Targeting the Hedgehog Pathway in Rhabdomyosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
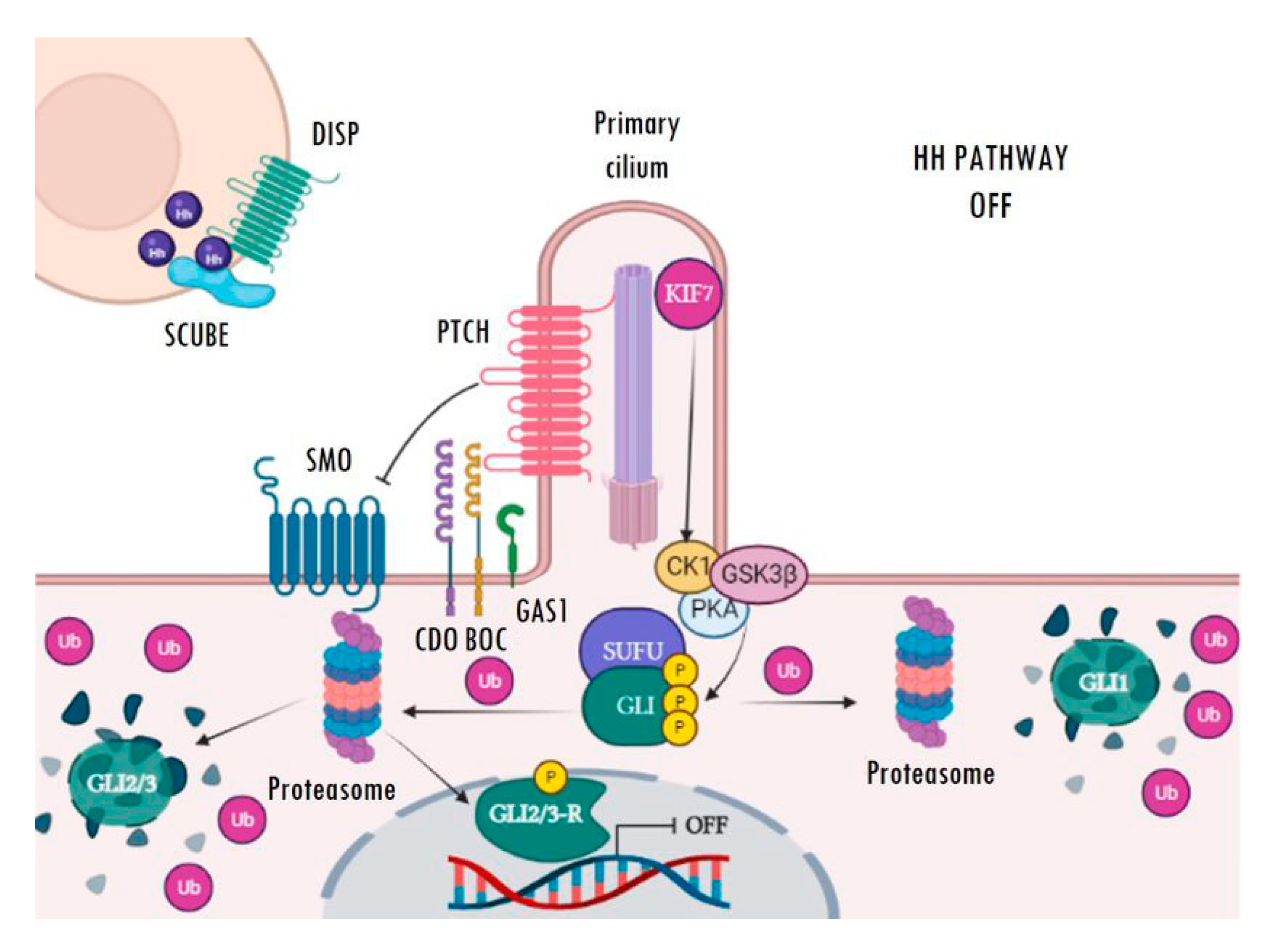
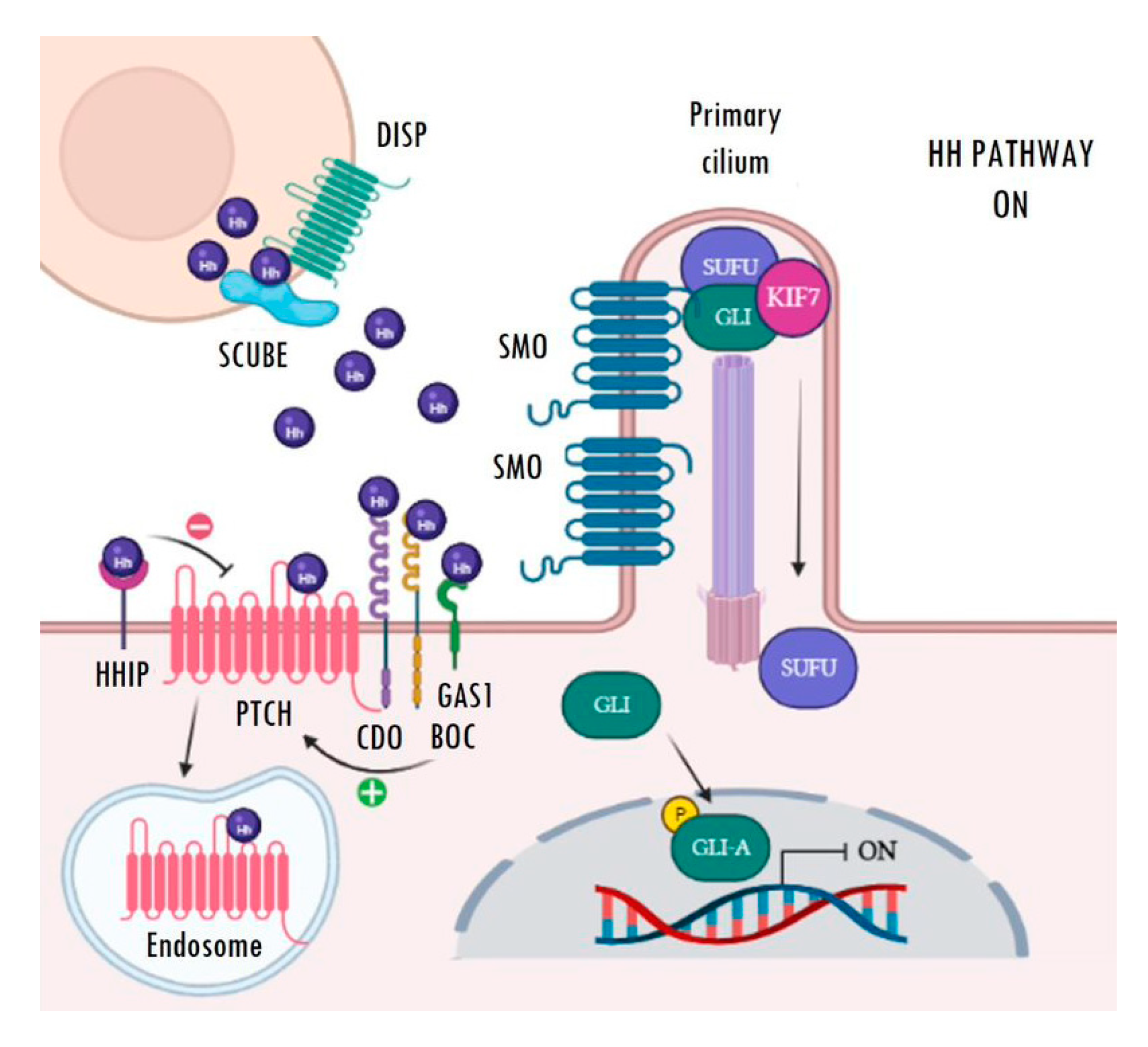
2. Overview of the Hedgehog Signalling Pathway in Mammals
3. General Models of Oncogenic Hh Pathway Activation
3.1. Ligand-Independent Hh Activation (Mutational)
3.2. Ligand-Dependent Hh Activation (Non-Mutational)
3.3. Non-Canonical Hh Activation (Non-Mutational)
4. Oncogenic Role of the Hedgehog Pathway in RMS
5. Hedgehog Inhibitors and Clinical Trials
5.1. SMO Inhibitors
| Study | Clinical Trials. gov Identifier | Inhibitor Name | Activity | Tumour Type | Phase | Outcome |
|---|---|---|---|---|---|---|
| Vismodegib in Treating Patients with Advanced Chondrosarcomas | NCT01267955 | Vismodegib (GDC-0449) | SMO inhibitor | Chondrosarcoma | Phase II | Clinical benefit was achieved after 6 months in 25.6% of patients [91] |
| Vismodegib and Gamma-Secretase/Notch Signalling Pathway Inhibitor RO4929097 in Treating Patients with Advanced or Metastatic Sarcoma | NCT01154452 | Vismodegib (GDC-0449) | SMO inhibitor | Adult rhabdomyosarcoma and other advanced/metastatic sarcomas | Phase I Phase II | The combination therapy was safe but Vismodegib did not significantly improve the clinical efficacy of RO4929097 [93] |
| A Phase I Dose Finding and Safety Study of Oral LDE225 in Children and a Phase II Portion to Assess Preliminary Efficacy in Recurrent or Refractory MB | NCT01125800 | Sonidegib (LDE225) | SMO inhibitor | Rhabdomyosarcoma and other paediatric tumours potentially dependent on the Hh pathway | Phase I Phase II | Only the SHh subgroup of medulloblastoma patients, as defined by a five-gene signature RT-PCR assay, responded [95] |
| A Safety and Efficacy Study of Patients with Metastatic or Locally Advanced (Unresectable) Chondrosarcoma | NCT01310816 | Patidegib/Saridegib (IPI-926) | SMO inhibitor | Chondrosarcoma | Phase II | Ended prematurely. On 14 June 2012, a planned futility analysis of data from the study concluded that treatment with IPI-926 was similar to placebo and, therefore, the trial would not meet its primary endpoint |
| A Study of LY2940680 in Paediatric Medulloblastoma or Rhabdomyosarcoma | NCT01697514 | Taladegib (LY2940680) | SMO inhibitor | Medulloblastoma and rhabdomyosarcoma | Phase I | Withdrawn (Trial stopped early for poor accrual) |
| Arsenic Trioxide in Treating Patients with Advanced Neuroblastoma or Other Childhood Solid Tumours | NCT00024258 | Arsenic trioxide | GLI inhibitor | Sarcoma and other paediatric tumours | Phase II | The disease progressed in 72.7% and stabilized in 22.7% of the patients |
| Arsenic Trioxide Plus Radiation Therapy in Treating Patients with Newly Diagnosed Malignant Glioma | NCT00045565 | Arsenic trioxide | GLI inhibitor | Gliosarcoma and other malignant glioma | Phase I | No results posted |
| Study of Genistein in Paediatric Oncology Patients (UVA-Gen001) (UVA-Gen001) | NCT02624388 | Genistein | GLI inhibitor | Sarcoma and other paediatric tumours | Phase II | The therapy is safe and well tolerated |
5.2. GLI Inhibitors
6. Hh Inhibitors in RMS: From Encouraging Preclinical Data to Disappointing Clinical Results
