The Relationship between Cancer and Dementia: An Updated Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Lung Cancer and Dementia
3. Breast Cancer and Dementia
4. Head and Neck Cancer and Dementia
5. Gastric Cancer and Dementia
6. Prostate Cancer and Dementia
7. Colorectal Cancer and Dementia
8. Brain Tumors/Metastases and Dementia
9. Commonly Used Strategies to Reduce Dementia in Cancer Patients
10. Summary of Current Evidence of the Association between Cancer and Dementia
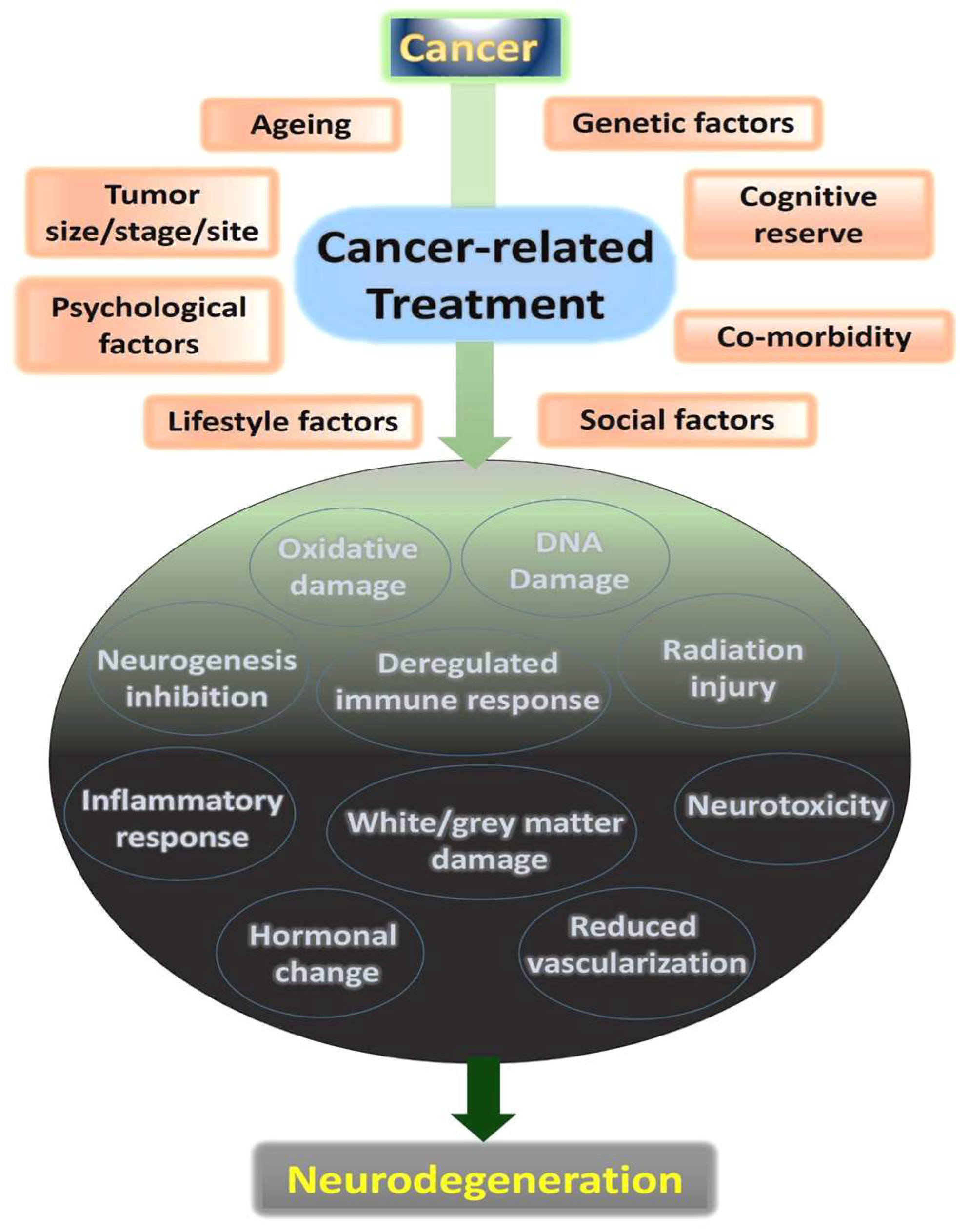
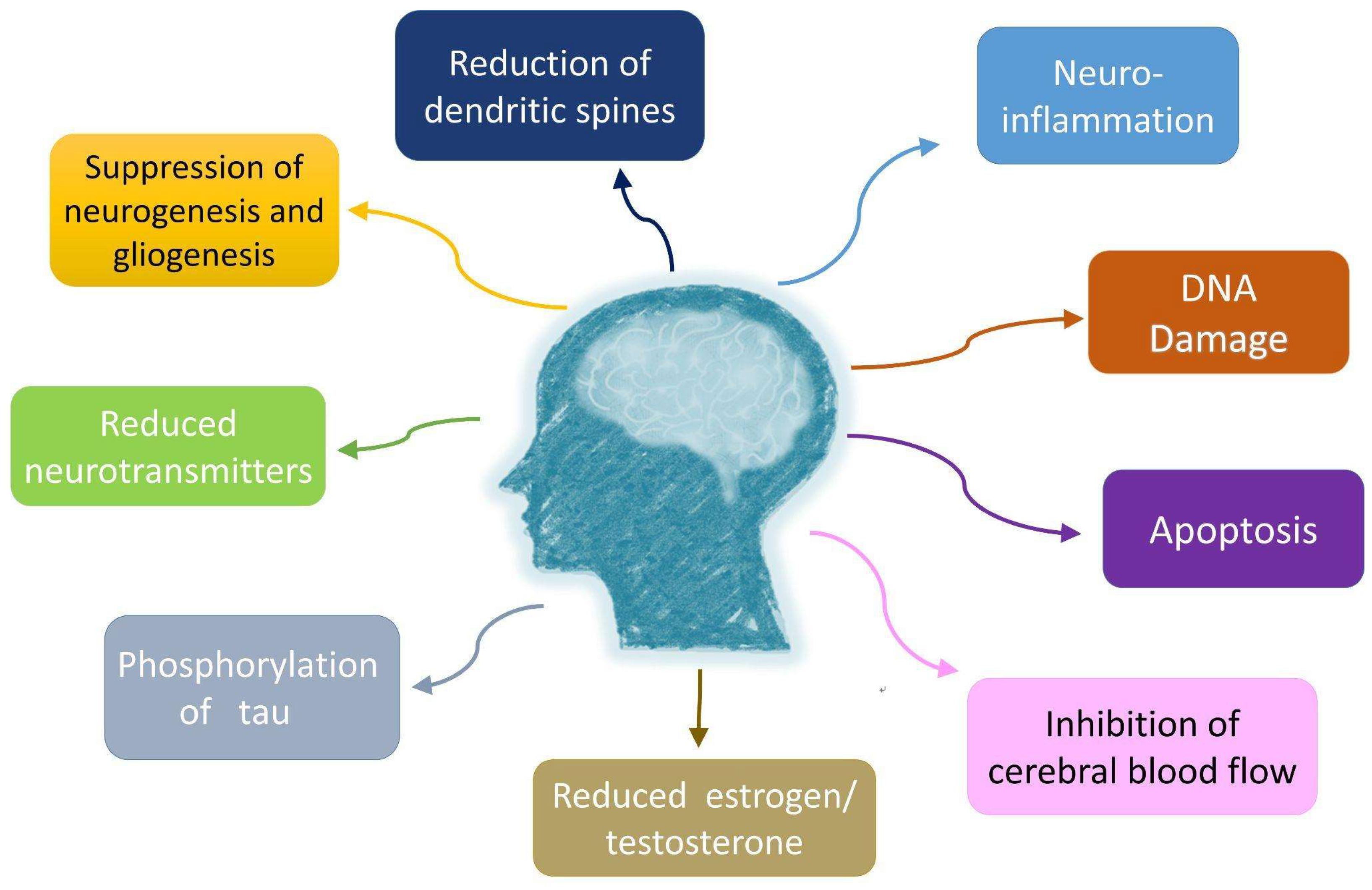
11. Cellular Mechanisms of Cancer-Related Effects on Neurodegeneration
12. Factors Predispose Patients for Dementia following Cancer Therapy
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Lu, L.Y.; Wu, M.Y.; Kao, Y.S.; Hung, C.H. Non-alcoholic fatty liver disease and the risk of dementia: A meta-analysis of cohort studies. Clin. Mol. Hepatol. 2022, 28, 931–932. [Google Scholar] [CrossRef]
- Edgerton-Fulton, M.; Ergul, A. Vascular contributions to cognitive impairment/dementia in diabetes: Role of endothelial cells and pericytes. Am. J. Physiol.-Cell Physiol. 2022, 323, C1177–C1189. [Google Scholar] [CrossRef]
- Du, X.L.; Xia, R.; Hardy, D. Relationship Between Chemotherapy Use and Cognitive Impairments in Older Women With Breast Cancer: Findings From a Large Population-Based Cohort. Am. J. Clin. Oncol. 2010, 33, 533–543. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients With Advanced Non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 14 November 2022).
- Spiro, S.G.; Douse, J.; Read, C.; Janes, S. Complications of lung cancer treatment. Semin. Respir. Crit. Care Med. 2008, 29, 302–317. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Simó, M.; Rifà-Ros, X.; Rodriguez-Fornells, A.; Bruna, J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Simó, M.; Root, J.C.; Vaquero, L.; Ripollés, P.; Jové, J.; Ahles, T.; Navarro, A.; Cardenal, F.; Bruna, J.; Rodríguez-Fornells, A. Cognitive and brain structural changes in a lung cancer population. J. Thoracic Oncol. 2015, 10, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef]
