Resection of Low-Grade Gliomas in the Face Area of the Primary Motor Cortex and Neurological Outcome
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
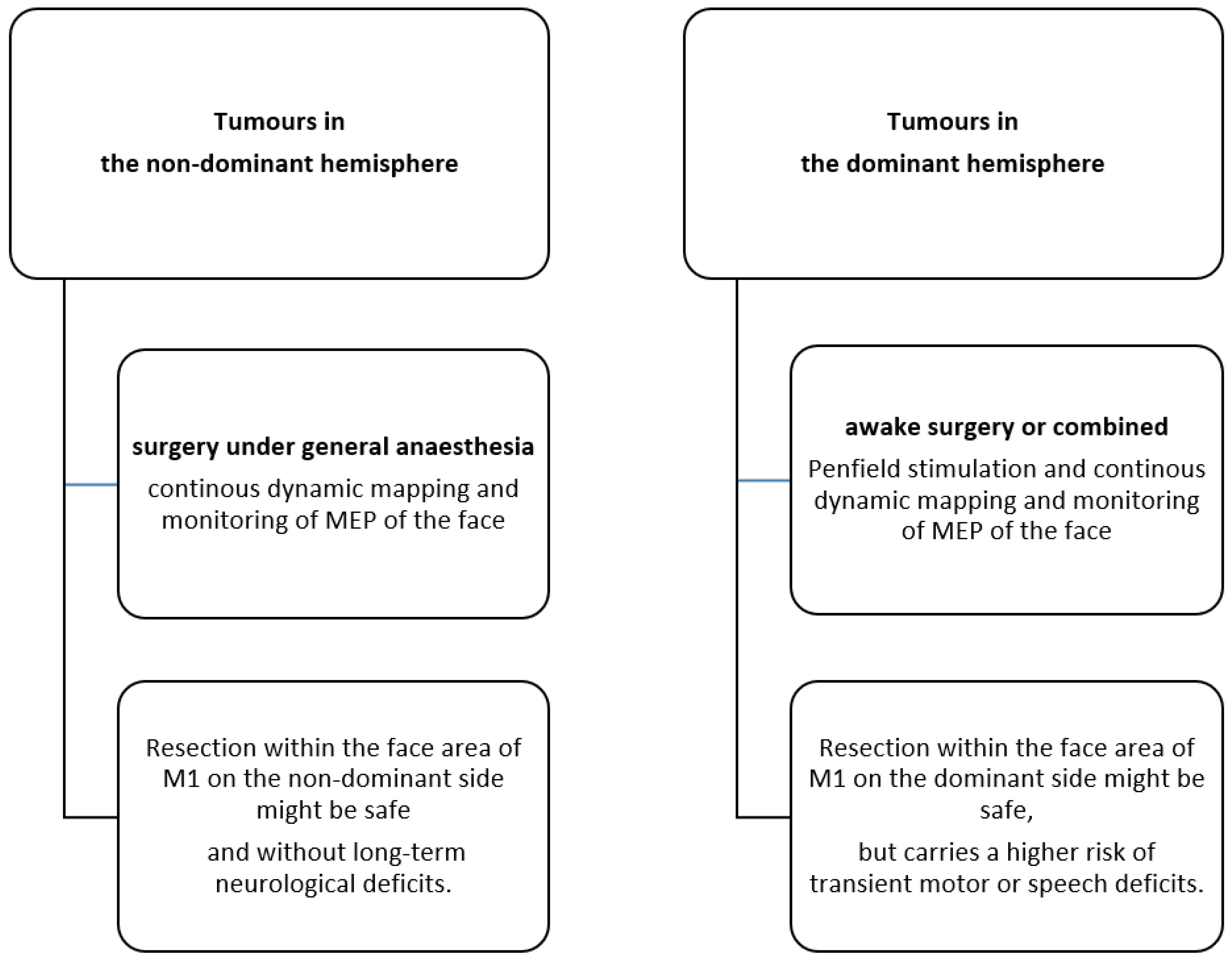
2.2. Surgical Procedure and Intraoperative Neurophysiological Monitoring
2.3. Postoperative Outcome and Clinical Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Population
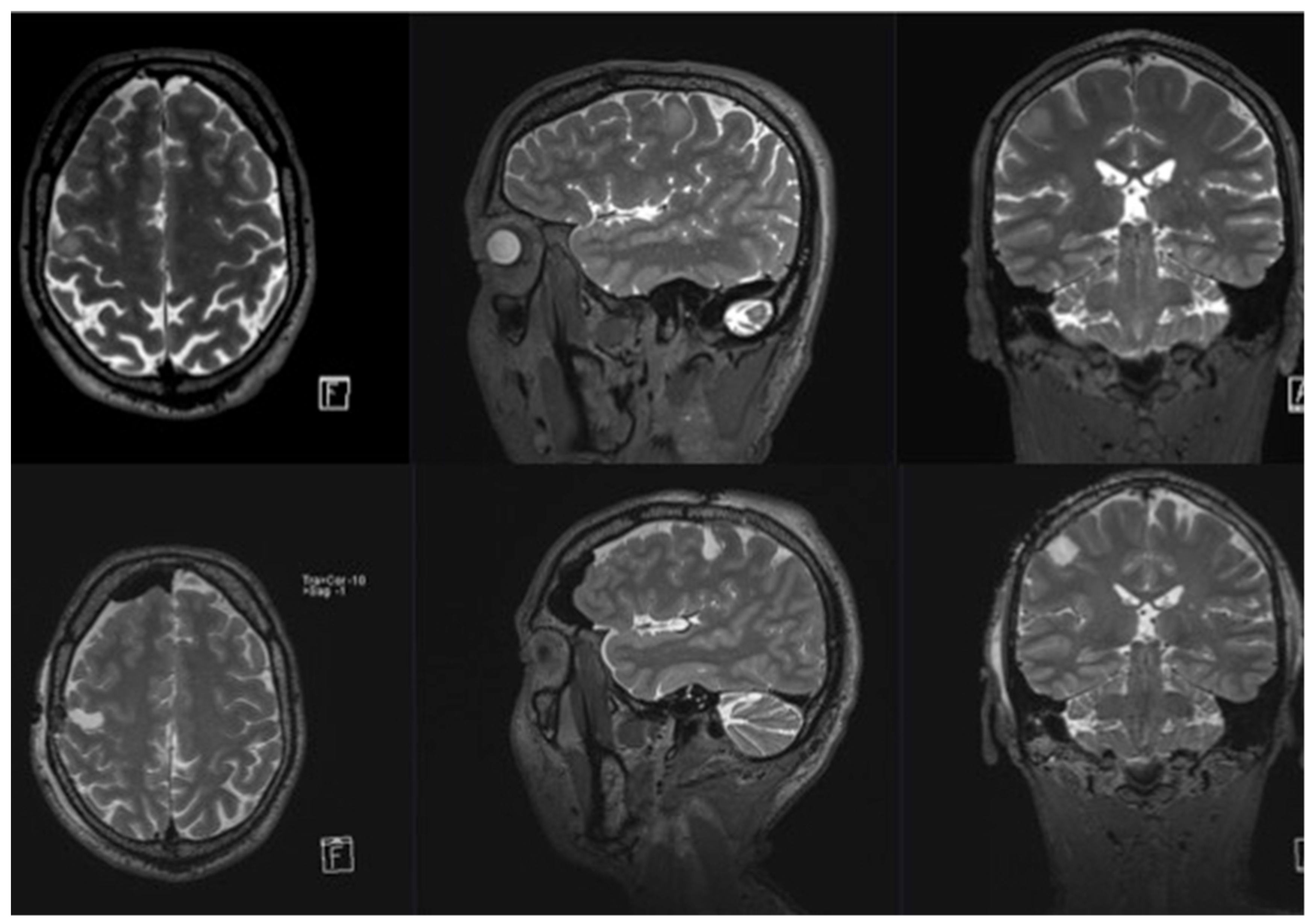
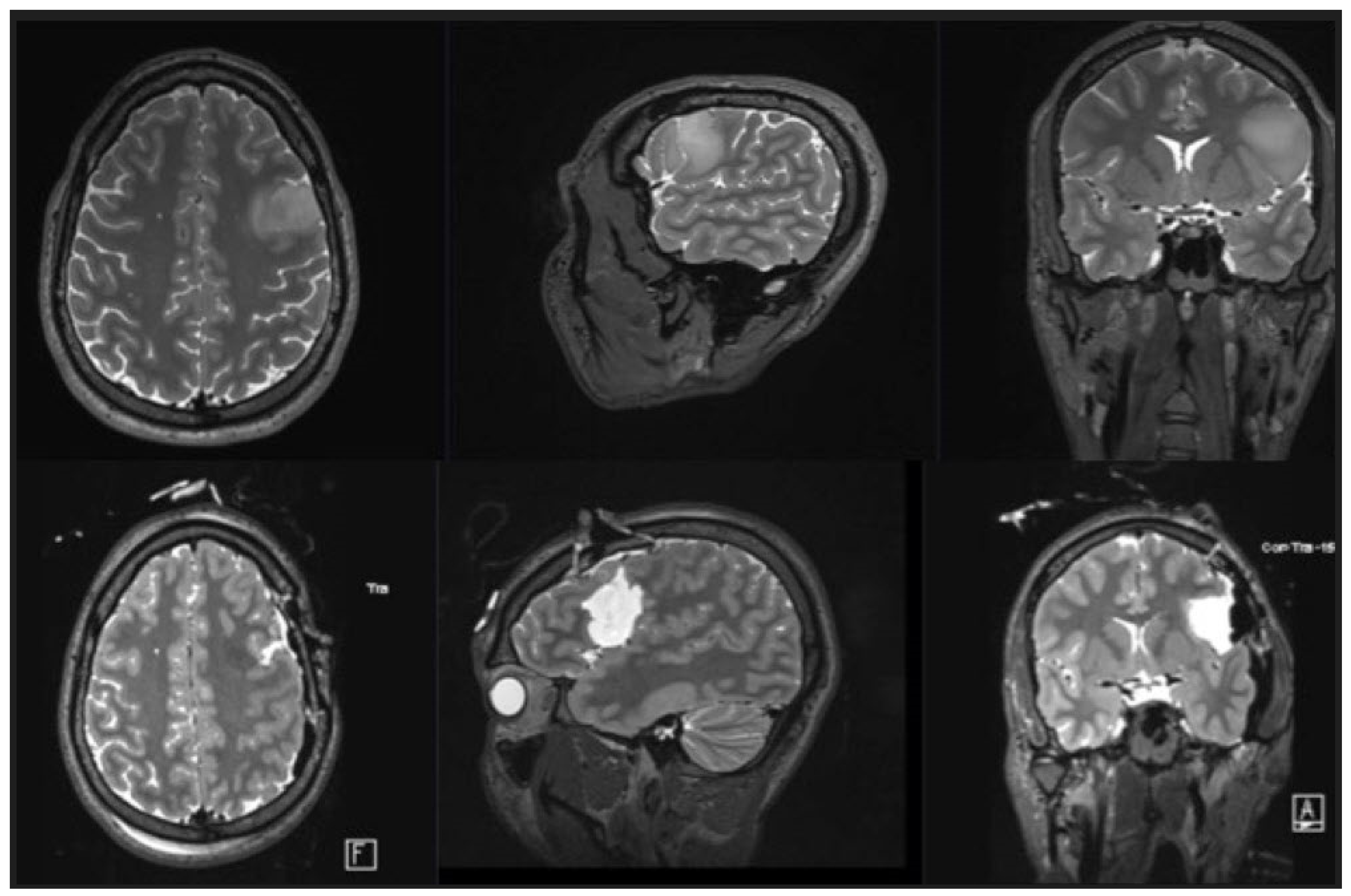
3.2. Intraoperative Findings
3.3. Clinical Findings
3.3.1. Facial Paresis
3.3.2. Aphasia
3.3.3. Dysarthria
3.3.4. Dysphagia
4. Discussion
4.1. Why Do All Patients Recover from Facial Palsy?
4.2. Bilateral Innervation Versus Plasticity
4.2.1. Bilateral Innervation
4.2.2. Imbalance in Inhibition and Excitation
4.2.3. Plasticity
4.2.4. Patient Age and the Likelihood of Recovery—Is There Any Connection?
4.3. The Role of Dominant Versus Non-Dominant Tumour Location
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carstam, L.; Smits, A.; Milos, P.; Corell, A.; Henriksson, R.; Bartek, J., Jr.; Jakola, A.S. Neurosurgical patterns of care for diffuse low-grade gliomas in Sweden between 2005 and 2015. Neurooncol. Pract. 2019, 6, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.B.; Afra, D.; Cornu, P.; Bleehan, N.; Schraub, S.; De Witte, O.; Darcel, F.; Stenning, S.; Pierart, M.; Van Glabbeke, M. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: An interim analysis. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 316–324. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Castellano, A.; Bailo, M.; Cicone, F.; Carideo, L.; Quartuccio, N.; Mortini, P.; Falini, A.; Cascini, G.L.; Minniti, G. Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Pintea, B.; Baumert, B.; Kinfe, T.M.; Gousias, K.; Parpaley, Y.; Boström, J.P. Early motor function after local treatment of brain metastases in the motor cortex region with stereotactic radiotherapy/radiosurgery or microsurgical resection: A retrospective study of two consecutive cohorts. Radiat. Oncol. 2017, 12, 177. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Gerbella, M.; Coudé, G.; Rozzi, S. Two different mirror neuron networks: The sensorimotor (hand) and limbic (face) pathways. Neuroscience 2017, 358, 300–315. [Google Scholar] [CrossRef]
