Clinical and Immunologic Characteristics of Non-Hematologic Cancers in Patients with Inborn Errors of Immunity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluation in IEI Patients
2.3. Genetic Investigation and Diagnoses in IEI Patients
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picard, C.; Bobby Gaspar, H.; Al-Herz, W.; Bousfiha, A.; Casanova, J.L.; Chatila, T.; Crow, Y.J.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J. Clin. Immunol. 2018, 38, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Tiri, A.; Masetti, R.; Conti, F.; Tignanelli, A.; Turrini, E.; Bertolini, P.; Esposito, S.; Pession, A. Inborn Errors of Immunity and Cancer. Biology 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.; Upton, J.; Warrington, R. Primary immunodeficiency. Allergy Asthma Clin. Immunol. 2018, 14, 61. [Google Scholar] [CrossRef]
- Abolhassani, H.; Azizi, G.; Sharifi, L.; Yazdani, R.; Mohsenzadegan, M.; Delavari, S.; Sohani, M.; Shirmast, P.; Chavoshzadeh, Z.; Mahdaviani, S.A.; et al. Global systematic review of primary immunodeficiency registries. Expert. Rev. Clin. Immunol. 2020, 16, 717–732. [Google Scholar] [CrossRef]
- Raje, N.; Dinakar, C. Overview of Immunodeficiency Disorders. Immunol. Allergy Clin. N. Am. 2015, 35, 599–623. [Google Scholar] [CrossRef]
- Filipovich, A.; Mathur, A.; Kamat, D.; Kersey, J.; Shapiro, R.J. Lymphoproliferative disorders and other tumors complicating immunodeficiencies. Immunodeficiency 1994, 5, 91–112. [Google Scholar] [PubMed]
- Bomken, S.; van der Werff Ten Bosch, J.; Attarbaschi, A.; Bacon, C.M.; Borkhardt, A.; Boztug, K.; Fischer, U.; Hauck, F.; Kuiper, R.P.; Lammens, T.; et al. Current Understanding and Future Research Priorities in Malignancy Associated With Inborn Errors of Immunity and DNA Repair Disorders: The Perspective of an Interdisciplinary Working Group. Front. Immunol. 2018, 9, 2912. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Wang, Y.; Hammarstrom, L.; Pan-Hammarstrom, Q. Hallmarks of Cancers: Primary Antibody Deficiency Versus Other Inborn Errors of Immunity. Front. Immunol. 2021, 12, 720025. [Google Scholar] [CrossRef] [PubMed]