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hibbitts, E.; Chi, Y.Y.; Hawkins, D.S.; Barr, F.G.; Bradley, J.A.; Dasgupta, R.; Meyer, W.H.; Rodeberg, D.A.; Rudzinski, E.R.; Spunt, S.L.; et al. Refinement of Risk Stratification for Childhood Rhabdomyosarcoma Using FOXO1 Fusion Status in Addition to Established Clinical Outcome Predictors: A Report from the Children’s Oncology Group. Cancer Med. 2019, 8, 6437–6448. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.; Zanetti, I.; Orbach, D.; Ranchère, D.; Shipley, J.; Zin, A.; Bergeron, C.; de Salvo, G.L.; Chisholm, J.; Ferrari, A.; et al. Fusion Status in Patients with Lymph Node-Positive (N1) Alveolar Rhabdomyosarcoma Is a Powerful Predictor of Prognosis: Experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer 2018, 124, 3201–3209. [Google Scholar] [CrossRef]
- Chen, C.; Dorado Garcia, H.; Scheer, M.; Henssen, A.G. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front. Oncol. 2019, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Helman, L.J. Rhabdomyosarcoma—Working out the Pathways. Oncogene 1999, 18, 5340–5348. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making Muscle: Skeletal Myogenesis in Vivo and in Vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Bryson-Richardson, R.J.; Currie, P.D. The Genetics of Vertebrate Myogenesis. Nat. Rev. Genet. 2008, 9, 632–646. [Google Scholar] [CrossRef]
- Manceau, L.; Albert, J.R.; Lollini, P.L.; Greenberg, M.V.C.; Gilardi-Hebenstreit, P.; Ribes, V. Divergent Transcriptional and Transforming Properties of PAX3-FOXO1 and PAX7-FOXO1 Paralogs. PLoS Genet. 2022, 18, e1009782. [Google Scholar] [CrossRef]
- Skapek, S.X.; Anderson, J.; Barr, F.G.; Bridge, J.A.; Gastier-Foster, J.M.; Parham, D.M.; Rudzinski, E.R.; Triche, T.; Hawkins, D.S. PAX-FOXO1 Fusion Status Drives Unfavorable Outcome for Children with Rhabdomyosarcoma: A Children’s Oncology Group Report. Pediatr. Blood Cancer 2013, 60, 1411–1417. [Google Scholar] [CrossRef]
- Hatley, M.E.; Tang, W.; Garcia, M.R.; Finkelstein, D.; Millay, D.P.; Liu, N.; Graff, J.; Galindo, R.L.; Olson, E.N. A Mouse Model of Rhabdomyosarcoma Originating from the Adipocyte Lineage. Cancer Cell 2012, 22, 536–546. [Google Scholar] [CrossRef]
- Drummond, C.J.; Hanna, J.A.; Garcia, M.R.; Devine, D.J.; Heyrana, A.J.; Finkelstein, D.; Rehg, J.E.; Hatley, M.E. Hedgehog Pathway Drives Fusion-Negative Rhabdomyosarcoma Initiated From Non-Myogenic Endothelial Progenitors. Cancer Cell 2018, 33, 108–124.e5. [Google Scholar] [CrossRef]
- Hu, J.K.H.; Mcglinn, E.; Harfe, B.D.; Kardon, G.; Tabin, C.J. Autonomous and Nonautonomous Roles of Hedgehog Signaling in Regulating Limb Muscle Formation. Genes Dev. 2012, 26, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Williams, V.C.; Moyon, B.; Daubas, P.; Tajbakhsh, S.; Buckingham, M.E.; Shiroishi, T.; Hughes, S.M.; Boryck, A.G. Sonic Hedgehog Acts Cell-Autonomously on Muscle Precursor Cells to Generate Limb Muscle Diversity. Genes Dev. 2012, 26, 2103–2117. [Google Scholar] [CrossRef]
- Voronova, A.; Coyne, E.; Al Madhoun, A.; Fair, J.V.; Bosiljcic, N.; St-Louis, C.; Li, G.; Thurig, S.; Wallace, V.A.; Wiper-Bergeron, N.; et al. Hedgehog Signaling Regulates MyoD Expression and Activity. J. Biol. Chem. 2013, 288, 4389–4404. [Google Scholar] [CrossRef]
- Bren-Mattison, Y.; Hausburg, M.; Olwin, B.B. Growth of Limb Muscle Is Dependent on Skeletal-Derived Indian Hedgehog. Dev. Biol. 2011, 356, 486–495. [Google Scholar] [CrossRef]
- Norris, A.M.; Johnson, C.D.; Zhou, L.Y.; Appu, A.; McKellar, D.W.; Cosgrove, B.D.; Kopinke, D. Hedgehog Signaling Acts as Cell Fate Determinant during Adult Tissue Repair. bioRxiv 2022. [Google Scholar] [CrossRef]