- Volk, G.F.; Steinerstauch, A.; Lorenz, A.; Modersohn, L.; Mothes, O.; Denzler, J.; Klingner, C.M.; Hamzei, F.; Guntinas-Lichius, O. Facial motor and non-motor disabilities in patients with central facial paresis: A prospective cohort study. J. Neurol. 2019, 266, 46–56. [Google Scholar] [CrossRef]
- Morecraft, R.J.; Stilwell-Morecraft, K.S.; Rossing, W.R. The motor cortex and facial expression: New insights from neuroscience. Neurologist 2004, 10, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Morecraft, R.J.; Binneboese, A.; Stilwell-Morecraft, K.S.; Ge, J. Localization of orofacial representation in the corona radiata, internal capsule and cerebral peduncle in Macaca mulatta. J. Comp. Neurol. 2017, 525, 3429–3457. [Google Scholar] [CrossRef] [PubMed]
- Rutten, G.J.; Verheul, J. Reorganization of left primary (face) motor cortex due to a low-grade glioma. Br. J. Neurosurg. 2014, 28, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Stålnacke, M.; Bergenheim, T.; Sjöberg, R.L. Neuropsychological Function and Quality of Life after Resection of Suspected Lower-Grade Glioma in the Face Primary Motor Area. J. Clin. Med. 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.T.; Berger, M.S.; Nagarajan, S.S. Motor field sensitivity for preoperative localization of motor cortex. J. Neurosurg. 2006, 105, 588–594. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, P.D.; Berger, M.S.; Haglund, M.M.; Pilcher, W.H.; Ojemann, G.A. Resection of intrinsic tumors from nondominant face motor cortex using stimulation mapping: Report of two cases. Surg. Neurol. 1991, 36, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, F.; Verheul, J.; Rutten, G.J. Functionality of glioma-infiltrated precentral gyrus: Experience from 14 patients. J. Neurosurg. Sci. 2017, 61, 140–150. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef]
- Seidel, K.; Beck, J.; Stieglitz, L.; Schucht, P.; Raabe, A. Low-threshold monopolar motor mapping for resection of primary motor cortex tumors. Neurosurgery 2012, 71, 104–115. [Google Scholar] [CrossRef]
