The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Decision Model Framework
2.2. Clinical Transition Probabilities
2.3. Studies on Diagnostic Accuracy for M3
2.4. Cost Estimates
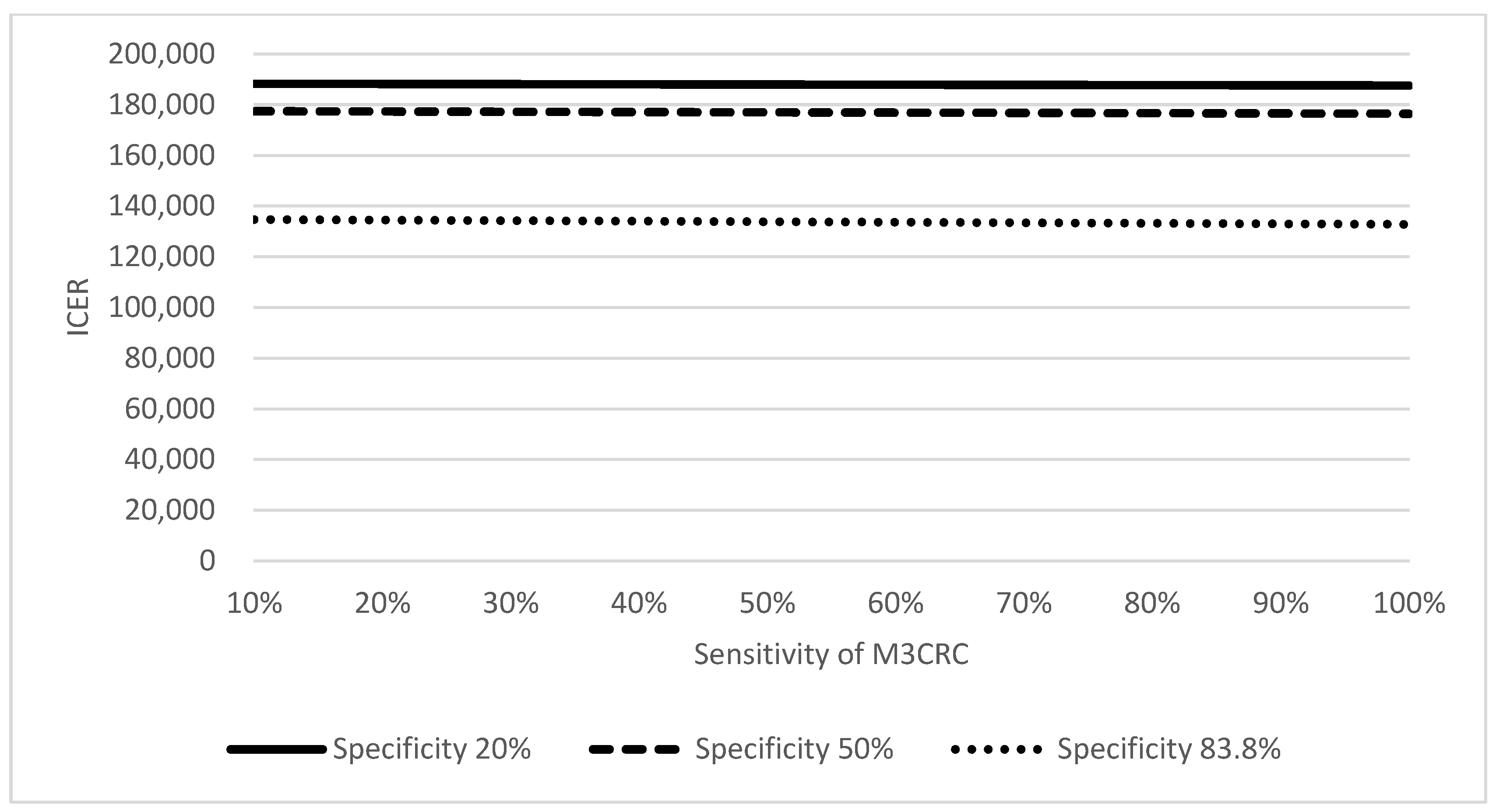
2.5. Sensitivity Analysis
2.6. Cost-Effectiveness Analysis
3. Results
4. Discussion
4.1. Major Findings
4.2. Limitations of FIT
4.3. Strengths of M3CRC When Compared with FIT and Colonoscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e61. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Huang, J.L.W.; Pang, T.W.Y.; Choi, P.; Wang, J.; Chiang, J.I.; Jiang, J.Y. Global prevalence of colorectal neoplasia: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 553–561.e10. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ngai, C.H.; Deng, Y.; Tin, M.S.; Lok, V.; Zhang, L.; Yuan, J.; Xu, W.; Zheng, Z.-J.; Wong, M.C.S. Cancer Incidence and Mortality in Asian Countries: A Trend Analysis. Cancer Control 2022, 29, 10732748221095955. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, J.D.; Chamberlain, J.O.; Robinson, M.H.; Moss, S.M.; Amar, S.S.; Balfour, T.W.; James, P.D.; Mangham, C.M. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996, 348, 1472–1477. [Google Scholar] [CrossRef]
- Kronborg, O.; Fenger, C.; Olsen, J.; Jørgensen, O.D.; Søndergaard, O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996, 348, 1467–1471. [Google Scholar] [CrossRef]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef]
- Ding, H.; Lin, J.; Xu, Z.; Chen, X.; Wang, H.H.X.; Huang, L.; Huang, J.; Zheng, Z.; Wong, M.C.S. A Global Evaluation of the Performance Indicators of Colorectal Cancer Screening with Fecal Immunochemical Tests and Colonoscopy: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1073. [Google Scholar] [CrossRef]
- Chan, F.K.L.; Wu, J.C.Y.; Ng, S.S.M.; Ng, S.C.; Wong, M.C.S. Evaluation of the Colorectal Cancer Screening Pilot Program in Hong Kong: Awareness, Knowledge, Attitude, Perception, Practice and Satisfaction of Stakeholders; The Health and Medical Research Fund (HMRF): Hong Kong, China, 2020.