- Yang, B.; Luo, C.; Yu, M.; Zhou, L.; Tao, B.; Tang, B.; Zhou, Y.; Zhu, J.; Huang, M.; Peng, F.; et al. Changes of Brain Structure in Patients With Metastatic Non-Small Cell Lung Cancer After Long-Term Target Therapy With EGFR-TKI. Front. Oncol. 2020, 10, 573512. [Google Scholar] [CrossRef]
- Kang, H.L.; Chen, V.C.; Hung, W.L.; Hsiao, H.P.; Wang, W.H. Preliminary comparison of neuropsychological performance in patients with non-small-cell lung cancer treated with chemotherapy or targeted therapy. Neuropsychiatr. Dis. Treat. 2019, 15, 753–761. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Bekele, T.; Renton, C.; Dodd, A.; Zhang, H.; Beale, P.; Clarke, S.; et al. Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. J. Clin. Oncol. 2015, 33, 4085–4092. [Google Scholar] [CrossRef]
- Bartels, F.; Wandrey, M.M.; Aigner, A.; Strönisch, T.; Farmer, K.; Rentzsch, K.; Tessmer, A.; Grohé, C.; Finke, C. Association Between Neuronal Autoantibodies and Cognitive Impairment in Patients With Lung Cancer. JAMA Oncol. 2021, 7, 1302–1310. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Mehta, M.P.; Tome, W.; Benzinger, T.; Bovi, J.A.; Corn, B.W.; Fogh, S.E.; Robinson, C.G.; Wefel, J.S.; et al. NRG Oncology CC003: A randomized phase II/III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small cell lung cancer. J. Clin. Oncol. 2019, 37, TPS8578. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Rao, V.; Ray, W.J.; Rao, A. Probability of Alzheimer’s disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement. 2017, 9, 67–75. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; de Ruiter, M.B.; van der Lijn, F.; Boogerd, W.; Seynaeve, C.; van der Lugt, A.; Vrooman, H.; Niessen, W.J.; Breteler, M.M.; Schagen, S.B. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res. Treat. 2012, 132, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Fuchsberger, T.; Giraldo, E.; Viña, J. Molecular mechanisms linking amyloid β toxicity and Tau hyperphosphorylation in Alzheimer’s disease. Free Radic. Biol. Med. 2015, 83, 186–191. [Google Scholar] [CrossRef]