- Seidel, K.; Beck, J.; Stieglitz, L.; Schucht, P.; Raabe, A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J. Neurosurg. 2013, 118, 287–296. [Google Scholar] [CrossRef]
- Spena, G.; Schucht, P.; Seidel, K.; Rutten, G.J.; Freyschlag, C.F.; D’Agata, F.; Costi, E.; Zappa, F.; Fontanella, M.; Fontaine, D.; et al. Brain tumors in eloquent areas: A European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg. Rev. 2017, 40, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Schucht, P.; Beck, J.; Raabe, A. Continuous Dynamic Mapping to Identify the Corticospinal Tract in Motor Eloquent Brain Tumors: An Update. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Seidel, K.; Jilch, A.; Beck, J.; Raabe, A. A review of monopolar motor mapping and a comprehensive guide to continuous dynamic motor mapping for resection of motor eloquent brain tumors. Neurochirurgie 2017, 63, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Häni, L.; Lutz, K.; Zbinden, C.; Redmann, A.; Consuegra, A.; Raabe, A.; Schucht, P. Postoperative navigated transcranial magnetic stimulation to predict motor recovery after surgery of tumors in motor eloquent areas. Clin. Neurophysiol. 2019, 130, 952–959. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Anamika, K.; Verma, S.P.; Jere, A.; Desai, A. Transcriptomic Profiling Using Next Generation Sequencing—Advances, Advantages, and Challenges; IntechOpen: London, UK, 2016. [Google Scholar]
- Mahadevappa, K.; Vora, A.; Graham, A.; Nesathurai, S. Facial paralysis: A critical review of accepted explanation. Med. Hypotheses 2010, 74, 508–509. [Google Scholar] [CrossRef]
- Morecraft, R.J.; Louie, J.L.; Herrick, J.L.; Stilwell-Morecraft, K.S. Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain 2001, 124, 176–208. [Google Scholar] [CrossRef]
- Müri, R.M. Cortical control of facial expression. J. Comp. Neurol. 2016, 524, 1578–1585. [Google Scholar] [CrossRef]
- Fornia, L.; Ferpozzi, V.; Montagna, M.; Rossi, M.; Riva, M.; Pessina, F.; Martinelli Boneschi, F.; Borroni, P.; Lemon, R.N.; Bello, L.; et al. Functional Characterization of the Left Ventrolateral Premotor Cortex in Humans: A Direct Electrophysiological Approach. Cereb. Cortex 2018, 28, 167–183. [Google Scholar] [CrossRef]