- Tak Manesh, A.; Azizi, G.; Heydari, A.; Kiaee, F.; Shaghaghi, M.; Hossein-Khannazer, N.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Epidemiology and pathophysiology of malignancy in common variable immunodeficiency? Allergol. Immunopathol. (Madr.) 2017, 45, 602–615. [Google Scholar] [CrossRef]
- Wang, Y.; Abolhassani, H. Updates of cancer hallmarks in patients with inborn errors of immunity. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S. Malignancies in the setting of primary immunodeficiency: Implications for hematologists/oncologists. Am. J. Hematol. 2011, 86, 48–55. [Google Scholar] [CrossRef]
- Ye, X.; Maglione, P.J.; Wehr, C.; Li, X.; Wang, Y.; Abolhassani, H.; Deripapa, E.; Liu, D.; Borte, S.; Du, L.; et al. Genomic characterization of lymphomas in patients with inborn errors of immunity. Blood Adv. 2022, 6, 5403–5414. [Google Scholar] [CrossRef]
- Kiaee, F.; Azizi, G.; Rafiemanesh, H.; Zainaldain, H.; Sadaat Rizvi, F.; Alizadeh, M.; Jamee, M.; Mohammadi, S.; Habibi, S.; Sharifi, L.; et al. Malignancy in common variable immunodeficiency: A systematic review and meta-analysis. Expert. Rev. Clin. Immunol. 2019, 15, 1105–1113. [Google Scholar] [CrossRef]
- Abolhassani, H.; Aghamohammadi, A.; Imanzadeh, A.; Mohammadinejad, P.; Sadeghi, B.; Rezaei, N. Malignancy phenotype in common variable immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2012, 22, 133–134. [Google Scholar] [PubMed]
- Vajdic, C.M.; Mao, L.; van Leeuwen, M.T.; Kirkpatrick, P.; Grulich, A.E.; Riminton, S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood 2010, 116, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Kebudi, R.; Kiykim, A.; Sahin, M.K. Primary Immunodeficiency and Cancer in Children; A Review of the Literature. Curr. Pediatr. Rev. 2019, 15, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Vacca, A.; Dammacco, F.; Racanelli, V. Common Variable Immunodeficiency and Gastric Malignancies. Int. J. Mol. Sci. 2018, 19, 451. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Rezaei, N.; Yazdani, R.; Delavari, S.; Kutukculer, N.; Topyildiz, E.; Ozen, A.; Baris, S.; Karakoc-Aydiner, E.; Kilic, S.S.; et al. Consensus Middle East and North Africa Registry on Inborn Errors of Immunity. J. Clin. Immunol. 2021, 41, 1339–1351. [Google Scholar] [CrossRef]
