Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference
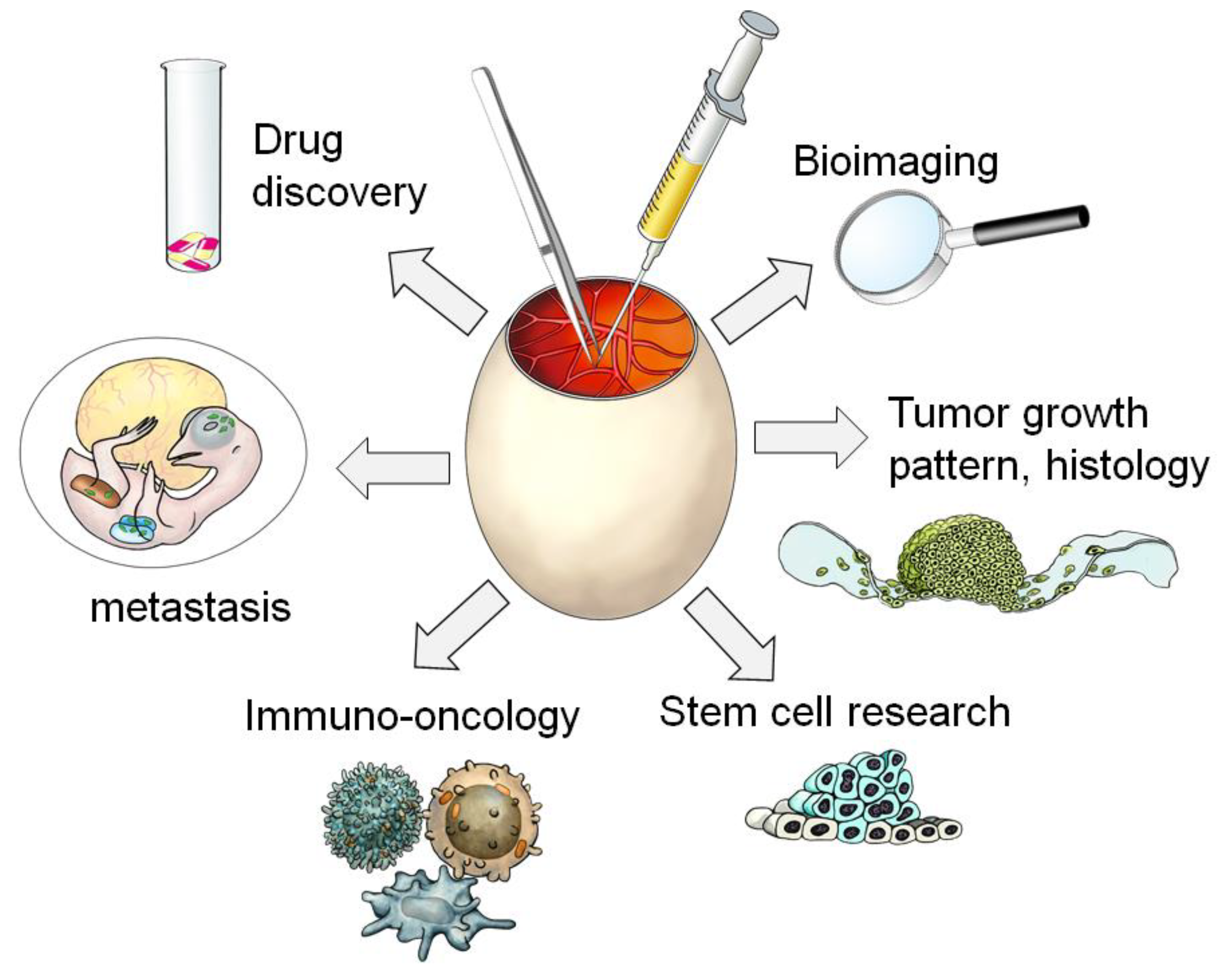
1. Introduction
2. Survey for Papers in This Special CAM Issue
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russel, W.; Burch, R. The Principles of Humane Experimental Technique. Med. J. Aust. 1959, 1, 500. [Google Scholar] [CrossRef]
- Arrowsmith, J.; Miller, P. Phase II and Phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Demcisakova, Z.; Luptakova, L.; Tirpakova, Z.; Kvasilova, A.; Medvecky, L.; De Spiegelaere, W.; Petrovova, E. Evaluation of Angiogenesis in an Acellular Porous Biomaterial Based on Polyhydroxybutyrate and Chitosan Using the Chicken Ex Ovo Chorioallantoic Membrane Model. Cancers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Wolint, P.; Jang, J.-H.; Itani, S.; Jungraithmayr, W.; Waschkies, C.F.; Meier-Bürgisser, G.; Andreoli, S.; Spanaus, K.; Schuepbach, R.A.; et al. High-Affinity Cu(I)-Chelator with Potential Anti-Tumorigenic Action—A Proof-of-Principle Experimental Study of Human H460 Tumors in the CAM Assay. Cancers 2022, 14, 5122. [Google Scholar] [CrossRef] [PubMed]
- Faihs, L.; Firouz, B.; Slezak, P.; Slezak, C.; Weißensteiner, M.; Ebner, T.; Ghaffari Tabrizi-Wizsy, N.; Schicho, K.; Dungel, P. A Novel Artificial Intelligence-Based Approach for Quantitative Assessment of Angiogenesis in the Ex Ovo CAM Model. Cancers 2022, 14, 4273. [Google Scholar] [CrossRef]
- Barnett, S.E.; Herrmann, A.; Shaw, L.; Gash, E.N.; Poptani, H.; Sacco, J.J.; Coulson, J.M. The Chick Embryo Xenograft Model for Malignant Pleural Mesothelioma: A Cost and Time Efficient 3Rs Model for Drug Target Evaluation. Cancers 2022, 14, 5836. [Google Scholar] [CrossRef]
- Benčurová, K.; Friske, J.; Anderla, M.; Mayrhofer, M.; Wanek, T.; Nics, L.; Egger, G.; Helbich, T.H.; Hacker, M.; Haug, A.; et al. CAM-Xenograft Model Provides Preclinical Evidence for the Applicability of [68Ga]Ga-Pentixafor in CRC Imaging. Cancers 2022, 14, 5549. [Google Scholar] [CrossRef]
- Löffler, J.; Herrmann, H.; Scheidhauer, E.; Wirth, M.; Wasserloos, A.; Solbach, C.; Glatting, G.; Beer, A.J.; Rasche, V.; Winter, G. Blocking Studies to Evaluate Receptor-Specific Radioligand Binding in the CAM Model by PET and MR Imaging. Cancers 2022, 14, 3870. [Google Scholar] [CrossRef]
