Efficacy and Safety of PD-1/PD-L1 Checkpoint Inhibitors versus Anti-PD-1/PD-L1 Combined with Other Therapies for Tumors: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Eligible Studies and Quality
3.2. Study Characteristics
3.3. Objective Response Rate (ORR)
3.4. Disease Control Rate (DCR)
3.5. Overall Survival (OS)
3.6. Progression-Free Survival (PFS)
3.7. Incidence of All-Grade and Grade 3–5 Adverse Events (AEs)
3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, 6477. [Google Scholar] [CrossRef] [PubMed]
- Abril-Rodriguez, G.; Ribas, A. SnapShot: Immune Checkpoint Inhibitors. Cancer Cell 2017, 31, 848–848.e1. [Google Scholar] [CrossRef]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2021, 21, 509–528. [Google Scholar] [CrossRef]
- Wolchok, J. Putting the Immunologic Brakes on Cancer. Cell 2018, 175, 1452–1454. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Upadhaya, S.; Neftelinov, S.T.; Hodge, J.; Campbell, J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat. Rev. Drug Discov. 2022, 21, 482–483. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Şendur, M.A.N.; Rodríguez-Abreu, D.; Park, K.; Lee, D.H.; Çiçin, I.; Yumuk, P.F.; Orlandi, F.J.; Leal, T.A.; Molinier, O.; et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J. Clin. Oncol. 2021, 39, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Bai, H.; Duan, J.; Wang, Z.; Wang, X.; Zhang, X.; Xu, J.; Fei, K.; Zhang, Z.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: A systematic review and meta-analysis. Lancet Oncol. 2021, 22, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Gu, L.; Su, R.; Chen, B.; Cao, H. Efficacy and safety of combination PD-1/PD-L1 checkpoint inhibitors for malignant solid tumours: A systematic review. J. Cell Mol. Med. 2020, 24, 13494–13506. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Bell, D.; Rubin, M.L.; Hutcheson, K.A.; Johnson, J.M.; Goepfert, R.P.; Phan, J.; Elamin, Y.Y.; Torman, D.K.; Warneke, C.L.; et al. Impact of Neoadjuvant Durvalumab with or without Tremelimumab on CD8+ Tumor Lymphocyte Density, Safety, and Efficacy in Patients with Oropharynx Cancer: CIAO Trial Results. Clin. Cancer Res. 2020, 26, 3211–3219. [Google Scholar] [CrossRef]
- Ferris, R.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.; Clement, P.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Redman, M.W.; Bazhenova, L.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Bradley, J.D.; Stinchcombe, T.E.; Leighl, N.B.; Ramalingam, S.S.; et al. Nivolumab Plus Ipilimumab vs Nivolumab for Previously Treated Patients With Stage IV Squamous Cell Lung Cancer: The Lung-MAP S1400I Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1368. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.-N.; Lee, S.S.; Yavuz, B.G.; Mohamed, Y.I.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Lee, J.; Bang, Y.-J.; Almhanna, K.; Blum-Murphy, M.; Catenacci, D.V.; Chung, H.C.; Wainberg, Z.A.; Gibson, M.K.; Lee, K.-W.; et al. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2020, 26, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.-E.; Lee, J.-S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 2021, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Reinmuth, N.; Orlov, S.; Fischer, J.; Sugawara, S.; Mandziuk, S.; Marquez-Medina, D.; Novello, S.; Takeda, Y.; Soo, R.; et al. ARCTIC: Durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann. Oncol. 