- Tokuno, H.; Takada, M.; Nambu, A.; Inase, M. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J. Comp. Neurol. 1997, 389, 34–48. [Google Scholar] [CrossRef]
- Simonyan, K.; Jürgens, U. Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neuroscience 2005, 130, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, D.; Capelle, L.; Duffau, H. Somatotopy of the supplementary motor area: Evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery 2002, 50, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.M.; Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 1991, 251, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012, 59, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.U.; Werhahn, K.; Rothwell, J.C.; Roericht, S.; Fauth, C. Functional organisation of corticonuclear pathways to motoneurones of lower facial muscles in man. Exp. Brain Res. 1994, 101, 465–472. [Google Scholar] [CrossRef]
- Yildiz, N.; Ertekin, C.; Ozdemirkiran, T.; Yildiz, S.K.; Aydogdu, I.; Uludag, B.; Secil, Y. Corticonuclear innervation to facial muscles in normal controls and in patients with central facial paresis. J. Neurol. 2005, 252, 429–435. [Google Scholar] [CrossRef]
- Hallett, M.; de Haan, W.; Deco, G.; Dengler, R.; Di Iorio, R.; Gallea, C.; Gerloff, C.; Grefkes, C.; Helmich, R.C.; Kringelbach, M.L.; et al. Human brain connectivity: Clinical applications for clinical neurophysiology. Clin. Neurophysiol. 2020, 131, 1621–1651. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Cicinelli, P.; Pasqualetti, P.; Zaccagnini, M.; Traversa, R.; Oliveri, M.; Rossini, P.M. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: A paired-pulse transcranial magnetic stimulation study. Stroke 2003, 34, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, T.; Parikh, S.; Bruno, M.K.; Hallett, M. Finger and face representations in the ipsilateral precentral motor areas in humans. J. Neurophysiol. 2005, 93, 2950–2958. [Google Scholar] [CrossRef]