- Moysich, K.B.; Freudenheim, J.L.; Baker, J.A.; Ambrosone, C.B.; Bowman, E.D.; Schisterman, E.F.; Vena, J.E.; Shields, P.G. Apolipoprotein E genetic polymorphism, serum lipoproteins, and breast cancer risk. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center. Mol. Carcinog. 2000, 27, 2–9. [Google Scholar] [CrossRef]
- Van Dyk, K.; Crespi, C.M.; Bower, J.E.; Carroll, J.E.; Petersen, L.; Ganz, P.A. Association of APOE4 genotype and treatment with cognitive outcomes in breast cancer survivors over time. NPJ Breast Cancer 2021, 7, 112. [Google Scholar] [CrossRef]
- Baxter, N.N.; Durham, S.B.; Phillips, K.A.; Habermann, E.B.; Virning, B.A. Risk of dementia in older breast cancer survivors: A population-based cohort study of the association with adjuvant chemotherapy. J. Am. Geriatr. Soc. 2009, 57, 403–411. [Google Scholar] [CrossRef]
- Raji, M.A.; Tamborello, L.P.; Kuo, Y.F.; Ju, H.; Freeman, J.L.; Zhang, D.D.; Giordano, S.H.; Goodwin, J.S. Risk of subsequent dementia diagnoses does not vary by types of adjuvant chemotherapy in older women with breast cancer. Med. Oncol. 2009, 26, 452–459. [Google Scholar] [CrossRef]
- Branigan, G.L.; Soto, M.; Neumayer, L.; Rodgers, K.; Brinton, R.D. Association Between Hormone-Modulating Breast Cancer Therapies and Incidence of Neurodegenerative Outcomes for Women With Breast Cancer. JAMA Netw. Open 2020, 3, e201541. [Google Scholar] [CrossRef]
- Blanchette, P.S.; Lam, M.; Le, B.; Richard, L.; Shariff, S.Z.; Pritchard, K.I.; Raphael, J.; Vandenberg, T.; Fernandes, R.; Desautels, D.; et al. The association between endocrine therapy use and dementia among post-menopausal women treated for early-stage breast cancer in Ontario, Canada. J. Geriatr. Oncol. 2020, 11, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.M.; Chen, H.J.; Liang, J.A.; Kao, C.H. Long-term use of tamoxifen reduces the risk of dementia: A nationwide population-based cohort study. Qjm 2016, 109, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ording, A.G.; Jensen, A.B.; Cronin-Fenton, D.; Pedersen, L.; Sørensen, H.T.; Lash, T.L. Null Association between Tamoxifen Use and Dementia in Danish Breast Cancer PatientsTamoxifen Prescriptions and Dementia. Cancer Epidemiol. Biomark. Prevent. 2013, 22, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Niu, J.; Lei, X.; Nowakowska, M.; Wehner, M.R.; Giordano, S.H.; Nead, K.T. Association of Endocrine Therapy and Dementia in Women with Breast Cancer. Breast Cancer (Dove Med. Press) 2021, 13, 219–224. [Google Scholar] [CrossRef]