2020, 31, 609–618. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.-L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Ready, N.E.; Ott, P.A.; Hellmann, M.D.; Zugazagoitia, J.; Hann, C.L.; de Braud, F.; Antonia, S.J.; Ascierto, P.A.; Moreno, V.; Atmaca, A.; et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J. Thorac. Oncol. 2020, 15, 426–435. [Google Scholar] [CrossRef]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Dô, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.-H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma. A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siefker-Radtke, A.; De Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616, Erratum in J. Clin. Oncol. 2019, 37, 2094. [Google Scholar] [CrossRef]
- Singh, A.S.; Hecht, J.R.; Rosen, L.; Wainberg, Z.A.; Wang, X.; Douek, M.; Hagopian, A.; Andes, R.; Sauer, L.; Brackert, S.R.; et al. A Randomized Phase II Study of Nivolumab Monotherapy or Nivolumab Combined with Ipilimumab in Patients with Advanced Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2022, 28, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Even, C.; Mesía, R.; Remenar, E.; Daste, A.; Delord, J.-P.; Krauss, J.; Saba, N.F.; Nabell, L.; Ready, N.E.; et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC. The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019, 5, 195–203. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. New Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J., Jr.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Gogas, H.; Dréno, B.; Larkin, J.; Demidov, L.; Stroyakovskiy, D.; Eroglu, Z.; Ferrucci, P.F.; Pigozzo, J.; Rutkowski, P.; Mackiewicz, J.; et al. Cobimetinib plus atezolizumab in BRAFV600 wild-type melanoma: Primary results from the randomized phase III IMspire170 study. Ann. Oncol. 2021, 32, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Molinaro, A.M.; Peters, K.B.; Clarke, J.L.; Jordan, J.T.; de Groot, J.F.; Nghiemphu, P.L.; Kaley, T.J.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Betts, C.B.; Maloney, L.; Nadler, E.; Algazi, A.; Guarino, M.J.; Nemunaitis, J.; Jimeno, A.; Patel, P.; Munugalavadla, V.; et al. Safety and Efficacy of Pembrolizumab in Combination with Acalabrutinib in Advanced Head and Neck Squamous Cell Carcinoma: Phase 2 Proof-of-Concept Study. Clin. Cancer Res. 2021, 28, 903–914. [Google Scholar] [CrossRef]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Zhang, T.; Harrison, M.R.; O’Donnell, P.H.; Alva, A.S.; Hahn, N.M.; Appleman, L.J.; Cetnar, J.; Burke, J.M.; Fleming, M.T.; Milowsky, M.I.; et al. A randomized phase 2 trial of pembrolizumab versus pembrolizumab and acalabrutinib in patients with platinum-resistant metastatic urothelial cancer. Cancer 2020, 126, 4485–4497. [Google Scholar] [CrossRef]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef]
- McBride, S.; Sherman, E.; Tsai, C.J.; Baxi, S.; Aghalar, J.; Eng, J.; Zhi, W.I.; McFarland, D.; Michel, L.S.; Young, R.; et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2021, 39, 30–37. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Johnson, M.L.; Lockhart, A.C.; Moore, K.N.; Falchook, G.S.; Formenti, S.C.; Naing, A.; Carvajal, R.D.; Rosen, L.S.; Weiss, G.J.; et al. First-In-Human Study of Cemiplimab Alone or In Combination with Radiotherapy and/or Low-dose Cyclophosphamide in Patients with Advanced Malignancies. Clin. Cancer Res. 2020, 26, 1025–1033. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; Van Der Noort, V.; De Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; De Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non–Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, Y.; Ma, Y.; Hong, S.; Lin, L.; He, X.; Xiong, J.; Li, P.; Zhao, H.; Huang, Y.; et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol. 2018, 19, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Levy, B.P.; Giaccone, G.; Besse, B.; Felip, E.; Garassino, M.C.; Gomez, M.D.; Garrido, P.; Piperdi, B.; Ponce-Aix, S.; Menezes, D.; et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur. J. Cancer 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Nie, J.; Wang, C.; Liu, Y.; Yang, Q.; Mei, Q.; Dong, L.; Li, X.; Liu, J.; Ku, W.; Zhang, Y.; et al. Addition of Low-Dose Decitabine to Anti–PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 1479–1489. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Gutierrez, M.; Moreno, V.; Heinhuis, K.M.; Olszanski, A.J.; Spreafico, A.; Ong, M.; Chu, Q.S.; Carvajal, R.D.; Trigo, J.; Ochoa de Olza, M.; et al. OX40 Agonist BMS-986178 Alone or in Combination With Nivolumab and/or Ipilimumab in Patients With Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 460–472. [Google Scholar] [CrossRef]
- Spigel, D.; Jotte, R.; Nemunaitis, J.; Shum, M.; Schneider, J.; Goldschmidt, J.; Eisenstein, J.; Berz, D.; Seneviratne, L.; Socoteanu, M.; et al. Randomized Phase 2 Studies of Checkpoint Inhibitors Alone or in Combination With Pegilodecakin in Patients With Metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J. Thorac. Oncol. 2021, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2021, 41, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Research C for DE and. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. U.S. Food and Drug Ad-ministration. Published 25 January 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics (accessed on 7 May 2022).
- Xu, Y.; Hezam, K.; Ali, M.G.; Wang, Y.; Zhang, J. The efficacy and safety of Nivolumab combined with Ipilimumab in the immunotherapy of cancer: A meta-analysis. Immunopharmacol. Immunotoxicol. 2021, 43, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Chemotherapy Plus Immune Check-Point Inhibitors in Metastatic Bladder Cancer—IOS Press. Available online: https://content.iospress.com/articles/bladder-cancer/blc190260 (accessed on 7 May 2022).
- Yang, Y.; Jin, G.; Pang, Y.; Huang, Y.; Wang, W.; Zhang, H.; Tuo, G.; Wu, P.; Wang, Z.; Zhu, Z. Comparative Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Advanced Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Espíndola, L.M.; Salhab, R.M.; Dos Anjos, C.; Garicochea, B.; Munhoz, R.R. Is It Better to Use Ipilimumab Combined With a PD-1 Inhibitor or a PD-1 Inhibitor Alone as Initial Immunotherapy in Patients With Metastatic Melanoma? Clin. Ski. Cancer 2017, 2, 10–17. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2020, 371, 602–609. [Google Scholar] [CrossRef]
- Janney, A.; Powrie, F.; Mann, E.H. Host–microbiota maladaptation in colorectal cancer. Nature 2020, 585, 509–517. [Google Scholar] [CrossRef]
- Zhou, S.; Khanal, S.; Zhang, H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther. Clin. Risk Manag. 2019, 15, 211–221. [Google Scholar] [CrossRef]