- Buschmann, J.; Heuberger, D.M.; Kivrak Pfiffner, F.; Wolint, P.; Jang, J.-H.; Jungraithmayr, W.; Giovanoli, P.; Calcagni, M.; Waschkies, C.F. Probing Vasoreactivity and Hypoxic Phenotype in Different Tumor Grafts Grown on the Chorioallantoic Membrane of the Chicken Embryo In Ovo Using MRI. Cancers 2022, 14, 3114. [Google Scholar] [CrossRef]
- Kunze, P.; Kreiss, L.; Novosadová, V.; Roehe, A.V.; Steinmann, S.; Prochazka, J.; Geppert, C.I.; Hartmann, A.; Schürmann, S.; Friedrich, O.; et al. Multiphoton Microscopy Reveals DAPK1-Dependent Extracellular Matrix Remodeling in a Chorioallantoic Membrane (CAM) Model. Cancers 2022, 14, 2364. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Legrand, C.; Hunault, M.; Genest, L.; Babin, D.; Froget, G.; Castagné, V. A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity. Cancers 2022, 14, 3548. [Google Scholar] [CrossRef]
- Wang, Y.; Rousset, X.; Prunier, C.; Garcia, P.; Dosda, E.; Leplus, E.; Viallet, J. PD-1/PD-L1 Checkpoint Inhibitors Are Active in the Chicken Embryo Model and Show Antitumor Efficacy In Ovo. Cancers 2022, 14, 3095. [Google Scholar] [CrossRef]
- Rousset, X.; Maillet, D.; Grolleau, E.; Barthelemy, D.; Calattini, S.; Brevet, M.; Balandier, J.; Raffin, M.; Geiguer, F.; Garcia, J.; et al. Embryonated Chicken Tumor Xenografts Derived from Circulating Tumor Cells as a Relevant Model to Study Metastatic Dissemination: A Proof of Concept. Cancers 2022, 14, 4085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider-Stock, R.; Flügen, G. Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference. Cancers 2023, 15, 772. https://doi.org/10.3390/cancers15030772
Schneider-Stock R, Flügen G. Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference. Cancers. 2023; 15(3):772. https://doi.org/10.3390/cancers15030772
Chicago/Turabian StyleSchneider-Stock, Regine, and Georg Flügen. 2023. "Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference" Cancers 15, no. 3: 772. https://doi.org/10.3390/cancers15030772
APA StyleSchneider-Stock, R., & Flügen, G. (2023). Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference. Cancers, 15(3), 772. https://doi.org/10.3390/cancers15030772