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171–181. [Google Scholar] [CrossRef]
- Duran-Sanchon, S.; Herrera-Pariente, C.; Moreira, L. New non-invasive biomarkers for colorectal cancer screening. Rev. Esp. Enfermadades Dig. (REED) 2020, 112, 642–649. [Google Scholar] [CrossRef]
- Normanno, N.; Cervantes, A.; Ciardiello, F.; De Luca, A.; Pinto, C. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat. Rev. 2018, 70, 1–8. [Google Scholar] [CrossRef]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.-X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp-Vogelaar, I.; Goede, S.L.; Bosch, L.J.; Melotte, V.; Carvalho, B.; van Engeland, M.; Meijer, G.A.; de Koning, H.J.; van Ballegooijen, M. Cost-effectiveness of high-performance biomarker tests vs fecal immunochemical test for noninvasive colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2018, 16, 504–512.e11. [Google Scholar] [CrossRef]
- Imperiale, T.F. Noninvasive screening tests for colorectal cancer. Dig. Dis. 2012, 30 (Suppl. S2), 16–26. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Allen, J.; Wandell, M.; Ramsey, S. Colorectal Cancer Screening with Blood-Based Biomarkers: Cost-Effectiveness of Methylated Septin 9 DNA versus Current StrategiesColorectal Cancer Screening: Cost-Effectiveness of Biomarker Septin 9. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology 2016, 151, 427–439.e6. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Knudsen, A.B.; Brenner, H. Cost-effectiveness of colorectal cancer screening. Epidemiol. Rev. 2011, 33, 88–100. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Kuntz, K.M.; Knudsen, A.B.; Wilschut, J.A.; Zauber, A.G.; Van Ballegooijen, M. Stool DNA testing to screen for colorectal cancer in the Medicare population: A cost-effectiveness analysis. Ann. Intern. Med. 2010, 153, 368–377. [Google Scholar] [CrossRef]
- Parekh, M.; Fendrick, A.M.; Ladabaum, U. As tests evolve and costs of cancer care rise: Reappraising stool-based screening for colorectal neoplasia. Aliment. Pharmacol. Ther. 2008, 27, 697–712. [Google Scholar] [CrossRef]
- Skally, M.; Hanly, P.; Sharp, L. Cost effectiveness of fecal DNA screening for colorectal cancer: A systematic review and quality appraisal of the literature. Appl. Health Econ. Health Policy 2013, 11, 181–192. [Google Scholar] [CrossRef]
- Song, K.; Fendrick, A.M.; Ladabaum, U. Fecal DNA testing compared with conventional colorectal cancer screening methods: A decision analysis. Gastroenterology 2004, 126, 1270–1279. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Ching, J.Y.L.; Chan, V.C.W.; Lam, T.Y.T.; Luk, A.K.C.; Wong, S.H.; Ng, S.C.; Ng, S.S.M.; Wu, J.C.Y.; Chan, F.K.L.; et al. Colorectal cancer screening based on age and gender: A cost-effectiveness analysis. Medicine 2016, 95, e2739. [Google Scholar] [CrossRef]
- Wong, M.; Ching, J.Y.; Chan, V.C.; Sung, J.J. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci. Rep. 2015, 5, 13568. [Google Scholar] [CrossRef]
- Wong, M.C.; Ching, J.Y.; Wong, S.H.; Ng, S.C.; Shum, J.P.; Chan, V.C.; Lam, T.; Luk, A.; Ng, S.; Wu, J.; et al. The cost-effectiveness of adopting risk-scoring systems for population-based colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2015, 13, e86. [Google Scholar] [CrossRef]
- Wong, M.C.; Ching, J.Y.; Chan, V.C.; Lam, T.Y.; Luk, A.K.; Wong, S.H.; Ng, S.C.; Wong, V.W.; Ng, S.S.; Wu, J.C.; et al. Screening strategies for colorectal cancer among patients with nonalcoholic fatty liver disease and family history. Int. J. Cancer 2016, 138, 576–583. [Google Scholar] [CrossRef]