- Ji, H.-H.; Tang, X.-W.; Dong, Z.; Song, L.; Jia, Y.-T. Adverse Event Profiles of Anti-CTLA-4 and Anti-PD-1 Monoclonal Antibodies Alone or in Combination: Analysis of Spontaneous Reports Submitted to FAERS. Clin. Drug Investig. 2019, 39, 319–330. [Google Scholar] [CrossRef]
- Abdelhafeez, A.; Shohdy, K.S.; Ibrahim, W. Safety of Combination Immune Checkpoint Inhibitors Compared to Monotherapy; A Systematic Review and Meta-Analysis. Cancer Investig. 2020, 38, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Vareki, S.M.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Tran, D.M.; McDowell, L.C.; Keyvani, D.; Barcelon, J.A.; Merino, O.; Wilson, L. Cost-Effectiveness of Nivolumab-Ipilimumab Combination Therapy Compared with Monotherapy for First-Line Treatment of Metastatic Melanoma in the United States. J. Manag. Care Spéc. Pharm. 2017, 23, 653–664. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Serviddio, G.; Vendemiale, G.; Muscatiello, N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J. Gastroenterol. 2016, 22, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Zheng, J.; Ma, Y.; Zhao, J.; Wang, C.; Liu, Z. The neutrophil-to-lymphocyte ratio as a predictor for recurrence of colorectal liver metastases following radiofrequency ablation. Med. Oncol. 2014, 31, 855. [Google Scholar] [CrossRef]
- Jia, W.; Wu, J.; Jia, H.; Yang, Y.; Zhang, X.; Chen, K.; Su, F. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS ONE 2015, 10, e0143061. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Ito, T.; Akagi, M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur. J. Histochem. 2021, 65, 3203. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Neftelino, S.T.; Hodge, J.P.; Oliva, C.; Campbell, J.R.; Yu, J.X. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat. Rev. Drug Discov. 2021, 20, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
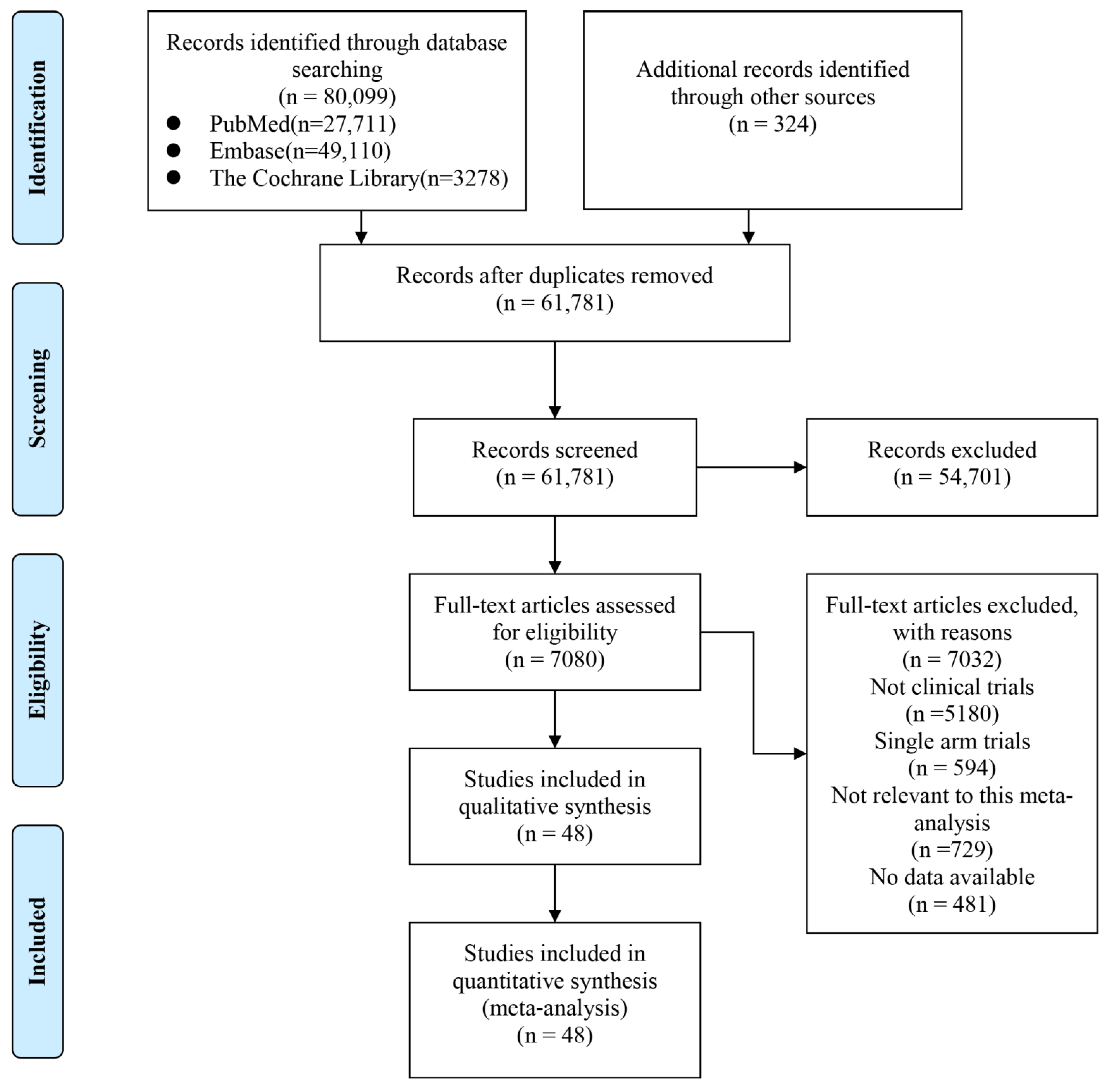
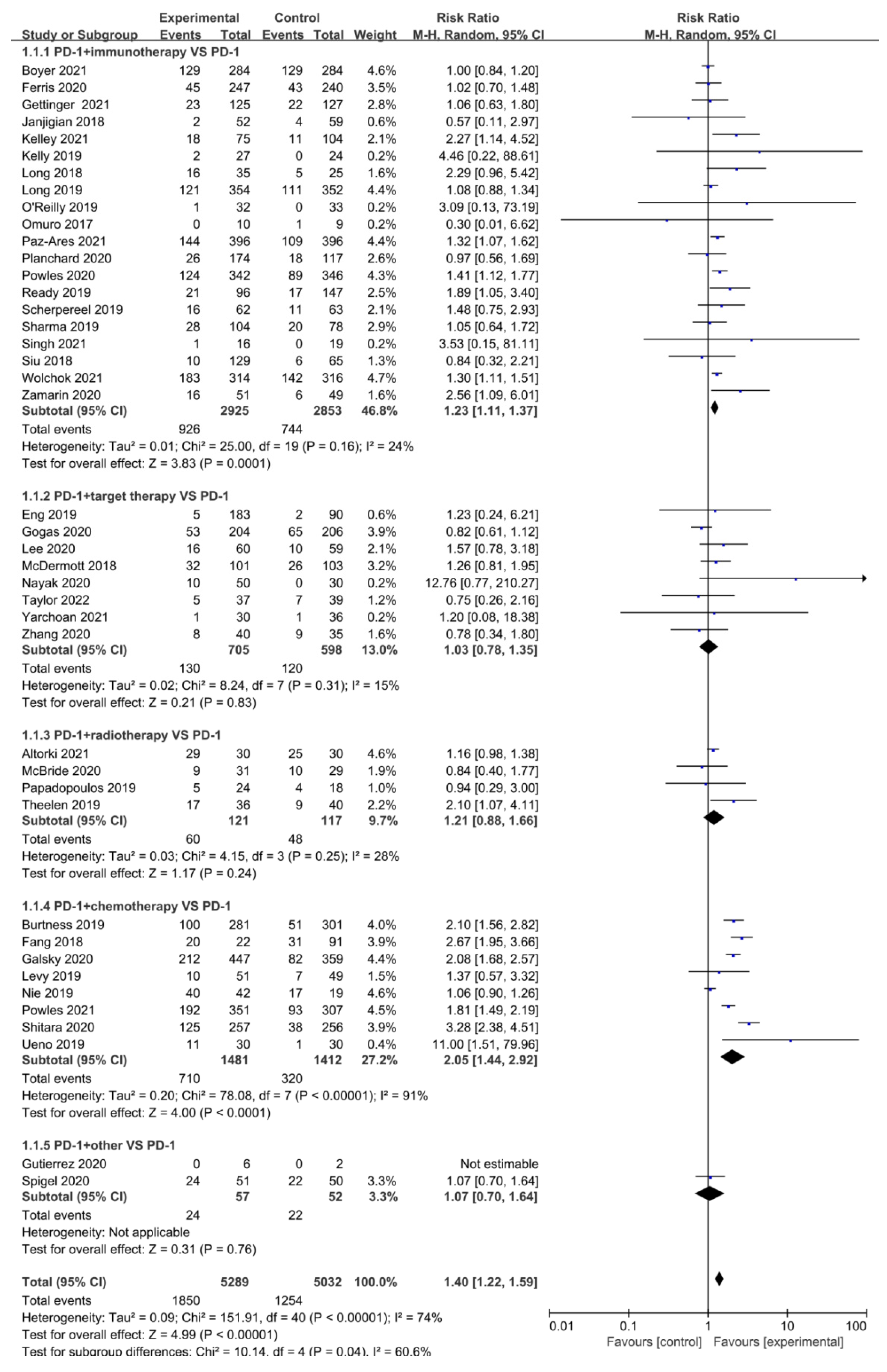
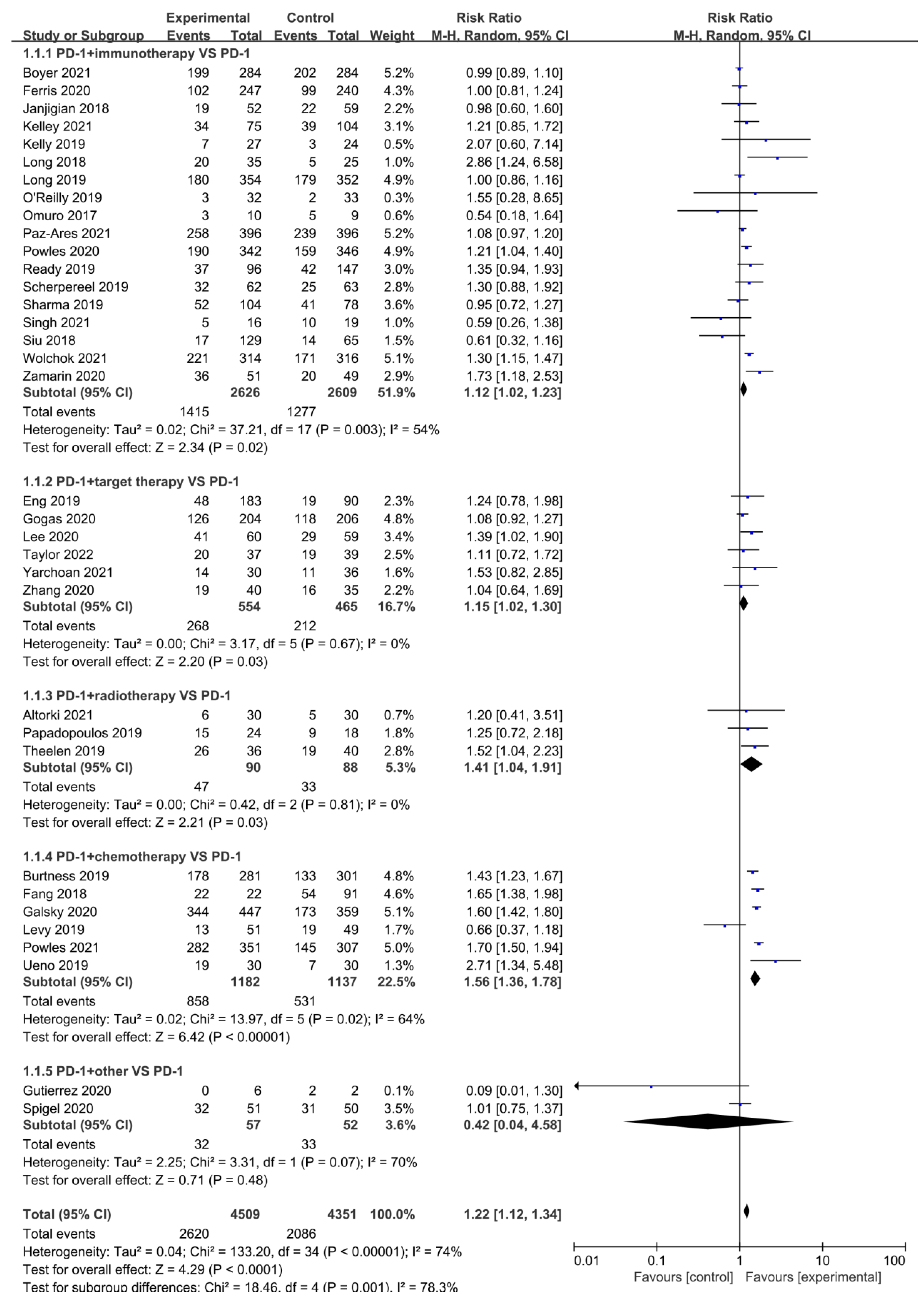
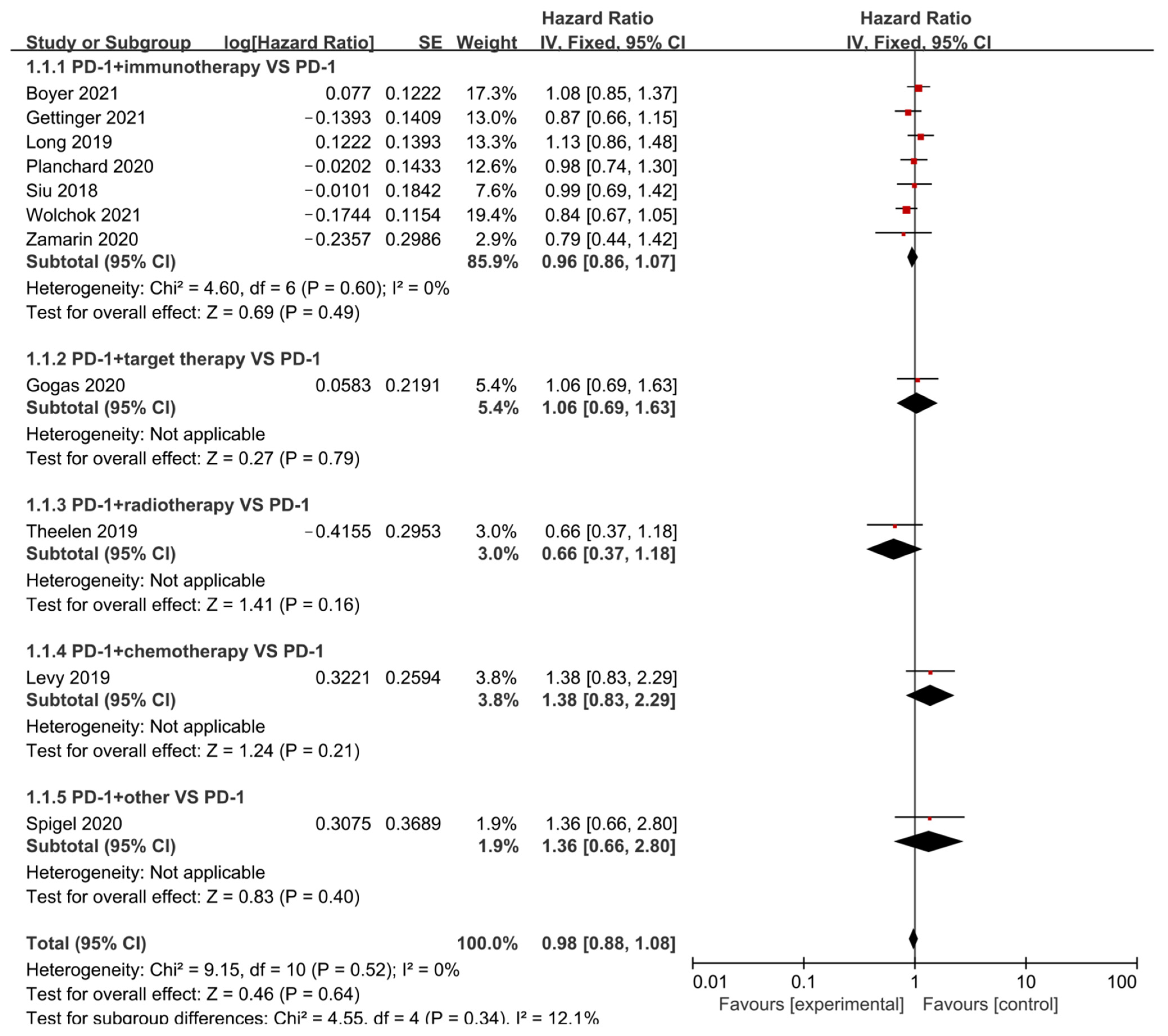
| Author | Phase | Tumor | Sample Size | Interventions | PMID | RN | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Control | |||||||
| Boyer 2021 [12] | III | NSCLC | 284 | 284 | Pembrolizumab + Ipilimumab | Pembrolizumab + Placebo | 33513313 | NCT03302234 |
| D’Angelo 2018 [15] | II | Sarcoma | 42 | 43 | Nivolumab + Ipilimumab | Nivolumab | 29370992 | NCT02500797 |
| Ferrarotto 2020 [16] | I | Oropharynx cancer | 14 | 15 | Durvalumab + Tremelimumab | Durvalumab | 32269052 | NCT03144778 |
| Ferris 2020 [17] | III | HNSCC | 247 | 240 | Durvalumab + Tremelimumab | Durvalumab | 32294530 | NCT02369874 |
| Gettinger 2021 [18] | III | NSCLC | 125 | 127 | Nivolumab + ipilimumab | Nivolumab | 34264316 | NCT02785952 |
| Janjigian 2018 [19] | I/II | Esophagogastric cancer | 52 | 59 | Nivolumab + ipilimumab | Nivolumab | 30110194 | NCT01928394 |
| Kaseb 2022 [20] | II | HCC | 14 | 13 | Nivolumab + ipilimumab | Nivolumab | 35065057 | NCT03222076 |
| Kelley 2021 [21] | I/II | HCC | 75 | 104 | Durvalumab + tremelimumab | Durvalumab | 34292792 | NCT02519348 |
| Kelly 2019 [22] | Ib/II | Gastric or GEJ cancer | 27 | 24 | Durvalumab + tremelimumab | Durvalumab | 31676670 | NCT02340975 |
| Long 2018 [23] | II | Melanoma | 35 | 25 | Nivolumab + ipilimumab | Nivolumab | 29602646 | NCT02374242 |
| Long 2019 [24] | III | Melanoma | 354 | 352 | Pembrolizumab + Epacadostat | Pembrolizumab + Placebo | 31221619 | NCT02752074 |