- Abolhassani, H.; Kiaee, F.; Tavakol, M.; Chavoshzadeh, Z.; Mahdaviani, S.A.; Momen, T.; Yazdani, R.; Azizi, G.; Habibi, S.; Gharagozlou, M.; et al. Fourth Update on the Iranian National Registry of Primary Immunodeficiencies: Integration of Molecular Diagnosis. J. Clin. Immunol. 2018, 38, 816–832. [Google Scholar] [CrossRef]
- Baris, S.; Abolhassani, H.; Massaad, M.J.; Al-Nesf, M.; Chavoshzadeh, Z.; Keles, S.; Reisli, I.; Tahiat, A.; Shendi, H.M.; Elaziz, D.A.; et al. The Middle East and North Africa Diagnosis and Management Guidelines for Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract 2022, 11, 158–180.e11. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, J.; Kindle, G.; Nieters, A.; Rusch, S.; Seppanen, M.R.J.; Fischer, A.; Grimbacher, B.; Edgar, D.; Buckland, M.; Mahlaoui, N.; et al. Initial presenting manifestations in 16,486 patients with inborn errors of immunity include infections and noninfectious manifestations. J. Allergy Clin. Immunol. 2021, 148, 1332–1341.e5. [Google Scholar] [CrossRef]
- Abolhassani, H.; Chou, J.; Bainter, W.; Platt, C.D.; Tavassoli, M.; Momen, T.; Tavakol, M.; Eslamian, M.H.; Gharagozlou, M.; Movahedi, M.; et al. Clinical, immunologic, and genetic spectrum of 696 patients with combined immunodeficiency. J. Allergy Clin. Immunol. 2018, 141, 1450–1458. [Google Scholar] [CrossRef]
- Abolhassani, H.; El-Sherbiny, Y.M.; Arumugakani, G.; Carter, C.; Richards, S.; Lawless, D.; Wood, P.; Buckland, M.; Heydarzadeh, M.; Aghamohammadi, A.; et al. Expanding Clinical Phenotype and Novel Insights into the Pathogenesis of ICOS Deficiency. J. Clin. Immunol. 2020, 40, 277–288. [Google Scholar] [CrossRef]