- Shibayama, O.; Yoshiuchi, K.; Inagaki, M.; Matsuoka, Y.; Yoshikawa, E.; Sugawara, Y.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; et al. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med. 2014, 3, 702–709. [Google Scholar] [CrossRef]
- Smith, B.D.; Bellon, J.R.; Blitzblau, R.; Freedman, G.; Haffty, B.; Hahn, C.; Halberg, F.; Hoffman, K.; Horst, K.; Moran, J.; et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 2018, 8, 145–152. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Kao, Y.S.; Hsu, Y.; Hsu, C.Y. Radiotherapy Increases the Incidence of Herpes Zoster in Oral Cavity Cancer Patients—A National Population-based Cohort Study. In Vivo 2021, 35, 3547–3553. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yen, Y.-C.; Liu, S.-H.; Lee, F.-P.; Lin, K.-C.; Lai, M.-T.; Wu, C.-C.; Chen, T.-M.; Yuan, S.-P.; Chang, C.-L. Dementia risk in irradiated patients with head and neck cancer. Medicine 2015, 94, e1983. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Yeh, S.A.; Chang, C.C.; Tsai, P.C.; Wu, J.M.; Gau, J.S. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 722–726. [Google Scholar] [CrossRef]
- Penn, I.W.; Chung, C.H.; Huang, Y.C.; Chen, M.C.; Sun, C.A.; Yip, P.K.; Chien, W.C. Increased risk of dementia in patients with nasopharyngeal cancer treated with radiation therapy: A nationwide population-based cohort study. Arch. Gerontol. Geriatr. 2021, 93, 104303. [Google Scholar] [CrossRef]
- Sharma, M.B.; Jensen, K.; Amidi, A.; Eskildsen, S.F.; Johansen, J.; Grau, C. Late toxicity in the brain after radiotherapy for sinonasal cancer: Neurocognitive functioning, MRI of the brain and quality of life. Clin. Transl. Radiat. Oncol. 2020, 25, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Lindholm, J.; Cook, D.; Siddiqui, F.; Ghanem, T.A.; Chang, S.S. Association between cognitive function and quality of life in patients with head and neck cancer. JAMA Otolaryngol.