- Qi, X.; Li, X. Mechanistic Insights into the Generation and Transduction of Hedgehog Signaling. Trends Biochem. Sci 2020, 45, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, G.; Fan, Q.; Xie, J. Activation of the Hedgehog-Signaling Pathway in Human Cancer and the Clinical Implications. Oncogene 2010, 29, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; McMahon, A.P. Hedgehog Signaling in Animal Development: Paradigms and Principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef]
- Lee, R.T.H.; Zhao, Z.; Ingham, P.W. Hedgehog Signalling. Development 2016, 143, 367–372. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Li, X. Structure of Human Dispatched-1 Provides Insights into Hedgehog Ligand Biogenesis. Life Sci. Alliance 2020, 3, e202000776. [Google Scholar] [CrossRef]
- Tukachinsky, H.; Kuzmickas, R.P.; Jao, C.Y.; Liu, J.; Salic, A. Dispatched and Scube Mediate the Efficient Secretion of the Cholesterol-Modified Hedgehog Ligand. Cell Rep. 2012, 2, 308–320. [Google Scholar] [CrossRef]
- Cheung, H.O.L.; Zhang, X.; Ribeiro, A.; Mo, R.; Makino, S.; Puviindran, V.; Lo Law, K.K.; Briscoe, J.; Hui, C.C. The Kinesin Protein Kif7 Is a Critical Regulator of Gli Transcription Factors in Mammalian Hedgehog Signaling. Sci. Signal. 2009, 2, 13377–13382. [Google Scholar] [CrossRef]
- Ryan, K.E.; Chiang, C. Hedgehog Secretion and Signal Transduction in Vertebrates. J. Biol. Chem. 2012, 287, 17905–17913. [Google Scholar] [CrossRef]
- Bangs, F.; Anderson, K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef]
- Teglund, S.; Toftgård, R. Hedgehog beyond Medulloblastoma and Basal Cell Carcinoma. Biochim. Biophys. Acta 2010, 1805, 181–208. [Google Scholar] [CrossRef]
- Heretsch, P.; Tzagkaroulaki, L.; Giannis, A. Modulators of the Hedgehog Signaling Pathway. Bioorg Med. Chem. 2010, 18, 6613–6624. [Google Scholar] [CrossRef] [PubMed]
- Matise, M.P.; Wang, H. Sonic Hedgehog Signaling in the Developing CNS. Where It Has Been and Where It Is Going. Curr. Top. Dev. Biol. 2011, 97, 75–117. [Google Scholar] [CrossRef]
- Sigafoos, A.N.; Paradise, B.D.; Fernandez-Zapico, M.E. Hedgehog/Gli Signaling Pathway: Transduction, Regulation, and Implications for Disease. Cancers 2021, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Wojnowski, L.; Zimmer, A.M.; Hall, J.; Miller, G.; Zimmer, A. Rhabdomyosarcomas and Radiation Hypersensitivity in a Mouse Model of Gorlin Syndrome. Nat. Med. 1998, 4, 619–622. [Google Scholar] [CrossRef]
- Reifenberger, J.; Wolter, M.; Knobbe, C.B.; Köhler, B.; Schönicke, A.; Scharwächter, C.; Kumar, K.; Blaschke, B.; Ruzicka, T.; Reifenberger, G. Somatic Mutations in the PTCH, SMOH, SUFUH and TP53 Genes in Sporadic Basal Cell Carcinomas. Br. J. Dermatol. 2005, 152, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The Role of the Hedgehog Signaling Pathway in Cancer: A Comprehensive Review. Bosn J. Basic. Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Manore, S.G.; Wong, G.L.; Lo, H.W. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells 2020, 9, 2114. [Google Scholar] [CrossRef]
- Northcott, P.A.; Hielscher, T.; Dubuc, A.; MacK, S.; Shih, D.; Remke, M.; Al-Halabi, H.; Albrecht, S.; Jabado, N.; Eberhart, C.G.; et al. Pediatric and Adult Sonic Hedgehog Medulloblastomas Are Clinically and Molecularly Distinct. Acta Neuropathol. 2011, 122, 231–240. [Google Scholar] [CrossRef]
- Kool, M.; Jones, D.T.W.; Jäger, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.J.; Walsh, M.; Rajagopalan, B.; Finlay, M.; Trainer, A.H.; Bonnet, F.; Sevenet, N.; Winship, I.M. Mutations in SUFU and PTCH1 Genes May Cause Different Cutaneous Cancer Predisposition Syndromes: Similar, but Not the Same. Fam. Cancer 2018, 17, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kawagoe, R.; Sasai, K.; Li, Y.; Russell, H.R.; Curran, T.; McKinnon, P.J. Loss of Suppressor-of-Fused Function Promotes Tumorigenesis. Oncogene 2007, 26, 6442–6447. [Google Scholar] [CrossRef]