| Omuro 2017 [25] | I | Mlioblastoma | 10 | 10 | Nivolumab + Ipilimumab | Nivolumab | 29106665 | NCT02017717 |
| O’Reilly 2019 [26] | II | mPDAC | 32 | 32 | Durvalumab + Tremelimumab | Durvalumab | 31318392 | NCT02558894 |
| Paz-Ares 2021 [27] | III | NSCLC | 396 | 396 | Nivolumab + Ipilimumab | Nivolumab | 34648948 | NCT02477826 |
| Planchard 2020 [28] | III | NSCLC | 174 | 117 | Durvalumab + Tremelimumab | Durvalumab | 32201234 | NCT02352948 |
| Powles 2020 [29] | III | Urothelial carcinoma | 342 | 346 | Durvalumab + Tremelimumab | Durvalumab | 32971005 | NCT02516241 |
| Ready 2019 [30] | I/II | SCLC | 96 | 147 | Nivolumab + Ipilimumab | Nivolumab | 31629915 | NCT01928394 |
| Scherpereel 2019 [31] | II | MPM | 62 | 63 | Nivolumab + Ipilimumab | Nivolumab | 30660609 | NCT02716272 |
| Schoenfeld 2020 [32] | II | OCSCC | 15 | 14 | Nivolumab + Ipilimumab | Nivolumab | 32852531 | NCT02919683 |
| Sharma 2019 [33] | I/II | Urothelial carcinoma | 104 | 78 | Nivolumab + Ipilimumab | Nivolumab | 31100038 | NCT01928394 |
| Singh 2021 [34] | II | GIST | 16 | 19 | Nivolumab + Ipilimumab | Nivolumab | 34407970 | NCT02880020 |
| Siu 2018 [35] | II | HNSCC | 133 | 67 | Durvalumab + Tremelimumab | Durvalumab | 30383184 | NCT02319044 |
| Tawbi 2022 [36] | II/III | Melanoma | 355 | 359 | Nivolumab + Relatlimab | Nivolumab | 34986285 | NCT03470922 |
| Wolchok 2021 [37] | III | Melanoma | 314 | 316 | Nivolumab + Ipilimumab | Nivolumab | 34818112 | NCT01844505 |
| Zamarin 2020 [38] | II | EOC | 51 | 49 | Nivolumab + Ipilimumab | Nivolumab | 32275468 | NCT02498600 |
| Zimmer 2020 [39] | II | Melanoma | 56 | 59 | Nivolumab + Ipilimumab | Nivolumab | 32416781 | NCT02523313 |
| Eng 2019 [40] | III | Colorectal cancer | 183 | 90 | Atezolizumab + Cobimetinib | Atezolizumab | 31003911 | NCT02788279 |
| Gogas 2020 [41] | III | Melanoma | 222 | 224 | Atezolizumab + Cobimetinib | Pembrolizumab | 33309774 | NCT03273153 |
| Lee 2020 [42] | Ib | HCC | 60 | 59 | Atezolizumab + Bevacizumab | Atezolizumab | 32502443 | NCT02715531 |
| McDermott 2018 [43] | II | RCC | 101 | 103 | Atezolizumab + Bevacizumab | Atezolizumab | 29867230 | NCT01984242 |
| Nayak 2020 [44] | II | Glioblastoma | 50 | 30 | Pembrolizumab + Bevacizumab | Pembrolizumab | 33199490 | NCT02337491 |
| Taylor 2022 [45] | II | HNSCC | 37 | 39 | Pembrolizumab + Acalabrutinib | Pembrolizumab | 34862248 | NCT02454179 |
| Yarchoan 2021 [46] | II | BTC | 38 | 39 | Atezolizumab + Cobimetinib | Atezolizumab | 34907910 | NCT03201458 |
| Zhang 2020 [47] | II | Urothelial carcinoma | 40 | 35 | Pembrolizumab + Acalabrutinib | Pembrolizumab | 32757302 | NCT02351739 |
| Altorki 2021 [48] | II | NSCLC | 30 | 30 | Durvalumab + SBRT | Durvalumab | 34015311 | NCT02904954 |
| McBride 2020 [49] | II | HNSCC | 32 | 30 | Nivolumab + SBRT | Nivolumab | 32822275 | NCT02684253 |
| Papadopoulos 2019 [50] | I | Solid Tumors | 24 | 18 | Cemiplimab + hfRT | Cemiplimab | 31796520 | NCT02383212 |
| Theelen 2019 [51] | II | NSCLC | 36 | 40 | Pembrolizumab + SBRT | Pembrolizumab | 31294749 | NCT02492568 |
| Burtness 2019 [52] | III | HNSCC | 281 | 301 | Pembrolizumab + Chemotherapy | Pembrolizumab | 31679945 | NCT02358031 |
| Fang 2018 [53] | I | NPC | 23 | 93 | Camrelizumab + Chemotherapy | Camrelizumab | 30213452 | NCT02721589NCT03121716 |
| Galsky 2020 [54] | III | Urothelial carcinoma | 451 | 362 | Atezolizumab + Chemotherapy | Atezolizumab | 32416780 | NCT02807636 |