- Jenkins, W.M.; Merzenich, M.M.; Recanzone, G. Neocortical representational dynamics in adult primates: Implications for neuropsychology. Neuropsychologia 1990, 28, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc. Natl. Acad. Sci. USA 1993, 90, 10413–10420. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. New concepts in surgery of WHO grade II gliomas: Functional brain mapping, connectionism and plasticity—A review. J. Neurooncol. 2006, 79, 77–115. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Han, S.J.; Hervey-Jumper, S.L.; Pekmezci, M.; Troncon, I.; Chang, S.M.; Butowski, N.A.; Berger, M.S. Molecular features and clinical outcomes in surgically treated low-grade diffuse gliomas in patients over the age of 60. J. Neurooncol. 2019, 141, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.K.; Nguyen, M.H. Elderly Stroke Rehabilitation: Overcoming the Complications and Its Associated Challenges. Curr. Gerontol. Geriatr. Res. 2018, 2018, 9853837. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Acute functional reorganisation of the human motor cortex during resection of central lesions: A study using intraoperative brain mapping. J. Neurol. Neurosurg. Psychiatry 2001, 70, 506–513. [Google Scholar] [CrossRef] [PubMed]

| Pre- and Postoperative Deficits | Tumor Volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Hemisphere/Dominance | Age (yrs.) | Lowest Motor Threshold (mA) | Facial Paresis | Aphasia | Dysarthria | Dysphagia | Preoperative (mL) | Postoperative (mL) |
| 1 | left (dominant) | 29 | 2.5 (awake) | no/yes | no/yes | no/no | no/no | 103.54 | 45.87 |
| 2 | right (non-dominant) | 40 | 1 | no/no | no/no | no/no | no/no | 7.29 | <1 |
| 3 | left (dominant) | 33 | 5 (awake: 3) | no/yes | no/yes | yes/yes | no/no | 28.23 | 9.53 |
| 4 | left (dominant) | 24 | 2 (awake: 2) | no/yes | no/yes | no/no | no/yes | 37.78 | 1.52 |
| 5 | right (non-dominant) | 22 | 1 | no/no | no/no | no/no | no/no | 17.27 | <1 |
| 6 | right (non-dominant) | 34 | 5 | no/yes | no | no/no | no/no | 28.43 | 7.16 |
| 7 | left (dominant) | 63 | 15 | no/yes | yes/yes | no/no | no/no | 9.13 | 2.11 |
| 8 | right (non-dominant) | 48 | 4 | no/no | no/no | no/no | no/no | 1.14 | <1 |
| 9 | right (non-dominant) | 41 | 3 | no/yes | no/no | no/no | no/no | 2.25 | <1 |