- Roberts, W.M.; Douglass, E.C.; Peiper, S.C.; Houghton, P.J.; Look, A.T. Amplification of the Gli Gene in Childhood Sarcomas. Cancer Res. 1989, 49, 5407–5413. [Google Scholar]
- Wasson, J.C.; Saylors, R.L.; Zeltzer, P.; Friedman, H.S.; Bigner, S.H.; Burger, P.C.; Bigner, D.D.; Look, A.T.; Douglass, E.C.; Brodeur, G.M. Oncogene Amplification in Pediatric Brain Tumors. Cancer Res. 1990, 50, 2987–2990. [Google Scholar]
- Snijders, A.M.; Schmidt, B.L.; Fridlyand, J.; Dekker, N.; Pinkel, D.; Jordan, R.C.K.; Albertson, D.G. Rare Amplicons Implicate Frequent Deregulation of Cell Fate Specification Pathways in Oral Squamous Cell Carcinoma. Oncogene 2005, 24, 4232–4242. [Google Scholar] [CrossRef]
- Raju, G.P.; Pham, D. Hedgehog Inhibition as an Anti-Cancer Strategy. Vitam Horm. 2012, 88, 507–522. [Google Scholar] [CrossRef]
- Sjöblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The Consensus Coding Sequences of Human Breast and Colorectal Cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- Lascorz, J.; Försti, A.; Chen, B.; Buch, S.; Steinke, V.; Rahner, N.; Holinski-Feder, E.; Morak, M.; Schackert, H.K.; Görgens, H.; et al. Genome-Wide Association Study for Colorectal Cancer Identifies Risk Polymorphisms in German Familial Cases and Implicates MAPK Signalling Pathways in Disease Susceptibility. Carcinogenesis 2010, 31, 1612–1619. [Google Scholar] [CrossRef]
- Cao, X.; Geradts, J.; Dewhirst, M.W.; Lo, H.W. Upregulation of VEGF-A and CD24 Gene Expression by the TGLI1 Transcription Factor Contributes to the Aggressive Behavior of Breast Cancer Cells. Oncogene 2012, 31, 104–115. [Google Scholar] [CrossRef]
- Lo, H.W.; Zhu, H.; Cao, X.; Aldrich, A.; Ali-Osman, F. A Novel Splice Variant of GLI1 That Promotes Glioblastoma Cell Migration and Invasion. Cancer Res. 2009, 69, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Ruppert, J.M.; Bigner, S.H.; Vogelstein, B. The GLI Gene Is a Member of the Kruppel Family of Zinc Finger Proteins. Nature 1988, 332, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Wicking, C.; Zaphiropoulos, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the Human Homolog of Drosophila Patched in the Nevoid Basal Cell Carcinoma Syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, J.; Wolter, M.; Weber, R.G.; Megahed, M.; Ruzicka, T.; Lichter, P.; Reifenberger, G. Missense Mutations in SMOH in Sporadic Basal Cell Carcinomas of the Skin and Primitive Neuroectodermal Tumors of the Central Nervous System. Cancer Res. 1998, 58, 1798–1803. [Google Scholar]
- Taylor, M.D.; Liu, L.; Raffel, C.; Hui, C.C.; Mainprize, T.G.; Zhang, X.; Agatep, R.; Chiappa, S.; Gao, L.; Lowrance, A.; et al. Mutations in SUFU Predispose to Medulloblastoma. Nat. Genet. 2002, 31, 306–310. [Google Scholar] [CrossRef]
- Tian, H.; Callahan, C.A.; Dupree, K.J.; Darbonne, W.C.; Ahn, C.P.; Scales, S.J.; De Sauvage, F.J. Hedgehog Signaling Is Restricted to the Stromal Compartment during Pancreatic Carcinogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 4254–4259. [Google Scholar] [CrossRef]