| Levy 2019 [55] | II | NSCLC | 51 | 49 | Pembrolizumab + CC-486 | Pembrolizumab + Placebo | 30654297 | NCT02546986 |
| Nie 2019 [56] | II | cHL | 42 | 19 | Camrelizumab + Decitabine | Camrelizumab | 31039052 | NCT02961101NCT03250962 |
| Powles 2021 [57] | III | Urothelial carcinoma | 351 | 307 | Pembrolizumab + Chemotherapy | Pembrolizumab | 34051178 | NCT02853305 |
| Shitara 2020 [58] | III | Gastric Cancer | 257 | 256 | Pembrolizumab + Chemotherapy | Pembrolizumab | 32880601 | NCT02494583 |
| Ueno 2019 [59] | I | BTC | 30 | 30 | Nivolumab + Chemotherapy | Nivolumab | 31109808 | JapicCTI-153098 |
| Gutierrez 2020 [60] | I/IIa | Bladder cancer | 6 | 2 | BMS-986178 + Nivolumab | Nivolumab | 33148673 | NCT02737475 |
| Spigel 2020 [61] | II | NSCLC | 51 | 50 | Pembrolizumab + Pegilodecakin | Pembrolizumab | 33166722 | NCT03382899NCT03382912 |
| Subgroup and Author (Year) | ES | [95% Conf. Interval] | %Weight | |
|---|---|---|---|---|
| PD-1 + Immunotherapy vs. PD-1 | ||||
| Omuro 2017 [25] | 0.885 | 0.564 | 1.387 | 2.17 |
| Schrpereel 2019 [31] | 1.336 | 1.121 | 1.592 | 3.35 |
| Singh 2021 [34] | 0.327 | 0.235 | 0.456 | 2.68 |
| Zamarin 2020 [38] | 1.289 | 1.060 | 1.568 | 3.27 |
| Planchard 2020 [28] | 1.150 | 1.025 | 1.290 | 3.55 |
| O’Reilly 2019 [26] | 0.861 | 0.675 | 1.098 | 3.08 |
| Wolchok 2021 [37] | 1.954 | 1.807 | 2.113 | 3.64 |
| Siu 2018 [35] | 1.267 | 1.100 | 1.458 | 3.48 |
| Paz-Ares 2021 [27] | 1.089 | 1.016 | 1.168 | 3.66 |
| Boyer 2021 [12] | 0.977 | 0.900 | 1.061 | 3.63 |
| Ready 2019 [30] | 0.825 | 0.727 | 0.935 | 3.52 |
| Sharma 2019 [33] | 0.747 | 0.646 | 0.864 | 3.46 |
| Ferris 2020 [17] | 0.855 | 0.783 | 0.935 | 3.62 |
| Kelley 2021 [21] | 1.238 | 1.070 | 1.434 | 3.46 |
| Kelly 2019 [22] | 2.706 | 2.056 | 3.560 | 2.94 |
| D’Angelo 2018 [15] | 1.336 | 1.081 | 1.653 | 3.21 |
| Gettinger 2021 [18] | 0.909 | 0.804 | 1.029 | 3.53 |
| Powles 2020 [29] | 1.144 | 1.062 | 1.233 | 3.65 |
| Janjigian 2018 [19] | 0.774 | 0.643 | 0.932 | 3.31 |
| Subgroup, DL | 1.059 | 0.920 | 1.220 | 63.20 |
| PD-1 + target therapy vs. PD-1 | ||||
| Eng 2019 [40] | 1.249 | 1.110 | 1.407 | 3.54 |
| Nayak 2020 [44] | 0.854 | 0.686 | 1.064 | 3.18 |
| Taylor 2022 [45] | 1.010 | 0.807 | 1.265 | 3.16 |
| Zhang 2020 [47] | 0.553 | 0.441 | 0.693 | 3.15 |
| Subgroup, DL | 0.885 | 0.620 | 1.262 | 13.03 |
| PD-1 + radiotherapy vs. PD-1 | ||||
| McBride 2020 [49] | 0.979 | 0.760 | 1.261 | 3.03 |
| Theelen 2019 [51] | 2.092 | 1.671 | 2.620 | 3.16 |
| Subgroup, DL | 1.434 | 0.681 | 3.019 | 6.19 |
| PD-1 + chemotherapy vs. PD-1 | ||||
| Burtness 2019 [52] | 0.872 | 0.804 | 0.946 | 3.63 |
| Shitara 2020 [58] | 1.179 | 1.081 | 1.286 | 3.62 |
| Ueno 2019 [59] | 2.962 | 2.299 | 3.814 | 3.03 |
| Galsky 2020 [54] | 1.019 | 0.951 | 1.092 | 3.66 |
| Powles 2021 [57] | 1.090 | 1.010 | 1.176 | 3.64 |
| Subgroup, DL | 1.222 | 1.006 | 1.484 | 17.59 |
| Overall, DL | 1.086 | 0.980 | 1.203 | 100.00 |
| Subgroup and Author (Year) | ES | [95% Conf. Interval] | % Weight | |
|---|---|---|---|---|
| PD-1 + Immunotherapy vs. PD-1 | ||||
| Kaseb 2022 [20] | 2.078 | 1.425 | 3.030 | 2.29 |
| Omuro 2017 [25] | 0.789 | 0.504 | 1.238 | 2.13 |
| Scherpereel 2019 [31] | 1.400 | 1.175 | 1.668 | 2.67 |
| Singh 2021 [34] | 0.710 | 0.510 | 0.989 | 2.39 |
| Zamarin 2020 [38] | 1.950 | 1.603 | 2.372 | 2.64 |
