow’s node on the left. -Start PET scan 50–100 min after the injection. |
| PET Agents | Fluorine-18 Fluciclovine | PSMA-Ligand Agents (18F DCFPyL, Ga-68 PSMA-11) |
|---|---|---|
| Physiologic Distribution | Pancreas, liver, salivary glands, pituitary glands, small bowel, red marrow, and muscles | Lacrimal glands, salivary glands, liver, spleen, small intestine, colon, rectum, and kidneys |
| PET Agents | Advantages | Disadvantages | Comments |
|---|---|---|---|
| 11C or 18F Choline | Min urinary excretion availability | Lower sensitivity Non-specific uptake | 11C Choline approved by the US FDA |
| 18F FACBC | Improved sensitivity local recurrence | Lower sensitivity Non-specific uptake | 18FACBC is approved by US FDA (Axumin) |
| 68Ga or 18F PSMA | High sensitivity High specificity | False positive uptake | Available in the US, Current gold standard |
| 18F DHT | Reports AR activity | Difficult synthesis Noisy scans | Not commercially available |
| 68Ga or 18F Bombesin | High sensitivity | False positive uptake | Not commercially available |
| 18F FDG | Prognostic indicator Widely available | Insensitive in early disease | 18F FDG is approved by US FDA |
| Modality | CT Pelvis and Bone Scintigraphy | Multiparametric Pelvic MRI | PSMA PET/CT | PSMA PET/MRI |
|---|---|---|---|---|
| Advantages | -Availability and cost | -T staging -No ionizing radiation | -Extrapelvic metastases -N staging | -Superior TNM staging and tissue characterization |
| Disadvantages | -Extrapelvic non-osseous metastases -Limited specificity of bone scintigraphy -Iodinated contrast exposure | -N staging reliant on morphology -Extrapelvic metastases -Gadolinium contrast exposure | -Less optimal T staging -Ionizing radiation | -Limited availability -Ionizing radiation |
| Estimated Dose (mSv) | ~8–10 | 0 | ~12 | ~4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jetty, S.; Loftus, J.R.; Patel, A.; Gupta, A.; Puri, S.; Dogra, V. Prostate Cancer—PET Imaging Update. Cancers 2023, 15, 796. https://doi.org/10.3390/cancers15030796
Jetty S, Loftus JR, Patel A, Gupta A, Puri S, Dogra V. Prostate Cancer—PET Imaging Update. Cancers. 2023; 15(3):796. https://doi.org/10.3390/cancers15030796
Chicago/Turabian StyleJetty, Sankarsh, James Ryan Loftus, Abhinav Patel, Akshya Gupta, Savita Puri, and Vikram Dogra. 2023. "Prostate Cancer—PET Imaging Update" Cancers 15, no. 3: 796. https://doi.org/10.3390/cancers15030796
APA StyleJetty, S., Loftus, J. R., Patel, A., Gupta, A., Puri, S., & Dogra, V. (2023). Prostate Cancer—PET Imaging Update. Cancers, 15(3), 796. https://doi.org/10.3390/cancers15030796