- Scales, S.J.; de Sauvage, F.J. Mechanisms of Hedgehog Pathway Activation in Cancer and Implications for Therapy. Trends Pharmacol. Sci. 2009, 30, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Moga, A.; Zarzosa, P.; Molist, C.; Velasco, P.; Pyczek, J.; Simon-Keller, K.; Giralt, I.; Vidal, I.; Navarro, N.; Segura, M.F.; et al. Ligand-Dependent Hedgehog Pathway Activation in Rhabdomyosarcoma: The Oncogenic Role of the Ligands. Br. J. Cancer 2017, 117, 1314–1325. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-Canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors beyond Smoothened. Front Genet 2019, 10, 556. [Google Scholar] [CrossRef]
- Graab, U.; Hahn, H.; Fulda, S. Identification of a Novel Synthetic Lethality of Combined Inhibition of Hedgehog and PI3K Signaling in Rhabdomyosarcoma. Oncotarget 2015, 6, 8722–8735. [Google Scholar] [CrossRef]
- Petricoin, E.F.; Espina, V.; Araujo, R.P.; Midura, B.; Yeung, C.; Wan, X.; Eichler, G.S.; Johann, D.J.; Qualman, S.; Tsokos, M.; et al. Phosphoprotein Pathway Mapping: Akt/Mammalian Target of Rapamycin Activation Is Negatively Associated with Childhood Rhabdomyosarcoma Survival. Cancer Res. 2007, 67, 3431–3440. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Q.; Yen, C.J.; Xia, W.; Izzo, J.G.; Lang, J.Y.; Li, C.W.; Hsu, J.L.; Miller, S.A.; Wang, X.; et al. The Crosstalk of MTOR/S6K1 and Hedgehog Pathways. Cancer Cell 2012, 21, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Geyer, N.; Ridzewski, R.; Bauer, J.; Kuzyakova, M.; Dittmann, K.; Dullin, C.; Rosenberger, A.; Schildhaus, H.U.; Uhmann, A.; Fulda, S.; et al. Different Response of Ptch Mutant and Ptch Wildtype Rhabdomyosarcoma Toward SMO and PI3K Inhibitors. Front. Oncol. 2018, 8, 396. [Google Scholar] [CrossRef]
- Stecca, B.; Mas, C.; Clement, V.; Zbinden, M.; Correa, R.; Piguet, V.; Beermann, F.; Ruiz, I.; Altaba, A. Melanomas Require HEDGEHOG-GLI Signaling Regulated by Interactions between GLI1 and the RAS-MEK/AKT Pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 5895–5900. [Google Scholar] [CrossRef]
- Ji, Z.; Mei, F.C.; Xie, J.; Cheng, X. Oncogenic KRAS Activates Hedgehog Signaling Pathway in Pancreatic Cancer Cells. J. Biol. Chem. 2007, 282, 14048–14055. [Google Scholar] [CrossRef]
- Dehner, C.A.; Armstrong, A.E.; Yohe, M.; Shern, J.F.; Hirbe, A.C. Genetic Characterization, Current Model Systems and Prognostic Stratification in PAX Fusion-Negative vs. PAX Fusion-Positive Rhabdomyosarcoma. Genes 2021, 12, 1500. [Google Scholar] [CrossRef]
- Bauer, J.; Cuvelier, N.; Ragab, N.; Simon-Keller, K.; Nitzki, F.; Geyer, N.; Botermann, D.S.; Elmer, D.P.; Rosenberger, A.; Rando, T.A.; et al. Context-Dependent Modulation of Aggressiveness of Pediatric Tumors by Individual Oncogenic RAS Isoforms. Oncogene 2021, 40, 4955–4966. [Google Scholar] [CrossRef]
- Dennler, S.; André, J.; Alexaki, I.; Li, A.; Magnaldo, T.; Ten Dijke, P.; Wang, X.J.; Verrecchia, F.; Mauviel, A. Induction of Sonic Hedgehog Mediators by Transforming Growth Factor-Beta: Smad3-Dependent Activation of Gli2 and Gli1 Expression in Vitro and in Vivo. Cancer Res. 2007, 67, 6981–6986. [Google Scholar] [CrossRef]
- Tang, Y.A.; Chen, Y.F.; Bao, Y.; Mahara, S.; Yatim, S.M.J.M.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic Tumor Microenvironment Activates GLI2 via HIF-1α and TGF-Β2 to Promote Chemoresistance in Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef]
- Atwood, S.X.; Li, M.; Lee, A.; Tang, J.Y.; Oro, A.E. GLI Activation by Atypical Protein Kinase C ι/λ Regulates the Growth of Basal Cell Carcinomas. Nature 2013, 494, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Zwerner, J.P.; Joo, J.; Warner, K.L.; Christensen, L.; Hu-Lieskovan, S.; Triche, T.J.; May, W.A. The EWS/FLI1 Oncogenic Transcription Factor Deregulates GLI1. Oncogene 2008, 27, 3282–3291. [Google Scholar] [CrossRef]