| Planchard 2020 [28] | 1.129 | 1.006 | 1.266 | 2.74 |
| O’Reilly 2019 [26] | 1.000 | 0.784 | 1.275 | 2.56 |
| Long 2019 [24] | 0.959 | 0.891 | 1.033 | 2.77 |
| Long 2018 [23] | 5.308 | 4.121 | 6.836 | 2.54 |
| Tawbi 2022 [36] | 2.196 | 2.040 | 2.363 | 2.77 |
| Wolchok 2021 [37] | 1.667 | 1.541 | 1.802 | 2.77 |
| Siu 2018 [35] | 1.053 | 0.914 | 1.212 | 2.71 |
| Paz-Ares 2021 [27] | 1.214 | 1.133 | 1.302 | 2.77 |
| Boyer 2021 [12] | 0.976 | 0.899 | 1.060 | 2.76 |
| Ready 2019 [30] | 1.071 | 0.945 | 1.215 | 2.73 |
| Sharma 2019 [33] | 0.929 | 0.803 | 1.074 | 2.70 |
| Kelley 2021 [21] | 1.048 | 0.905 | 1.214 | 2.70 |
| Kelly 2019 [22] | 1.125 | 0.855 | 1.480 | 2.50 |
| D’Angelo 2018 [15] | 2.412 | 1.950 | 2.983 | 2.61 |
| Gettinger 2021 [18] | 1.310 | 1.158 | 1.483 | 2.73 |
| Powles 2020 [29] | 1.609 | 1.493 | 1.734 | 2.77 |
| Janjigian 2018 [19] | 1.143 | 0.949 | 1.377 | 2.65 |
| Subgroup, DL | 1.337 | 1.157 | 1.544 | 57.88 |
| PD-1 + target therapy vs. PD-1 | ||||
| Eng 2019 [40] | 0.985 | 0.874 | 1.109 | 2.73 |
| McDermott 2018 [43] | 1.918 | 1.672 | 2.200 | 2.71 |
| Gogas 2020 [41] | 0.965 | 0.876 | 1.063 | 2.75 |
| Nayak 2020 [44] | 2.867 | 2.303 | 3.570 | 2.60 |
| Yarchoan 2021 [46] | 1.952 | 1.533 | 2.484 | 2.56 |
| Taylor 2022 [45] | 1.588 | 1.268 | 1.989 | 2.59 |
| Lee 2020 [42] | 1.647 | 1.376 | 1.971 | 2.66 |
| Zhang 2020 [47] | 1.375 | 1.097 | 1.724 | 2.59 |
| Subgroup, DL | 1.559 | 1.189 | 2.044 | 21.20 |
| PD-1 + radiotherapy vs. PD-1 | ||||
| Papadopoulos 2019 [50] | 1.583 | 1.170 | 2.142 | 2.45 |
| McBride 2020 [49] | 1.368 | 1.063 | 1.762 | 2.54 |
| Theelen 2019 [51] | 3.474 | 2.774 | 4.349 | 2.59 |
| Subgroup, DL | 1.968 | 1.068 | 3.625 | 7.58 |
| PD-1 + chemotherapy vs. PD-1 | ||||
| Burtness 2019 [52] | 2.130 | 1.964 | 2.311 | 2.76 |
| Levy 2019 [55] | 0.725 | 0.596 | 0.882 | 2.64 |
| Shitara 2020 [58] | 3.450 | 3.164 | 3.762 | 2.76 |
| Ueno 2019 [59] | 3.000 | 2.329 | 3.864 | 2.54 |
| Subgroup, DL | 2.004 | 1.178 | 3.408 | 10.70 |
| PD-1 + other vs. PD-1 | ||||
| Spigel 2020 [61] | 1.033 | 0.850 | 1.255 | 2.64 |
| Subgroup, DL | 1.033 | 0.850 | 1.255 | 2.64 |
| Overall, DL | 1.475 | 1.290 | 1.688 | 100.00 |
| Experimental vs. Control | No. of Studies | RR | 95% CI | p | Heterogeneity (I2) |
|---|---|---|---|---|---|
| Any grade adverse events | 38 | 1.13 | [1.08, 1.18] | <0.001 | 89% |
| Any grade fatigue | 42 | 1.31 | [1.15, 1.50] | <0.001 | 62% |
| Any grade diarrhea | 37 | 1.87 | [1.58, 2.21] | <0.001 | 59% |
| Any grade rash | 37 | 1.81 | [1.51, 2.16] | <0.001 | 64% |
| Any grade pruritus | 34 | 1.48 | [1.34, 1.64] | <0.001 | 39% |
| Any grade nausea | 31 | 1.75 | [1.32, 2.33] | <0.001 | 80% |
| Any grade thyroid abnormalities | 31 | 1.22 | [1.11, 1.35] | <0.001 | 26% |
| Any grade decreased appetite | 30 | 1.59 | [1.27, 2.00] | <0.001 | 69% |
| Any grade elevated enzymes | 29 | 1.93 | [1.61, 2.33] | <0.001 | 58% |
| Any grade hypothyroidism | 27 | 1.2 | [1.06, 1.35] | 0.003 | 7% |
| Any grade pain | 27 | 1.24 | [1.03, 1.49] | 0.02 | 56% |
| Any grade anemia | 26 | 2.03 | [1.54, 2.67] | <0.001 | 68% |
| Any grade pyrexia | 25 | 1.89 | [1.44, 2.48] | <0.001 | 55% |
| Any grade vomiting | 22 | 2.34 | [1.77, 3.08] | <0.001 | 56% |
| Any grade decreased white-cell count | 20 | 2.56 | [1.16, 5.67] | 0.02 | 83% |
| Any grade decreased platelet count | 19 | 2.83 | [1.27, 6.29] | 0.01 | 80% |
| Any grade constipation | 18 | 1.79 | [1.35, 2.38] | <0.001 | 63% |