- Azizi, G.; Tavakol, M.; Yazdani, R.; Delavari, S.; Moeini Shad, T.; Rasouli, S.E.; Jamee, M.; Pashangzadeh, S.; Kalantari, A.; Shariat, M.; et al. Autoimmune manifestations among 461 patients with monogenic inborn errors of immunity. Pediatr. Allergy Immunol. 2021, 32, 1335–1348. [Google Scholar] [CrossRef]
- Karimi, E.; Mahmoudian, F.; Reyes, S.O.L.; Bargir, U.A.; Madkaikar, M.; Artac, H.; Sabzevari, A.; Lu, N.; Azizi, G.; Abolhassani, H. Approach to genetic diagnosis of inborn errors of immunity through next-generation sequencing. Mol. Immunol. 2021, 137, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Abolhassani, H.; Kiaee, F.; Habibi, S.; Azizi, G.; Tavakol, M.; Chavoshzadeh, Z.; Mahdaviani, S.A.; Momen, T.; Gharagozlou, M.; et al. Comparison of Common Monogenic Defects in a Large Predominantly Antibody Deficiency Cohort. J. Allergy Clin. Immunol. Pract. 2019, 7, 864–878.e9. [Google Scholar] [CrossRef]
- Li, Q.; Wang, K. InterVar: Clinical Interpretation of Genetic Variants by the 2015 ACMG-AMP Guidelines. Am. J. Hum. Genet. 2017, 100, 267–280. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Hammarstrom, L.; Cunningham-Rundles, C. Current genetic landscape in common variable immune deficiency. Blood 2020, 135, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Jamee, M.; Azizi, G.; Baris, S.; Karakoc-Aydiner, E.; Ozen, A.; Kilic, S.S.; Kose, H.; Chavoshzadeh, Z.; Mahdaviani, S.A.; Momen, T.; et al. Clinical, immunological, molecular and therapeutic findings in monogenic immune dysregulation diseases: Middle East and North Africa registry. Clin. Immunol. 2022, 244, 109131. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salehiniya, H.; Mohammadian-Hafshejani, A. Some Facts on Incidence and Mortality of Cancer in Iran. Iran J. Public Health 2017, 46, 1446–1447. [Google Scholar]
- Available online: https://www.iarc.who.int/news-events/cancer-in-iran-2008-to-2025-recent-incidence-trends-and-short-term-predictions-of-the-future-burden/ (accessed on 17 October 2022).
