A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When?
Abstract
Simple Summary
Abstract
1. Background
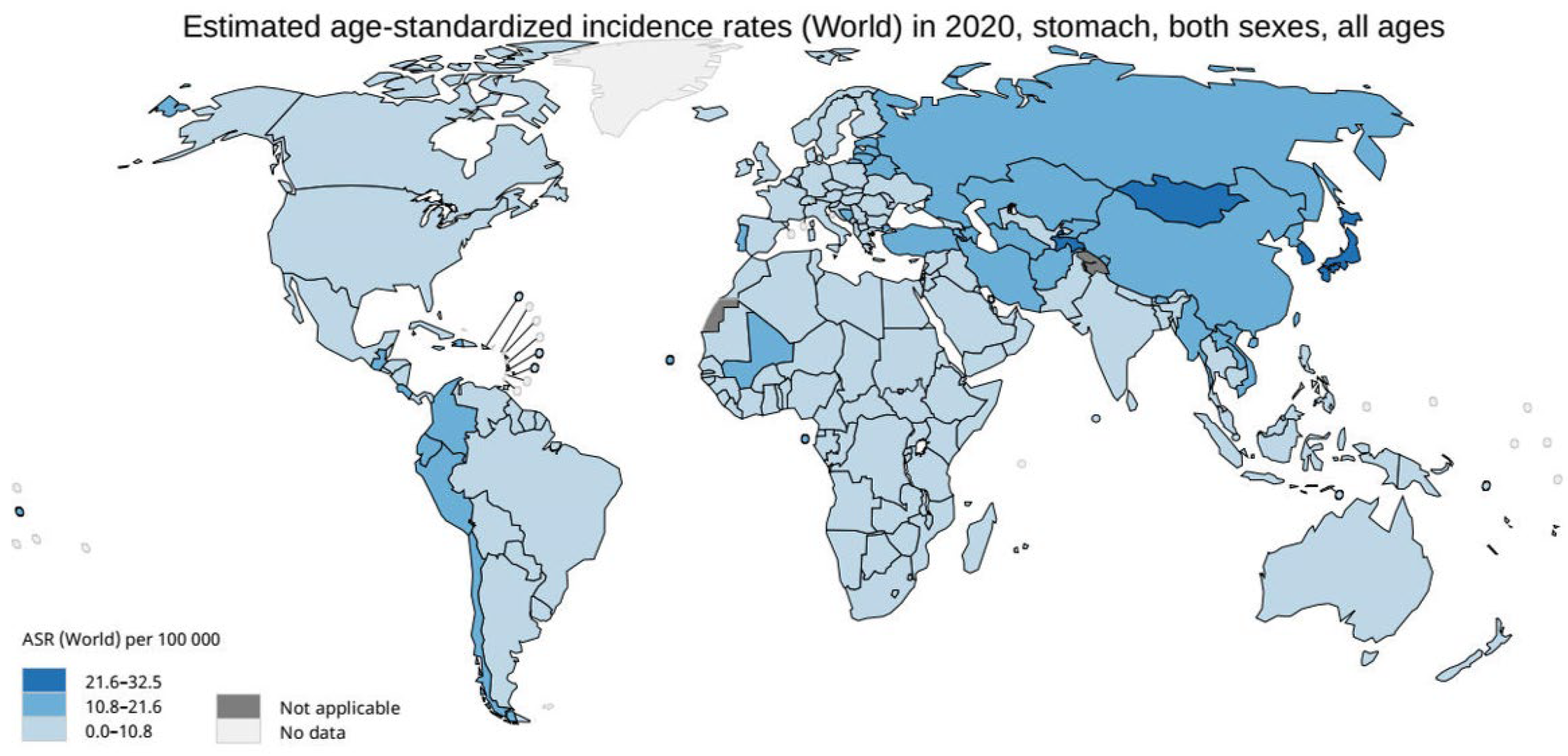
2. The Rationale for Gastric Cancer Screening
3. Primary Endoscopic Screening in High-Risk Areas
4. Gastric Cancer Screening in Low- to Intermediate-Risk Countries
Serological Biomarkers
5. Future Perspectives of Gastric Cancer Screening
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M. Global surveillance of trends in cancer survival: Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers during 2000–2014 from 322 population-based registries in 71 countries (CONCORD-3). Lancet 2018, 391, 1023. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Mori, G.; Nonaka, S.; Yoshinaga, S.; Saito, Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar]
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A. Management of helicobacter pylori infection-the maastricht V/florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The maastricht VI/florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Correa, P.; Haenszel, W.; Cuello, C.; Tannenbaum, S.; Archer, M. A model for gastric cancer epidemiology. Lancet 1975, 306, 58–60. [Google Scholar] [CrossRef]
- Hansson, L.-E.; Engstrand, L.; Nyrén, O.; Lindgren, A. Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology 1995, 109, 885–888. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; De Martel, C. Global burden of gastric cancer attributable to pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.B.; Pinto, C.B.; Lopez, A.D. Age standardization of rates: A new WHO standard. GPE Discuss Pap. Ser. 2001, 9, 1–14. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Chen, S.; Hu, J.; Guo, Q.; Liu, R. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: A meta-analysis and systematic review. Gastroenterology 2018, 155, 347–354.e9. [Google Scholar] [CrossRef]
- Hamashima, C.; Kato, K.; Miyashiro, I.; Nishida, H.; Takaku, R.; Terasawa, T. Update version of the Japanese guidelines for gastric cancer screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 2018, 48, 278–286. [Google Scholar] [CrossRef]
- Park, E.C.; Gwak, M.S.; Lee, J.Y.; Choe, G.S.; Sin, H.R. The present and challenges of national cancer screening program. J. Korea Assoc. Health Promot. 2005, 3, 280–287. [Google Scholar]