- Schneider, P.; Miguel Bayo-Fina, J.; Singh, R.; Kumar Dhanyamraju, P.; Holz, P.; Baier, A.; Fendrich, V.; Ramaswamy, A.; Baumeister, S.; Martinez, E.D.; et al. Identification of a Novel Actin-Dependent Signal Transducing Module Allows for the Targeted Degradation of GLI1. Nat. Commun. 2015, 6, 8023. [Google Scholar] [CrossRef] [PubMed]
- Jagani, Z.; Mora-Blanco, E.L.; Sansam, C.G.; McKenna, E.S.; Wilson, B.; Chen, D.; Klekota, J.; Tamayo, P.; Nguyen, P.T.L.; Tolstorukov, M.; et al. Loss of the Tumor Suppressor Snf5 Leads to Aberrant Activation of the Hedgehog-Gli Pathway. Nat. Med. 2010, 16, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- DeCristofaro, M.F.; Betz, B.L.; Wang, W.; Weissman, B.E. Alteration of HSNF5/INI1/BAF47 Detected in Rhabdoid Cell Lines and Primary Rhabdomyosarcomas but Not Wilms’ Tumors. Oncogene 1999, 18, 7559–7565. [Google Scholar] [CrossRef]
- Zibat, A.; Missiaglia, E.; Rosenberger, A.; Pritchard-Jones, K.; Shipley, J.; Hahn, H.; Fulda, S. Activation of the Hedgehog Pathway Confers a Poor Prognosis in Embryonal and Fusion Gene-Negative Alveolar Rhabdomyosarcoma. Oncogene 2010, 29, 6323–6330. [Google Scholar] [CrossRef]
- Pressey, J.G.; Anderson, J.R.; Crossman, D.K.; Lynch, J.C.; Barr, F.G. Hedgehog Pathway Activity in Pediatric Embryonal Rhabdomyosarcoma and Undifferentiated Sarcoma: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 930–938. [Google Scholar] [CrossRef]
- Satheesha, S.; Manzella, G.; Bovay, A.; Casanova, E.A.; Bode, P.K.; Belle, R.; Feuchtgruber, S.; Jaaks, P.; Dogan, N.; Koscielniak, E.; et al. Targeting Hedgehog Signaling Reduces Self-Renewal in Embryonal Rhabdomyosarcoma. Oncogene 2016, 35, 2020–2030. [Google Scholar] [CrossRef]
- Manzella, G.W.; Schäfer, B. Interfering with Hedgehog Pathway: New Avenues for Targeted Therapy in Rhabdomyosarcoma. Curr. Drug Targets 2016, 17, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Lamm, M.; Chandler, C.; Iannaccone, P.; Walterhouse, D. Up-Regulation of GLI1 in Vincristine-Resistant Rhabdomyosarcoma and Ewing Sarcoma. BMC Cancer 2020, 20, 511. [Google Scholar] [CrossRef]
- Calzada-Wack, J.; Schnitzbauer, U.; Walch, A.; Wurster, K.H.; Kappler, R.; Nathrath, M.; Hahn, H. Analysis of the PTCH Coding Region in Human Rhabdomyosarcoma. Hum. Mutat. 2002, 20, 233–234. [Google Scholar] [CrossRef]
- Bridge, J.A.; Liu, J.; Qualman, S.J.; Suijkerbuijk, R.; Wenger, G.; Zhang, J.; Wan, X.; Baker, K.S.; Sorensen, P.; Barr, F.G. Genomic Gains and Losses Are Similar in Genetic and Histologic Subsets of Rhabdomyosarcoma, Whereas Amplification Predominates in Embryonal with Anaplasia and Alveolar Subtypes. Genes Chromosom. Cancer 2002, 33, 310–321. [Google Scholar] [CrossRef]
- Tostar, U.; Malm, C.J.; Meis-Kindblom, J.M.; Kindblom, L.G.; Toftgård, R.; Undén, A.B. Deregulation of the Hedgehog Signalling Pathway: A Possible Role for the PTCH and SUFU Genes in Human Rhabdomyoma and Rhabdomyosarcoma Development. J. Pathol. 2006, 208, 17–25. [Google Scholar] [CrossRef]
- Teot, L.A.; Schneider, M.; Thorner, A.R.; Tian, J.; Chi, Y.Y.; Ducar, M.; Lin, L.; Wlodarski, M.; Grier, H.E.; Fletcher, C.D.M.; et al. Clinical and Mutational Spectrum of Highly Differentiated, Paired Box 3:Forkhead Box Protein O1 Fusion–Negative Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Cancer 2018, 124, 1973–1981. [Google Scholar] [CrossRef]
- Georg-August-Universität Göttingen. Available online: https://ediss.uni-goettingen.de/handle/11858/00-1735-0000-0028-8687-C (accessed on 10 November 2022).