—Head Neck Surg. 2017, 143, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Kitpanit, S.; Lee, A.; Pitter, K.L.; Fan, D.; Chow, J.C.H.; Neal, B.; Han, Z.; Fox, P.; Sine, K.; Mah, D.; et al. Temporal Lobe Necrosis in Head and Neck Cancer Patients after Proton Therapy to the Skull Base. Int. J. Part Ther. 2020, 6, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Schuppert, C.; Paul, A.; Nill, S.; Schwahofer, A.; Debus, J.; Sterzing, F. A treatment planning study of combined carbon ion-beam plus photon intensity-modulated radiotherapy. Phys. Imaging Radiat. Oncol. 2020, 15, 16–22. [Google Scholar] [CrossRef]
- Leong, Y.H.; Soon, Y.Y.; Lee, K.M.; Wong, L.C.; Tham, I.W.K.; Ho, F.C.H. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: A meta-analysis. Head Neck 2018, 40, 622–631. [Google Scholar] [CrossRef]
- Voon, N.S.; Abdul Manan, H.; Yahya, N. Cognitive decline following radiotherapy of head and neck cancer: Systematic review and meta-analysis of MRI correlates. Cancers 2021, 13, 6191. [Google Scholar] [CrossRef]
- Ma, J.; Shen, H.; Kapesa, L.; Zeng, S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol. Lett. 2016, 11, 2959–2964. [Google Scholar] [CrossRef]
- Chen, Y.C.; Fang, W.L.; Wang, R.F.; Liu, C.A.; Yang, M.H.; Lo, S.S.; Wu, C.W.; Li, A.F.; Shyr, Y.M.; Huang, K.H. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol. Oncol. Res. 2016, 22, 197–202. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, D.W.; Jang, W.; Lee, D.H.; Jeong, S.M.; Park, S.; Han, K.D.; Park, Y.G. Risk of Dementia in Gastric Cancer Survivors Who Underwent Gastrectomy: A Nationwide Study in Korea. Ann. Surg. Oncol. 2019, 26, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Laganà, A.S. A Review of Vitamin B12. In Molecular Nutrition: Vitamins; Academic Press: Cambridge, MA, USA, 2020; pp. 105–129. [Google Scholar]
- Wang, H.X.; Wahlin, A.; Basun, H.; Fastbom, J.; Winblad, B.; Fratiglioni, L. Vitamin B(12) and folate in relation to the development of Alzheimer’s disease. Neurology 2001, 56, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology Prostate Cancer Version 1.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 8 November 2022).