- Lee, K.S.; Oh, D.K.; Han, M.A.; Lee, H.-Y.; Jun, J.K.; Choi, K.S.; Park, E.-C. Gastric cancer screening in Korea: Report on the national cancer screening program in 2008. Cancer Res. Treat. 2011, 43, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jun, J.K.; Suh, M.; Park, B.; Noh, D.K.; Jung, K.W. Gastric cancer screening uptake trends in Korea: Results for the nation-al cancer screening program from 2002 to 2011: A prospective cross-sectional study. Medicine 2015, 94, e533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Gastric cancer screening and subsequent risk of gastric cancer: A large-scale population-based cohort study, with a 13-year follow-up in Japan. Int. J. Cancer 2006, 118, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Kuriyama, S.; Nishino, Y.; Tsubono, Y.; Nakaya, N.; Ohmori, K.; Kurashima, K.; Shibuya, D.; Tsuji, I. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: A population-based cohort study. Prev. Med. 2007, 44, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Oshima, A.; Hirata, N.; Ubukata, T.; Umeda, K.; Fujimoto, I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int. J. Cancer 1986, 38, 829–833. [Google Scholar] [CrossRef]
- Fukao, A.; Tsubono, Y.; Tsuji, I.; Hisamichi, S.; Sugahara, N.; Takano, A. The evaluation of screening for gastric cancer in miyagi prefecture, Japan: A population-based case-control study. Int. J. Cancer 1995, 60, 45–48. [Google Scholar] [CrossRef]
- Huang, H.-L.; Leung, C.Y.; Saito, E.; Katanoda, K.; Hur, C.; Kong, C.Y.; Nomura, S.; Shibuya, K. Effect and cost-effectiveness of national gastric cancer screening in Japan: A microsimulation modeling study. BMC Med. 2020, 18, 257. [Google Scholar] [CrossRef]
- Suh, Y.S.; Lee, J.; Woo, H.; Shin, D.; Kong, S.H.; Lee, H.J. National cancer screening program for gastric cancer in Korea: Na-tionwide treatment benefit and cost. Cancer 2020, 126, 1929–1939. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.-Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.-C.; et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef]
- Cho, E.; Kang, M.H.; Choi, K.S.; Suh, M.; Jun, J.K.; Park, E.-C. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac. J. Cancer Prev. 2013, 14, 2533–2540. [Google Scholar] [CrossRef]
- Dong, Z.; Qian, Y. The practice and discussion of population-based cancer screening program in China. China Cancer 2009, 9. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZHLU200909002.htm (accessed on 27 April 2022).