- Chahal, K.K.; Parle, M.; Abagyan, R. Hedgehog Pathway and Smoothened Inhibitors in Cancer Therapies. Anti. Cancer Drugs 2018, 29, 387–401. [Google Scholar] [CrossRef]
- Axelson, M.; Liu, K.; Jiang, X.; He, K.; Wang, J.; Zhao, H.; Kufrin, D.; Palmby, T.; Dong, Z.; Russell, A.M.; et al. Food and Drug Administration Approval: Vismodegib for Recurrent, Locally Advanced, or Metastatic Basal Cell Carcinoma. Clin. Cancer Res. 2013, 19, 2289–2293. [Google Scholar] [CrossRef]
- Burness, C.B. Sonidegib: First Global Approval. Drugs 2015, 75, 1559–1566. [Google Scholar] [CrossRef]
- Hoy, S.M. Glasdegib: First Global Approval. Drugs 2019, 79, 207–213. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; Macdonald, T.J.; Mechinaud, F.; et al. Phase i Study of Oral Sonidegib (LDE225) in Pediatric Brain and Solid Tumors and a Phase II Study in Children and Adults with Relapsed Medulloblastoma. Neuro. Oncol. 2017, 19, 1542–1552. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Solomon, J.A.; Hainsworth, J.D.; Goldberg, L.; McKenna, E.; Day, B.M.; Chen, D.M.; Weiss, G.J. Expanded Access Study of Patients with Advanced Basal Cell Carcinoma Treated with the Hedgehog Pathway Inhibitor, Vismodegib. J. Am. Acad. Dermatol. 2014, 70, 60–69. [Google Scholar] [CrossRef]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-Term Efficacy and Safety of Sonidegib in Patients with Advanced Basal Cell Carcinoma: 42-Month Analysis of the Phase II Randomized, Double-Blind BOLT Study. Br J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Hann, C.L.; Laterra, J.; Yauch, R.L.; Callahan, C.A.; Fu, L.; Holcomb, T.; Stinson, J.; Gould, S.E.; Coleman, B.; et al. Treatment of Medulloblastoma with Hedgehog Pathway Inhibitor GDC-0449. N. Engl. J. Med. 2009, 361, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy against Recurrent Sonic Hedgehog—Subgroup Medulloblastoma: Results from Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.; Bendell, J.C.; Hart, L.L.; Firdaus, I.; Gore, I.; Hermann, R.C.; Mulcahy, M.F.; Zalupski, M.M.; Mackey, H.M.; Yauch, R.L.; et al. A Randomized Phase II Trial of Vismodegib versus Placebo with FOLFOX or FOLFIRI and Bevacizumab in Patients with Previously Untreated Metastatic Colorectal Cancer. Clin. Cancer Res. 2013, 19, 258–267. [Google Scholar] [CrossRef]
- Kaye, S.B.; Fehrenbacher, L.; Holloway, R.; Amit, A.; Karlan, B.; Slomovitz, B.; Sabbatini, P.; Fu, L.; Yauch, R.L.; Chang, I.; et al. A Phase II, Randomized, Placebo-Controlled Study of Vismodegib as Maintenance Therapy in Patients with Ovarian Cancer in Second or Third Complete Remission. Clin. Cancer Res. 2012, 18, 6509–6518. [Google Scholar] [CrossRef]
- De Jesus-Acosta, A.; Sugar, E.A.; O’Dwyer, P.J.; Ramanathan, R.K.; Von Hoff, D.D.; Rasheed, Z.; Zheng, L.; Begum, A.; Anders, R.; Maitra, A.; et al. Phase 2 Study of Vismodegib, a Hedgehog Inhibitor, Combined with Gemcitabine and Nab-Paclitaxel in Patients with Untreated Metastatic Pancreatic Adenocarcinoma. Br J. Cancer 2020, 122, 498–505. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01267955 (accessed on 24 September 2022).
- Italiano, A.; Le Cesne, A.; Bellera, C.; Piperno-Neumann, S.; Duffaud, F.; Penel, N.; Cassier, P.; Domont, J.; Takebe, N.; Kind, M.; et al. GDC-0449 in Patients with Advanced Chondrosarcomas: A French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase Ii Collaborative Study. Ann. Oncol. 2013, 24, 2922–2926. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01154452 (accessed on 10 November 2022).
- Gounder, M.M.; Rosenbaum, E.; Wu, N.; Dickson, M.A.; Sheikh, T.N.; D’Angelo, S.P.; Chi, P.; Keohan, M.L.; Erinjeri, J.P.; Antonescu, C.R.; et al. A Phase Ib/II Randomized Study of RO4929097, a Gamma-Secretase or Notch Inhibitor with or without Vismodegib, a Hedgehog Inhibitor, in Advanced Sarcoma. Clin. Cancer Res. 2022, 28, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01125800 (accessed on 10 November 2022).
- Nguyen, N.M.; Cho, J. Hedgehog Pathway Inhibitors as Targeted Cancer Therapy and Strategies to Overcome Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1733. [Google Scholar] [CrossRef]
- Xin, M.; Ji, X.; De La Cruz, L.K.; Thareja, S.; Wang, B. Strategies to Target the Hedgehog Signaling Pathway for Cancer Therapy. Med. Res. Rev. 2018, 38, 870–913. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, W.; Li, C.; Ueki, H.; Xue, R.; Sadahira, T.; Hu, H.; Wada, K.; Li, N.; Liu, C.; et al. Repurposing of Posaconazole as a Hedgehog/SMO Signaling Inhibitor for Embryonal Rhabdomyosarcoma Therapy. Am. J. Cancer Res. 2021, 11, 4528–4540. [Google Scholar]
- Peer, E.; Tesanovic, S.; Aberger, F. Next-Generation Hedgehog/GLI Pathway Inhibitors for Cancer Therapy. Cancers 2019, 11, 538. [Google Scholar] [CrossRef]
- Berardozzi, S.; Bernardi, F.; Infante, P.; Ingallina, C.; Toscano, S.; De Paolis, E.; Alfonsi, R.; Caimano, M.; Botta, B.; Mori, M.; et al. Synergistic Inhibition of the Hedgehog Pathway by Newly Designed Smo and Gli Antagonists Bearing the Isoflavone Scaffold. Eur. J. Med. Chem. 2018, 156, 554–562. [Google Scholar] [CrossRef]
- Severini, L.L.; Quaglio, D.; Basili, I.; Ghirga, F.; Bufalieri, F.; Caimano, M.; Balducci, S.; Moretti, M.; Romeo, I.; Loricchio, E.; et al. A Smo/Gli Multitarget Hedgehog Pathway Inhibitor Impairs Tumor Growth. Cancers 2019, 11, 1518. [Google Scholar] [CrossRef]
- Ng, J.M.Y.; Curran, T. The Hedgehog’s Tale: Developing Strategies for Targeting Cancer. Nat. Rev. Cancer 2011, 11, 493–501. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arsenic-trioxide-accord (accessed on 24 September 2022).
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14888 (accessed on 24 September 2022).
- Beauchamp, E.M.; Ringer, L.; Bulut, G.; Sajwan, K.P.; Hall, M.D.; Lee, Y.C.; Peaceman, D.; Özdemirli, M.; Rodriguez, O.; Macdonald, T.J.; et al. Arsenic Trioxide Inhibits Human Cancer Cell Growth and Tumor Development in Mice by Blocking Hedgehog/GLI Pathway. J. Clin. Investig. 2011, 121, 148–160. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.J.; Kim, J.; Gardner, D.; Beachy, P.A. Arsenic Antagonizes the Hedgehog Pathway by Preventing Ciliary Accumulation and Reducing Stability of the Gli2 Transcriptional Effector. Proc. Natl. Acad. Sci. USA 2010, 107, 13432–13437. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00024258 (accessed on 17 October 2022).