- Sari Motlagh, R.; Quhal, F.; Mori, K.; Miura, N.; Aydh, A.; Laukhtina, E.; Pradere, B.; Karakiewicz, P.I.; Enikeev, D.V.; Deuker, M.; et al. The Risk of New Onset Dementia and/or Alzheimer Disease among Patients with Prostate Cancer Treated with Androgen Deprivation Therapy: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jayadevappa, R.; Chhatre, S.; Malkowicz, S.B.; Parikh, R.B.; Guzzo, T.; Wein, A.J. Association between androgen deprivation therapy use and diagnosis of dementia in men with prostate cancer. JAMA Netw. Open 2019, 2, e196562. [Google Scholar] [CrossRef] [PubMed]
- Gandy, S.; Almeida, O.P.; Fonte, J.; Lim, D.; Waterrus, A.; Spry, N.; Flicker, L.; Martins, R.N. Chemical andropause and amyloid-β peptide. JAMA 2001, 285, 2195–2196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Chen, T.H.; Chuang, H.C.; Wu, C.T.; Hsu, R.J. Statin reduces the risk of dementia in diabetic patients receiving androgen deprivation therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 276–283. [Google Scholar] [CrossRef]
- Raji, M.A.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Effect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: Implications for cancer care. Arch. Intern Med. 2008, 168, 2033–2040. [Google Scholar] [CrossRef]
- Chiu, R.H.; Lu, S.R.; Liang, F.W.; Lin, C.L.; Ho, C.H.; Hsiao, P.C. Risk of dementia in colorectal cancer patients receiving chemotherapy: A nationwide cohort study. Cancer Epidemiol. 2022, 76, 102083. [Google Scholar] [CrossRef]
- Akushevich, I.; Yashkin, A.P.; Kravchenko, J.; Kertai, M.D. Chemotherapy and the Risk of Alzheimer’s Disease in Colorectal Cancer Survivors: Evidence From the Medicare System. JCO Oncol. Pract. 2021, 17, e1649–e1659. [Google Scholar] [CrossRef]
- Kuryba, A.J.; Boyle, J.M.; van der Meulen, J.; Aggarwal, A.; Walker, K.; Fearnhead, N.S.; Braun, M.S. Severity of Dementia and Survival in Patients Diagnosed with Colorectal Cancer: A National Cohort Study in England and Wales. Clin. Oncol. (R. Coll. Radiol.) 2022, 35, e67–e76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cress, R.D.; Stewart, S.L.; Semrad, T.J.; Harvey, D.; Tencredi, D.J.; Beckett, L. Mediating Effect of Postsurgical Chemotherapy on Presence of Dementia and Survival among Patients 65 and Older with Stage III Colon Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, X.; Jiang, X.; Li, M.; Lu, K. Impact of Alzheimer’s disease and related dementias on colorectal cancer screening utilization, knowledge, and associated health disparities. Front. Pharmacol. 2022, 13, 872702. [Google Scholar] [CrossRef] [PubMed]
- Flechl, B.; Konrath, L.; Lütgendorf-Caucig, C.; Achtaewa, M.; Hug, E.B.; Georg, P. Preservation of Neurocognition after Proton Beam Radiation Therapy for Intracranial Tumors: First Results from REGI-MA-002015. Int. J. Radiat. Oncol. Biol. Phys. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R. Radiation therapy for brain metastases: An ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1809–1815. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef]