| Any grade asthenia | 15 | 1.51 | [1.32, 1.72] | <0.001 | 23% |
| Any grade infection | 11 | 1.65 | [1.36, 2.01] | <0.001 | 12% |
| Any grade serious event | 11 | 1.44 | [1.21, 1.72] | <0.001 | 57% |
| Any grade treatment-related Adverse Events Leading to Discontinuation | 11 | 1.83 | [1.46, 2.29] | <0.001 | 0 |
| Any grade treatment-related serious adverse events | 10 | 2.35 | [1.97, 2.81] | <0.001 | 11% |
| Experimental vs. Control | No. of Studies | RR | 95% CI | p | Heterogeneity(I2) |
|---|---|---|---|---|---|
| 3–5 grade adverse events | 39 | 1.81 | [1.63, 2.01] | <0.001 | 77% |
| 3–5 grade fatigue | 41 | 1.93 | [1.71, 2.18] | <0.001 | 39% |
| 3–5 grade diarrhea | 34 | 3.35 | [2.46, 4.57] | <0.001 | 6% |
| 3–5 grade rash | 34 | 2.07 | [1.45, 2.93] | <0.001 | 31% |
| 3–5 grade pruritus | 31 | 2 | [0.89, 4.46] | =0.09 | 0% |
| 3–5 grade elevated enzymes | 30 | 2.05 | [1.44, 2.92] | <0.001 | 55% |
| 3–5 grade nausea | 29 | 3.06 | [2.02, 4.65] | <0.001 | 37% |
| 3–5 grade decreased appetite | 28 | 2.06 | [1.35, 3.16] | <0.001 | 24% |
| 3–5 grade thyroid abnormalities | 27 | 1.82 | [0.83, 3.97] | 0.13 | 0% |
| 3–5 grade anemia | 25 | 4.51 | [2.64, 7.70] | <0.001 | 60% |
| 3–5 grade pain | 25 | 0.97 | [0.66, 1.43] | 0.88 | 0% |
| 3–5 grade hypothyroidism | 24 | 1.13 | [0.40, 3.18] | 0.81 | 0% |
| 3–5 grade decreased white-cell count | 21 | 3.44 | [1.06, 11.19] | 0.04 | 54% |
| 3–5 grade pyrexia | 21 | 2.67 | [1.42, 5.05] | 0.002 | 0% |
| 3–5 grade vomiting | 20 | 3.91 | [1.51, 10.07] | 0.005 | 63% |
| 3–5 grade decreased platelet count | 19 | 6.12 | [1.86, 20.16] | 0.003 | 66% |
| 3–5 grade asthenia | 17 | 2.39 | [1.68, 3.40] | <0.001 | 8% |
| 3–5 grade constipation | 17 | 0.82 | [0.36, 1.90] | 0.65 | 0% |
| 3–5 grade infection | 12 | 1.25 | [1.00, 1.55] | 0.05 | 35% |
| 3–5 grade treatment-related Adverse Events Leading to Discontinuation | 8 | 3.4 | [2.26, 5.12] | <0.001 | 0% |
| 3–5 grade treatment-related serious adverse events | 6 | 2.84 | [1.65, 4.86] | <0.001 | 0% |
| 3–5 serious event | 4 | 2.29 | [1.48, 3.53] | <0.001 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yao, Q.; Pan, Y.; Fang, X.; Xu, H.; Zhao, T.; Zhu, G.; Jiang, T.; Li, S.; Cao, H. Efficacy and Safety of PD-1/PD-L1 Checkpoint Inhibitors versus Anti-PD-1/PD-L1 Combined with Other Therapies for Tumors: A Systematic Review. Cancers 2023, 15, 682. https://doi.org/10.3390/cancers15030682
Zhang Y, Yao Q, Pan Y, Fang X, Xu H, Zhao T, Zhu G, Jiang T, Li S, Cao H. Efficacy and Safety of PD-1/PD-L1 Checkpoint Inhibitors versus Anti-PD-1/PD-L1 Combined with Other Therapies for Tumors: A Systematic Review. Cancers. 2023; 15(3):682. https://doi.org/10.3390/cancers15030682
Chicago/Turabian StyleZhang, Yiru, Qigu Yao, Yong Pan, Xinru Fang, Haoying Xu, Tingxiao Zhao, Guangqi Zhu, Tianan Jiang, Shibo Li, and Hongcui Cao. 2023. "Efficacy and Safety of PD-1/PD-L1 Checkpoint Inhibitors versus Anti-PD-1/PD-L1 Combined with Other Therapies for Tumors: A Systematic Review" Cancers 15, no. 3: 682. https://doi.org/10.3390/cancers15030682
APA StyleZhang, Y., Yao, Q., Pan, Y., Fang, X., Xu, H., Zhao, T., Zhu, G., Jiang, T., Li, S., & Cao, H. (2023). Efficacy and Safety of PD-1/PD-L1 Checkpoint Inhibitors versus Anti-PD-1/PD-L1 Combined with Other Therapies for Tumors: A Systematic Review. Cancers, 15(3), 682. https://doi.org/10.3390/cancers15030682