- Clincosm. Available online: https://www.clincosm.com/trial/brain-and-cns-tumors-childhood-germ-cell-tumor-extragonadal-new-york (accessed on 17 October 2022).
- Zhang, L.; Li, L.; Jiao, M.; Wu, D.; Wu, K.; Li, X.; Zhu, G.; Yang, L.; Wang, X.; Hsieh, J.T.; et al. Genistein Inhibits the Stemness Properties of Prostate Cancer Cells through Targeting Hedgehog-Gli1 Pathway. Cancer Lett. 2012, 323, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Fan, S.; Wang, H.; Mao, J.; Shi, Y.; Ibrahim, M.M.; Ma, W.; Yu, X.; Hou, Z.; Wang, B.; et al. Genistein Decreases the Breast Cancer Stem-like Cell Population through Hedgehog Pathway. Stem Cell Res. Ther. 2013, 4, 146. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/show/NCT02624388 (accessed on 10 November 2022).
- Gorlin, R.J. Nevoid Basal-Cell Carcinoma Syndrome. Medicine 1987, 66, 98–113. [Google Scholar] [CrossRef]
- Ridzewski, R.; Rettberg, D.; Dittmann, K.; Cuvelier, N.; Fulda, S.; Hahn, H. Hedgehog Inhibitors in Rhabdomyosarcoma: A Comparison of Four Compounds and Responsiveness of Four Cell Lines. Front Oncol. 2015, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Curran, T. Reproducibility of Academic Preclinical Translational Research: Lessons from the Development of Hedgehog Pathway Inhibitors to Treat Cancer. Open Biol. 2018, 8, 180098. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Ray, H. Safety and Tolerability of Sonic Hedgehog Pathway Inhibitors in Cancer. Drug. Saf. 2019, 42, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Lauth, M.; Bergström, Å.; Shimokawa, T.; Toftgård, R. Inhibition of GLI-Mediated Transcription and Tumor Cell Growth by Small-Molecule Antagonists. Proc. Natl. Acad. Sci. USA 2007, 104, 8455–8460. [Google Scholar] [CrossRef] [PubMed]
- Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; et al. Interfering with Resistance to Smoothened Antagonists by Inhibition of the PI3K Pathway in Medulloblastoma. Sci. Transl. Med. 2010, 2, 51ra70. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, G.J.P.; Alicke, B.; Weinmann, L.; Januario, T.; West, K.; Modrusan, Z.; Burdick, D.; Goldsmith, R.; Robarge, K.; Sutherlin, D.; et al. Small Molecule Inhibition of GDC-0449 Refractory Smoothened Mutants and Downstream Mechanisms of Drug Resistance. Cancer Res. 2011, 71, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01576666 (accessed on 9 January 2023).
- Yauch, R.; Januario, T.; Fu, L.; Holcomb, T.; Stinson, J.; Pujara, K.; Callahan, C.; Koeppen, H.; Reddy, J.; Von Hoff, D.; et al. Abstract A44: Predictive Biomarkers of Efficacy to the Hedgehog Pathway Inhibitor, GDC-0449, in Advanced Basal Cell Carcinoma and Medulloblastoma in Phase I Studies. Mol. Cancer Ther. 2009, 8 (Suppl. 12), A44. [Google Scholar] [CrossRef]
- Kieran, M.W. Lessons Learned from Diffuse Intrinsic Pontine Glioma: How a Terrible Disease Forced Us to Think Better. Neuro Oncol. 2017, 19, 1017–1018. [Google Scholar] [CrossRef]
- Shou, Y.; Smithson, M. Evaluating Predictors of Dispersion: A Comparison of Dominance Analysis and Bayesian Model Averaging. Psychometrika 2015, 80, 236–256. [Google Scholar] [CrossRef]
- Rodon, J. An (Only) Partially Established Paradigm of Drug Development of Targeted Therapies. Eur. J. Cancer 2014, 50, 2037–2039. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarzosa, P.; Garcia-Gilabert, L.; Hladun, R.; Guillén, G.; Gallo-Oller, G.; Pons, G.; Sansa-Girona, J.; Segura, M.F.; Sánchez de Toledo, J.; Moreno, L.; et al. Targeting the Hedgehog Pathway in Rhabdomyosarcoma. Cancers 2023, 15, 727. https://doi.org/10.3390/cancers15030727
Zarzosa P, Garcia-Gilabert L, Hladun R, Guillén G, Gallo-Oller G, Pons G, Sansa-Girona J, Segura MF, Sánchez de Toledo J, Moreno L, et al. Targeting the Hedgehog Pathway in Rhabdomyosarcoma. Cancers. 2023; 15(3):727. https://doi.org/10.3390/cancers15030727
Chicago/Turabian StyleZarzosa, Patricia, Lia Garcia-Gilabert, Raquel Hladun, Gabriela Guillén, Gabriel Gallo-Oller, Guillem Pons, Julia Sansa-Girona, Miguel F. Segura, Josep Sánchez de Toledo, Lucas Moreno, and et al. 2023. "Targeting the Hedgehog Pathway in Rhabdomyosarcoma" Cancers 15, no. 3: 727. https://doi.org/10.3390/cancers15030727
APA StyleZarzosa, P., Garcia-Gilabert, L., Hladun, R., Guillén, G., Gallo-Oller, G., Pons, G., Sansa-Girona, J., Segura, M. F., Sánchez de Toledo, J., Moreno, L., Gallego, S., & Roma, J. (2023). Targeting the Hedgehog Pathway in Rhabdomyosarcoma. Cancers, 15(3), 727. https://doi.org/10.3390/cancers15030727