- Rule, W.G.; Foster, N.R.; Meyers, J.P.; Ashman, J.B.; Vora, S.A.; Kozelsky, T.F.; Garces, Y.I.; Urbanic, J.J.; Salama, J.K.; Schild, S.E. Prophylactic cranial irradiation in elderly patients with small cell lung cancer: Findings from a North Central Cancer Treatment Group pooled analysis. J. Geriatr. Oncol. 2015, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Pyo, H.; Ahn, Y.C.; Noh, J.M.; Oh, D. Role of prophylactic cranial irradiation for elderly patient with limiteddisease small-cell lung cancer: Inverse probability of treatment weighting using propensity score. J. Radiat. Res. 2019, 60, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and cisplatin induced cognitive impairment: The possible mechanisms and interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- Geraghty, A.C.; Gibson, E.M.; Ghanem, R.A.; Greene, J.J.; Ocampo, A.; Goldstein, A.K.; Ni, L.; Yang, T.; Marton, R.M.; Paşca, S.P.; et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 2019, 103, 250–265.e258. [Google Scholar] [CrossRef]
- Merriman, J.D.; Von Ah, D.; Miaskowski, C.; Aouizerat, B.E. Proposed mechanisms for cancer- and treatment-related cognitive changes. Semin. Oncol. Nurs. 2013, 29, 260–269. [Google Scholar] [CrossRef]
- Ng, T.; Cheung, Y.T.; Ng, Q.S.; Ho, H.K.; Chan, A. Vascular endothelial growth factor inhibitors and cognitive impairment: Evidence and controversies. Expert Opin. Drug Saf. 2014, 13, 83–92. [Google Scholar] [CrossRef]
- Lim, I.; Joung, H.Y.; Yu, A.R.; Shim, I.; Kim, J.S. PET Evidence of the Effect of Donepezil on Cognitive Performance in an Animal Model of Chemobrain. Biomed. Res. Int. 2016, 2016, 6945415. [Google Scholar] [CrossRef]
- Zhou, W.; Kavelaars, A.; Heijnen, C.J. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PloS One 2016, 11, e0151890. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Park, J.Y.; Lengacher, C.A.; Reich, R.R.; Park, H.Y.; Whiting, J.; Nguyen, A.T.; Rodríguez, C.; Meng, H.; Tinsley, S.; Chauca, K.; et al. Translational Genomic Research: The Association between Genetic Profiles and Cognitive Functioning or Cardiac Function Among Breast Cancer Survivors Completing Chemotherapy. Biol. Res. Nurs. 2022, 24, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hajj, A.; Khoury, R.; Hachem, R.; Awad, A.; Hallit, S.; Sacre, H.; Nasr, F.; Karak, F.E.; Chahine, G.; Kattan, J.; et al. Clinical and genetic factors associated with self-reported cognitive deficits in women with breast cancer: The “CAGE-Cog” study. BMC Cancer 2022, 22, 996. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.R.; Varma, A.; Flowers, S.A.; Rebeck, G.W. Cancer Chemotherapy Related Cognitive Impairment and the Impact of the Alzheimer’s Disease Risk Factor APOE. Cancers 2020, 12, 3842. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Rheinstein, P.H. Alzheimer gene BIN1 may simultaneously influence dementia risk and androgen deprivation therapy dosage in prostate cancer. Am. J. Clin. Oncol. 2020, 43, 685. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-C.; Chen, Y.-M. Post-stroke dementia: Epidemiology, mechanisms and management. Int. J. Gerontol. 2017, 11, 210–214. [Google Scholar] [CrossRef]
- Ahtiluoto, S.; Polvikoski, T.; Peltonen, M.; Solomon, A.; Tuomilehto, J.; Winblad, B.; Sulkava, R.; Kivipelto, M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 2010, 75, 1195–1202. [Google Scholar] [CrossRef]