- Roosan, M.R.; Mambetsariev, I.; Pharaon, R.; Fricke, J.; Baroz, A.R.; Chao, J.; Chen, C.; Nasser, M.W.; Chirravuri-Venkata, R.; Jain, M.; et al. Evaluation of Somatic Mutations in Solid Metastatic Pan-Cancer Patients. Cancers 2021, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. Tumors of the immunocompromised patient. Annu. Rev. Med. 1988, 39, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, M.; Notarangelo, L.D.; Schumacher, R.F.; Soncini, E.; Soresina, A.; Lanfranchi, A.; Porta, F. Primary Immunodeficiencies and Oncological Risk: The Experience of the Children’s Hospital of Brescia. Front. Pediatr. 2019, 7, 232. [Google Scholar] [CrossRef] [PubMed]
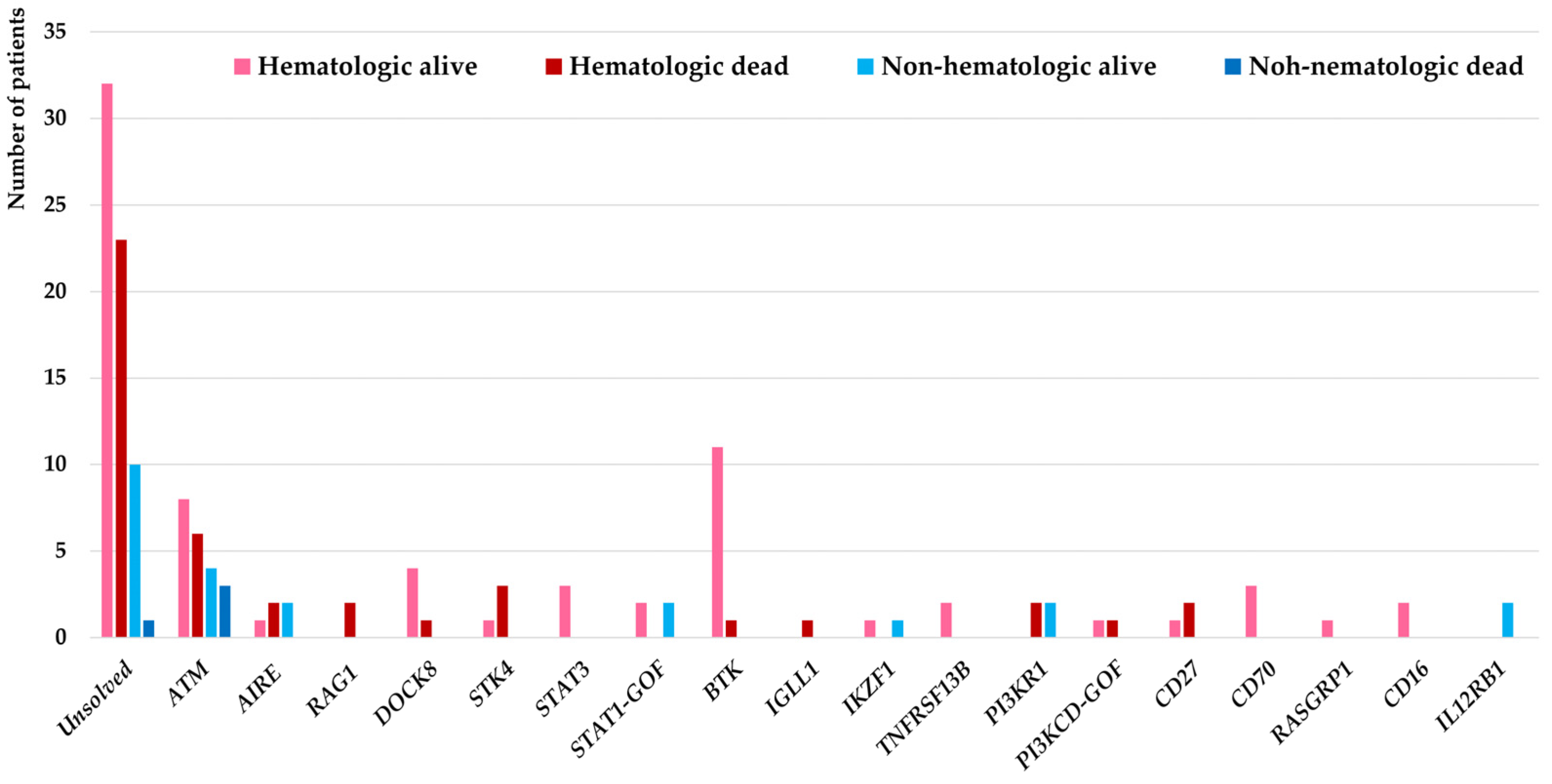
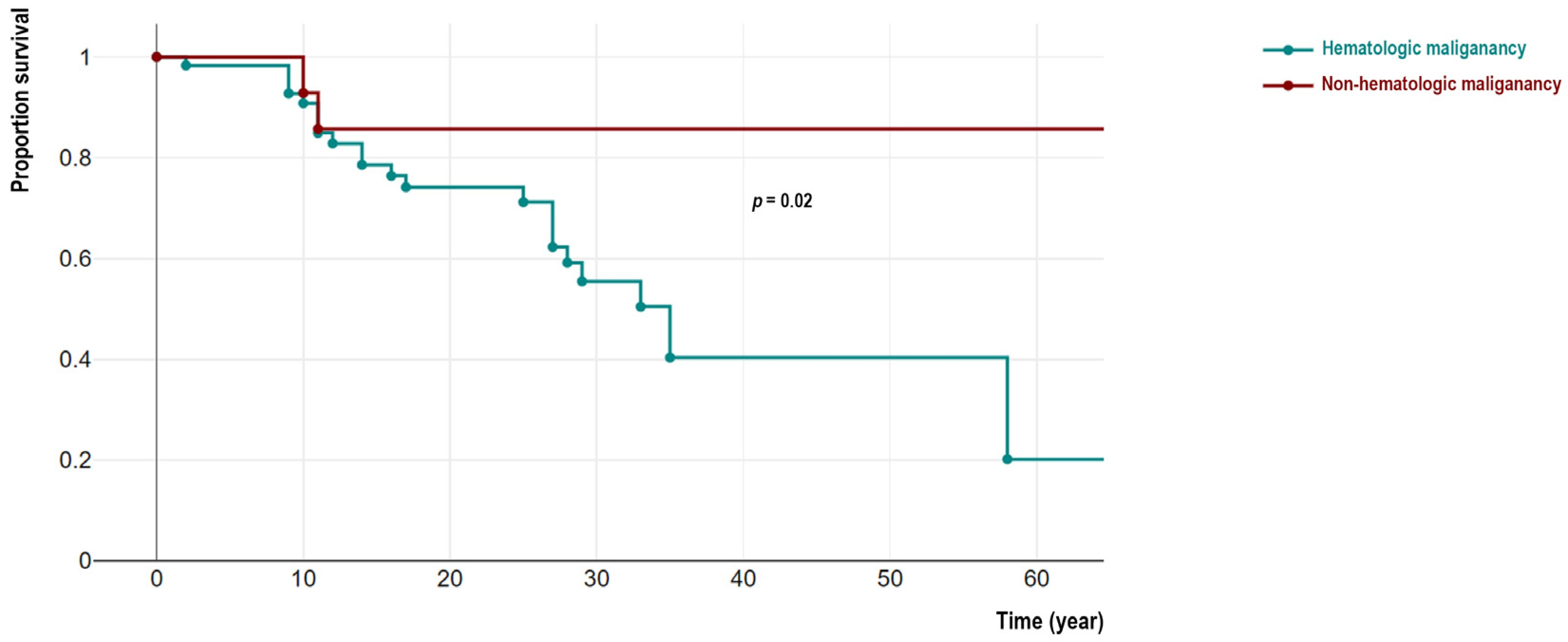
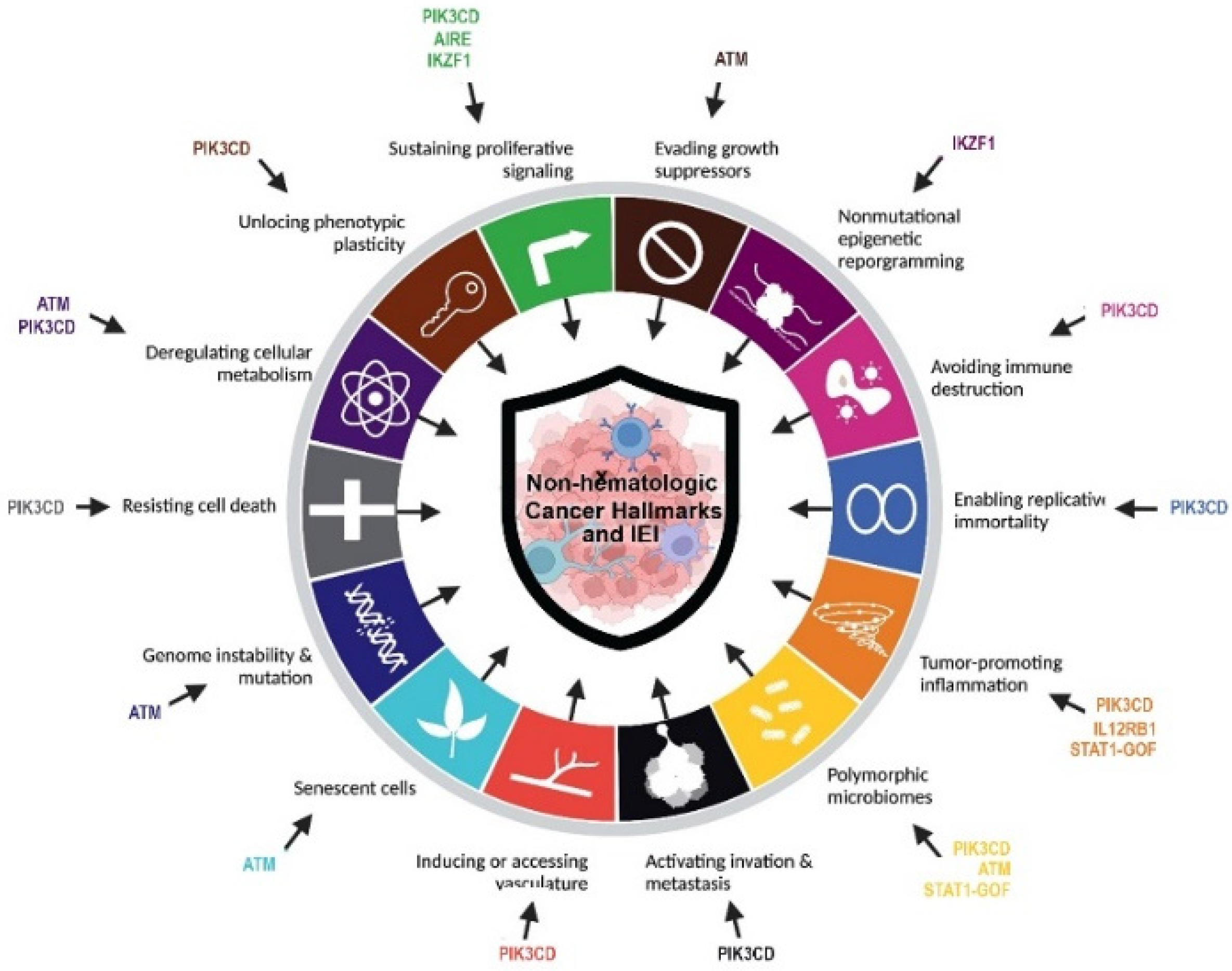
| Parameters | Hematologic Cancers (n = 117) | Non-Hematologic Cancers (n = 27) | p-Value |
|---|---|---|---|
| Sex ratio (M/F) | 66/51 | 18/9 | 0.32 |
| Parental consanguinity (%) | 77 (65.8) | 20 (74.0) | 0.40 |
| Mortality (%) | 43 (36.7) | 5(18.5) | 0.07 |
| Median age at IEI onset, year (IQR) | 3.5 (1.5–8.0) | 5.0(2.5–12.2) | 0.22 |
| Median age at the diagnosis of malignancy, year (IQR) | 13.0 (6.0–20.5) | 19.2 (12.0–28.0) | 0.08 |
| Median age at the time of study *, year (IQR) | 17.0 (10.9–24.2) | 27.4 (22.7–36.4) | 0.01 * |
| Otitis media (%) | 39 (33.3) | 7 (25.9) | 0.27 |
| Sinusitis (%) | 43 (36.7) | 12 (44.4) | 0.28 |
| Pneumonia (%) | 60 (51.3) | 13 (48.1) | 0.76 |
| Bronchiectasis (%) | 23 (19.6) | 11 (40.7) | 0.02 * |
| Severe infections (%) | 4 (3.4) | 0 | 0.98 |
| Chronic enteropathy (%) | 14 (11.9) | 11 (40.7) | <0.001 * |
| Failure to thrive (%) | 27 (23.0) | 7 (25.9) | 0.75 |
| Autoimmunity (%) | 18 (15.3) | 4 (14.8) | 1.00 |
| Allergy and atopic diseases (%) | 26 (22.2) | 4 (14.8) | 0.39 |
| Lymphoproliferation (%) | 37(31.6) | 6 (22.2) | 0.33 |
| Parameters | Hematologic Cancers (n = 117) | Non-Hematologic Cancers (n = 27) | p-Value |
|---|---|---|---|
| Leukocyte/uL, Median (IQR) | 6725 (4185–9074) | 8237 (4421–11097) | 0.51 |
| Neutrophils/uL, Median (IQR) | 3572 (2844–6820) | 3586 (2804–4502) | 0.86 |
| Lymphocyte/uL, Median (IQR) | 2039 (1517–3506) | 1548 (609–2467) | 0.07 |
| Absolute CD3+ T cells/uL, Median (IQR) | 1504 (757–2011) | 472 (349–674) | 0.11 |