| 10 | right (non-dominant) | 51 | 4 | no/no | no/no | no/no | no/no | 24.36 | 3.14 |
| 11 | right (non-dominant) | 19 | 3 | no/yes | no/no | no/no | no/no | 2.92 | <1 |
| 12 | left (dominant) | 53 | 5 | no/yes | no/yes | no/yes | no/no | 33.81 | 13.73 |
| Facial Weakness Postoperative | Aphasia (Severe) Postoperative | Dysarthria Postoperative | Dysphagia Postoperative | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day | 1 Week | 3 Month | 1 Year | 1 Day | 1 Week | 3 Month | 1 Year | 1 Day | 1 Week | 3 Month | 1 Year | 1 Day | 1 Week | 3 Month | 1 Year | |
| Dominance (tumor location) | ||||||||||||||||
| Non-dominant (n = 7) | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dominant (n = 5) | 5 | 3 | 1 | 0 | 4 | 3 | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Histology | ||||||||||||||||
| Astrocytoma WHO ° 2 (n = 6) | 4 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Oligodendroglioma WHO ° 2 (n = 6) | 4 | 3 | 1 | 0 | 3 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age | ||||||||||||||||
| <38 years (n = 6) | 5 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| >38 years (n = 6) | 3 | 3 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, K.; Häni, L.; Kissling, C.; Raabe, A.; Schucht, P.; Seidel, K. Resection of Low-Grade Gliomas in the Face Area of the Primary Motor Cortex and Neurological Outcome. Cancers 2023, 15, 781. https://doi.org/10.3390/cancers15030781
Lutz K, Häni L, Kissling C, Raabe A, Schucht P, Seidel K. Resection of Low-Grade Gliomas in the Face Area of the Primary Motor Cortex and Neurological Outcome. Cancers. 2023; 15(3):781. https://doi.org/10.3390/cancers15030781
Chicago/Turabian StyleLutz, Katharina, Levin Häni, Cédric Kissling, Andreas Raabe, Philippe Schucht, and Kathleen Seidel. 2023. "Resection of Low-Grade Gliomas in the Face Area of the Primary Motor Cortex and Neurological Outcome" Cancers 15, no. 3: 781. https://doi.org/10.3390/cancers15030781
APA StyleLutz, K., Häni, L., Kissling, C., Raabe, A., Schucht, P., & Seidel, K. (2023). Resection of Low-Grade Gliomas in the Face Area of the Primary Motor Cortex and Neurological Outcome. Cancers, 15(3), 781. https://doi.org/10.3390/cancers15030781