- Xia, R.; Zeng, H.; Liu, W.; Xie, L.; Shen, M.; Li, P.; Li, H.; Wei, W.; Chen, W.; Zhuang, G. Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China. JAMA Netw. Open 2021, 4, e2121403. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Canakis, A.; Peek, R.M.; Saumoy, M. Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 3026–3039. [Google Scholar] [CrossRef] [PubMed]
- Saumoy, M.; Schneider, Y.; Shen, N.; Kahaleh, M.; Sharaiha, R.Z.; Shah, S.C. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology 2018, 155, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, L.; Tsilegeridis-Legeris, T.; Lam, S. Clinical and endoscopic characteristics associated with post-endoscopy upper gastrointestinal cancers: A systematic review and meta-analysis. Gastroenterology 2022, 162, 1123–1135. [Google Scholar] [CrossRef]
- Luo, H.; Xu, G.; Li, C.; He, L.; Luo, L.; Wang, Z.; Jing, B.; Deng, Y.; Jin, Y.; Li, Y.; et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: A multicentre, case-control, diagnostic study. Lancet Oncol. 2019, 20, 1645–1654. [Google Scholar] [CrossRef]
- Jin, P.; Ji, X.; Kang, W.; Li, Y.; Liu, H.; Ma, F.; Ma, S.; Hu, H.; Li, W.; Tian, Y. Artificial intelligence in gastric cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Oura, H.; Matsumura, T.; Fujie, M.; Ishikawa, T.; Nagashima, A.; Shiratori, W.; Tokunaga, M.; Kaneko, T.; Imai, Y.; Oike, T.; et al. Development and evaluation of a double-check support system using artificial intelligence in endoscopic screening for gastric cancer. Gastric Cancer 2021, 25, 392–400. [Google Scholar] [CrossRef]
- Anta, J.A.; Dinis-Ribeiro, M. Early gastric cancer and artificial intelligence: Is it time for population screening? Best Pract. Res. Clin. Gastroenterol. 2020, 52–53, 101710. [Google Scholar] [CrossRef]
- Areia, M.; Spaander, M.C.; Kuipers, E.J.; Dinis-Ribeiro, M. Endoscopic screening for gastric cancer: A cost-utility analysis for countries with an intermediate gastric cancer risk. United Eur. Gastroenterol. J. 2018, 6, 192–202. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; Di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, P.; Cassone, M.; Luzzi, I.; Lucchetti, C.; Otvos, L.; Giordano, A. Helicobacter pylori as a class I carcinogen: Physio-pathology and management strategies. J. Cell. Biochem. 2007, 102, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-M.; Malfertheiner, P.; Lee, Y.-C.; Sheu, B.-S.; Sugano, K.; Cheng, H.-C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, T.-H.; Chiu, H.-M.; Wu, M.-S.; Yeh, Y.-P.; Chen, T.H.-H.; Chen, S.L.-S.; Yen, A.M.-F.; Fann, J.C.-Y.; Chiu, S.Y.-H.; et al. Community-based gastric cancer screening coupled with a national colorectal cancer screening program: Baseline results. Gastroenterology 2021, 160, 2159–2161.e4. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef]
- De Vries, A.C.; Van Grieken, N.C.T.; Looman, C.W.N.; Casparie, M.K.; De Vries, E.; Meijer, G.A. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European society of gastrointestinal endoscopy (ESGE), European helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and sociedade portuguesa de endoscopia digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; De Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European society of gastrointestinal endoscopy (ESGE), European helicobacter study group (EHSG), European society of pathology (ESP), and the sociedade portuguesa de endoscopia digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [CrossRef]
- Samloff, I.M.; Liebman, W.M. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology 1973, 65, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, M.; Miki, K.; Ichinose, M.; Kakei, N.; Yahagi, N.; Suzuki, T.; Shimizu, Y.; Ishihama, S.; Tsukada, S.; Kurokawa, K.; et al. The clinical application of the serum pepsinogen I And II levels as a mass screening method for gastric cancer. Adv. Exp. Med. Biol. 1995, 362, 139–143. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagata, Y.; Hiratsuka, R.; Kawase, Y.; Tominaga, T.; Takeuchi, S.; Sakagami, S.; Ishida, S. Gastric cancer screening by combined assay for serum anti-helicobacter pylori IgG antibody and serum pepsinogen levels—The ABC method. Digestion 2016, 93, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.M.; Hur, C.; Ward, Z.; Schrag, D.; Goldie, S.J. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: A cost-effectiveness analysis. Gut 2015, 65, 563–574. [Google Scholar] [CrossRef]
- In, H.; Sarkar, S.; Ward, J.; Friedmann, P.; Parides, M.; Yang, J. Serum pepsinogen as a biomarker for gastric cancer in the United States: A nested case–Control study using the PLCO cancer screening trial data. Cancer Epidemiol. Biomark. Prev. 2022, 31, OF1–OF7. [Google Scholar] [CrossRef] [PubMed]