| Absolute CD3+CD4+ T cells/uL, Median (IQR) | 417 (201–1154) | 335 (197–726) | 0.19 |
| Absolute CD3+CD8+ T cells/uL, Median (IQR) | 1533 (779–1845) | 512 (379–670) | 0.06 |
| Absolute CD16/CD64+ NK cells/uL, Median (IQR) | 249 (87–688) | 165 (67–228) | 0.35 |
| Absolute CD19+ B cells/uL, Median (IQR) | 192 (69–364) | 39 (25–117) | 0.09 |
| Serum IgG mg/dL, Median (IQR) * | 317 (84–603) | 448 (89–821) | 0.72 |
| Serum IgA mg/dL, Median (IQR) | 26 (13–47) | 21 (9–32) | 0.88 |
| Serum IgM mg/dL, Median (IQR) | 44 (22–115) | 65 (32–106) | 0.42 |
| Serum IgE IU/mL, Median (IQR) | 23 (5–80) | 34 (7–96) | 0.26 |
| ID | Age (Year) | Sex | Clinical Diagnosis | IUIS Classification | Type of Cancer/s | Molecular Diagnosis | Mutation | Type of Mutation | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 10 | M | Ataxia telangectasia | syndromic CID | Brain tumor + leukemia | ATM | Hom p.E1622X | Stop-gain | Death |
| P2 | 11 | M | Ataxia telangectasia | syndromic CID | Mandibular squamous cell carcinoma + NHL | ATM | Hom p.L1851fsX1856 | Frameshift | Death |
| P3 | 10 | F | Ataxia telangectasia | syndromic CID | Squamous cell carcinoma of tongue + NHL | ATM | Hom p.Q1862RfsX25 | Frameshift | Death |
| P4 | 10.5 | F | Ataxia telangectasia | syndromic CID | Ovarian cystadenoma | ATM | Hom p.Tyr2371X | Stop-gain | Death |
| P5 | 13 | M | Ataxia telangectasia | syndromic CID | Brain tumor | ATM | Hom c.2921+1G>T | Splicing | Alive |
| P6 | 9 | M | Ataxia telangectasia | syndromic CID | Gastric adenocarcinomas | ATM | Hom del EX61-EX62 | Large deletion | Alive |
| P7 | 22 | F | Ataxia telangectasia | syndromic CID | Invasive ductal carcinomas | ATM | Hom. p.D2016G | Missense | Alive |
| P8 | 28 | M | Common variable immunodeficiency | PAD | Gastric adenocarcinomas | AIRE | Het p.A500PfsX21 | Frameshift | Alive |
| P9 | 64 | M | Common variable immunodeficiency | PAD | Gastric adenocarcinomas | AIRE | Het p.R139X | Stop-gain | Alive |
| P10 | 20 | M | Common variable immunodeficiency | PAD | Gastric adenocarcinomas | IKZF1 | Het p.R143W | Missense | Alive |