- Williams, A.M.; Shah, R.; Shayne, M.; Huston, A.J.; Krebs, M.; Murray, N.; Thompson, B.D.; Doyle, K.; Korotkin, J.; Van Wijngaarden, E. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 2018, 314, 17–23. [Google Scholar] [CrossRef]
- Natori, A.; Ogata, T.; Sumitani, M.; Kogure, T.; Yamauchi, T.; Yamauchi, H. Potential Role of pNF-H, a Biomarker of Axonal Damage in the Central Nervous System, as a Predictive Marker of Chemotherapy-Induced Cognitive ImpairmentPotential Role of pNF-H as a Predictive Marker of Chemobrain. Clin. Cancer Res. 2015, 21, 1348–1352. [Google Scholar] [CrossRef]
- Kesler, S.R.; Watson, C.; Koovakkattu, D.; Lee, C.; O’Hara, R.; Mahaffey, M.L.; Wefel, J.S. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2013, 7, 501–510. [Google Scholar] [CrossRef]
| Condition | Commonly Used Strategy |
|---|---|
| Brain metastasis | Hippocampal-avoidance whole-brain radiotherapy |
| Brain metastasis | Memantine for patients receiving whole-brain radiotherapy |
| Brain metastasis | Stereotactic radiosurgery |
| Brain tumor | Proton therapy |
| Head and neck cancer | Proton therapy |
| Head and neck cancer | Carbon ion therapy |
| Lung cancer | Avoidance of prophylactic cranial irradiation in elderly patients |
| Prostate cancer | Use of statins in diabetic patients with prostate cancer |
| Gastric cancer | Vitamin B12 supplement for patients receiving gastrectomy |
| Cancer Type | Surgery | Radiotherapy | Chemotherapy | Hormone Therapy | Target Therapy |
|---|---|---|---|---|---|
| Lung cancer | ? | + | O | N/A | O |
| Breast cancer | ? | + | O | +/− | ? |
| Head and neck cancer | ? | + | ? | N/A | ? |
| Gastric cancer | + | ? | ? | N/A | ? |
| Prostate cancer | ? | ? | ? | + | ? |
| Colorectal cancer | ? | ? | + | N/A | ? |
| Brain tumor/ brain metastasis | ? | + | ? | N/A | ? |
| Possible Mechanism/Function | ||
|---|---|---|
| Genetic factors | APOE-4 | Blood brain barrier dysfunction, oxidative stress, and inflammation |
| IL-1R1 | Inflammation | |
| COMT | Neurotransmitter metabolism | |
| BDNF | Mediation of neuroplasticity | |
| BIN1 | tau pathology | |
| TOMM40 | α-synuclein accumulation, oxidative damage, mitochondrial dysfunction and neuroinflammation | |
| OPRM1 | Activation of the mu-receptor | |
| Morbidity | Stroke | Brain atrophy/brain damage |
| Diabetes | Induction of vascular damage | |
| Biomarkers | pNF-H | Serum marker of axonal damage |
| TNF-α, sTNFRI/II | Inflammatory cytokine and receptor | |
| IL-1β, IL-2, IL-10, IL-6, IL-8 | Alteration of blood brain barrier | |
| NAA/Cho, NAA/mI | Neurological markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-S.; Yeh, C.-C.; Chen, Y.-F. The Relationship between Cancer and Dementia: An Updated Review. Cancers 2023, 15, 640. https://doi.org/10.3390/cancers15030640
Kao Y-S, Yeh C-C, Chen Y-F. The Relationship between Cancer and Dementia: An Updated Review. Cancers. 2023; 15(3):640. https://doi.org/10.3390/cancers15030640
Chicago/Turabian StyleKao, Yung-Shuo, Cheng-Chang Yeh, and Yi-Fang Chen. 2023. "The Relationship between Cancer and Dementia: An Updated Review" Cancers 15, no. 3: 640. https://doi.org/10.3390/cancers15030640
APA StyleKao, Y.-S., Yeh, C.-C., & Chen, Y.-F. (2023). The Relationship between Cancer and Dementia: An Updated Review. Cancers, 15(3), 640. https://doi.org/10.3390/cancers15030640