- Robles, C.; Rudzite, D.; Polaka, I.; Sjomina, O.; Tzivian, L.; Kikuste, I.; Tolmanis, I.; Vanags, A.; Isajevs, S.; Liepniece-Karele, I.; et al. Assessment of serum pepsinogens with and without co-testing with gastrin-17 in gastric cancer risk assessment—Results from the GISTAR pilot study. Diagnostics 2022, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Selgrad, M.; Bornschein, J.; Kandulski, A.; Weigt, J.; Roessner, A.; Wex, T.; Malfertheiner, P. Combined gastric and colorectal cancer screening—A new strategy. Int. J. Mol. Sci. 2018, 19, 3854. [Google Scholar] [CrossRef]
- Yue, H.; Shan, L.; Bin, L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: A sys-tematic review and meta-analysis. Gastric Cancer 2018, 21, 579–587. [Google Scholar] [CrossRef]
- Den Hollander, W.J.; Holster, I.L.; Den Hoed, C.M.; Capelle, L.G.; Tang, T.J.; Anten, M.P. Surveillance of premalignant gastric lesions: A multicentre prospective cohort study from low incidence regions. Gut 2019, 68, 585–593. [Google Scholar] [CrossRef]
- Zagari, R.M.; Rabitti, S.; Greenwood, D.C.; Eusebi, L.H.; Vestito, A.; Bazzoli, F. Systematic review with meta-analysis: Diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment. Pharmacol. Ther. 2017, 46, 657–667. [Google Scholar] [CrossRef]
- Aikou, S.; Ohmoto, Y.; Gunji, T.; Matsuhashi, N.; Ohtsu, H.; Miura, H.; Kubota, K.; Yamagata, Y.; Seto, Y.; Nakajima, A.; et al. Tests for serum levels of trefoil factor family proteins can improve gastric cancer screening. Gastroenterology 2011, 141, 837–845.e7. [Google Scholar] [CrossRef] [PubMed]
- Januszewicz, W.; Fitzgerald, R.C.R.C. Early Detection and Therapeutics; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Liu, M.C. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Mencel, J.; Slater, S.; Cartwright, E.; Starling, N. The role of ctDNA in gastric cancer. Cancers 2022, 14, 5105. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Uedo, N.; Kamada, T.; Hirasawa, T.; Nagahama, T.; Yoshinaga, S.; Oka, M.; Inoue, K.; Mabe, K.; Yao, T.; et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig. Endosc. 2020, 32, 663–698. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef]
| Japan [18,19] | South Korea [20,21] | China—UGCED Program | China—CanSPUC Program | |
|---|---|---|---|---|
| Year of implementation: | 1983 (last updated in 2018) | 1999 (last updated in 2015) | 2008 (last updated in 2020) | 2012 |
| Coverage: | Nationwide | Nationwide | High-risk rural areas | High-risk urban areas |
| Screening test: | UGIS or EGD | UGIS or EGD (EGD recommended) | EGD | Questionnaire + HP and EGD |
| Target age for screening: | ≥50 years (no upper age limit) | ≥40 (no upper age limit) | 40–69 years | 40–69 years |
| Screening interval: | Every 2–3 years | Every 2 years | Individuals diagnosed with severe CAG/IM and LGD: repeated endoscopy within 3 years | CAG/IM/ gastric polyps: repeated endoscopy within 6–12 months; LGD: repeated endoscopy within 3–6 months |
| Compliance: | 48.0% (2019) | 45.4% (2011) [22] | NA. | NA |
| Low-Risk Areas (ASR < 10 per 100,000) | Intermediate-Risk Areas (ASR ≥ 10 and <20 per 100,000) | High-Risk Areas (ASR ≥ 20 per 100,000) |
|---|---|---|
| Targeted GC screening for at-risk individuals (postulated and potentially cost-effective) | Primary GC screening (established and cost-effective) | |
| H. pylori “screen-and-treat” method for at-risk individuals (e.g., family history of GC, precancerous gastric lesions) | H. pylori “screen-and-treat” general population | |
| Serological testing (serum pepsinogen) in high-risk individuals (e.g., smoking men over 50 years of age) | Upper GI endoscopy in FOBT-positive CRC screening individuals | Primary-imaging screening (upper GI endoscopy/gastrography) for individuals ≥40 (50) years old. |
| Stool antigen H. pylori testing combined with a FOBT-based CRC screening program | ||
| Serological testing (serum pepsinogen) coupled with CRC-screening program | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszewicz, W.; Turkot, M.H.; Malfertheiner, P.; Regula, J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers 2023, 15, 664. https://doi.org/10.3390/cancers15030664
Januszewicz W, Turkot MH, Malfertheiner P, Regula J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers. 2023; 15(3):664. https://doi.org/10.3390/cancers15030664
Chicago/Turabian StyleJanuszewicz, Wladyslaw, Maryla Helena Turkot, Peter Malfertheiner, and Jaroslaw Regula. 2023. "A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When?" Cancers 15, no. 3: 664. https://doi.org/10.3390/cancers15030664
APA StyleJanuszewicz, W., Turkot, M. H., Malfertheiner, P., & Regula, J. (2023). A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers, 15(3), 664. https://doi.org/10.3390/cancers15030664