| P11 | 25 | M | hyper IgM syndrome | PAD | Colorectal cancer | PI3KR1 | Het c.1425+1G>A | Splicing | Alive |
| P12 | 12 | F | Common variable immunodeficiency | PAD | Gastric adenocarcinomas | PI3KR1 | Het c.1425+1G>A | Splicing | Alive |
| P13 | 35 | M | Mendelian susceptibility to mycobacterial diseases | innate immunity | Thyroid cancer | IL12RB1 | Hom c.783+1G>A | Splicing | Alive |
| P14 | 37 | F | Mendelian susceptibility to mycobacterial diseases | innate immunity | In situ ductal carcinoma | IL12RB1 | Hom p.R212Q | Missense | Alive |
| P15 | 67 | M | Chronic mucocutaneous candidiasis | innate immunity | Squamous cell carcinoma | STAT1 | Het p.R274Q | Missense | Alive |
| P16 | 41 | F | Chronic mucocutaneous candidiasis | innate immunity | Squamous cell carcinoma | STAT1 | Het p.R274Q | Missense | Alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delavari, S.; Wang, Y.; Moeini shad, T.; Pashangzadeh, S.; Nazari, F.; Salami, F.; Abolhassani, H. Clinical and Immunologic Characteristics of Non-Hematologic Cancers in Patients with Inborn Errors of Immunity. Cancers 2023, 15, 764. https://doi.org/10.3390/cancers15030764
Delavari S, Wang Y, Moeini shad T, Pashangzadeh S, Nazari F, Salami F, Abolhassani H. Clinical and Immunologic Characteristics of Non-Hematologic Cancers in Patients with Inborn Errors of Immunity. Cancers. 2023; 15(3):764. https://doi.org/10.3390/cancers15030764
Chicago/Turabian StyleDelavari, Samaneh, Yating Wang, Tannaz Moeini shad, Salar Pashangzadeh, Farzad Nazari, Fereshte Salami, and Hassan Abolhassani. 2023. "Clinical and Immunologic Characteristics of Non-Hematologic Cancers in Patients with Inborn Errors of Immunity" Cancers 15, no. 3: 764. https://doi.org/10.3390/cancers15030764
APA StyleDelavari, S., Wang, Y., Moeini shad, T., Pashangzadeh, S., Nazari, F., Salami, F., & Abolhassani, H. (2023). Clinical and Immunologic Characteristics of Non-Hematologic Cancers in Patients with Inborn Errors of Immunity. Cancers, 15(3), 764. https://doi.org/10.3390/cancers15030764