- Tsoi, K.; Ng, S.; Leung, M.; Sung, J. Cost-effectiveness analysis on screening for colorectal neoplasm and management of colorectal cancer in Asia. Aliment. Pharmacol. Ther. 2008, 28, 353–363. [Google Scholar] [CrossRef]
- Wong, M.; Ching, J.Y.; Chan, V.C.; Lam, T.Y.; Shum, J.P.; Luk, A.K.; Wong, S.S.; Ng, S.C.; Ng, S.S.; Wu, J.C.; et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but not number of specimens. Clin. Gastroenterol. Hepatol. 2015, 13, 1472–1479. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Gao, Q.-Y.; Fang, J.-Y. Repeat colonoscopy every 10 years or single colonoscopy for colorectal neoplasm screening in average-risk Chinese: A cost-effectiveness analysis. Asian Pac. J. Cancer Prev. 2012, 13, 1761–1766. [Google Scholar] [CrossRef]
- Non-Communicable Disease Branch CfHPDoHHKSARG. Colorectal Cancer Screening Programme-Progress Report of the Screening Outcome for Participant Enrolled between 28 September 2016 and 27 September 2019; Center for Health Protection: Hong Kong, China, 2019.
- Reumkens, A.; Rondagh, E.J.; Bakker, M.C.; Winkens, B.; Masclee, A.A.; Sanduleanu, S. Post-colonoscopy complications: A systematic review, time trends, and meta-analysis of population-based studies. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 1092–1101. [Google Scholar] [CrossRef]
- Hong Kong Cancer Registry. Overview of Hong Kong Cancer Statistics of 2015. October 2017. Available online: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202015.pdf (accessed on 30 December 2022).
- Zhang, J.; Cheng, Z.; Ma, Y.; He, C.; Lu, Y.; Zhao, Y.; Chang, X.; Zhang, Y.; Bai, Y.; Cheng, N. Effectiveness of screening modalities in colorectal cancer: A network meta-analysis. Clin. Color. Cancer 2017, 16, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Hu, C.-Y.; Eng, C.; Skibber, J.M.; Rodriguez-Bigas, M.A. Practical application of a calculator for conditional survival in colon cancer. J. Clin. Oncol. 2009, 27, 5938. [Google Scholar] [CrossRef]
- Bir, A.; Tan, W.; Wilding, G.E.; Lombardo, J.; Fakih, M.G. 5-fluorouracil, leucovorin and oxaliplatin plus bevacizumab in the first-line treatment of metastatic colorectal cancer: A single-institute study. Oncology 2007, 72, 4–9. [Google Scholar] [CrossRef]
- Omura, K. Advances in chemotherapy against advanced or metastatic colorectal cancer. Digestion 2008, 77 (Suppl. S1), 13–22. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Lam, C.L.K.; Poon, J.T.C.; McGhee, S.M.; Law, W.-L.; Kwong, D.L.W.; Tsang, J.; Chan, P. Direct medical costs of care for Chinese patients with colorectal neoplasia: A health care service provider perspective. J. Eval. Clin. Pract. 2012, 18, 1203–1210. [Google Scholar] [CrossRef]
- Drummond, M.F.; Jefferson, T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996, 313, 275–283. [Google Scholar] [CrossRef]
- Zhong, G.-C.; Sun, W.-P.; Wan, L.; Hu, J.-J.; Hao, F.-B. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 91, 684–697.e15. [Google Scholar] [CrossRef]
- Cross, A.J.; Wooldrage, K.; Robbins, E.C.; Kralj-Hans, I.; MacRae, E.; Piggott, C.; Stenson, I.; Prendergast, A.; Patel, B.; Pack, K.; et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: A diagnostic accuracy and cost-effectiveness study. Gut 2019, 68, 1642–1652. [Google Scholar] [CrossRef]
- Peterse, E.F.P.; Meester, R.G.S.; de Jonge, L.; Omidvari, A.-H.; Alarid-Escudero, F.; Knudsen, A.B.; Zauber, A.G.; Lansdorp-Vogelaar, I. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. JNCI J. Natl. Cancer Inst. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef]
- Crouse, A.L.; De Koning, L.; Sadrzadeh, S.H.; Naugler, C. Sensitivity and specificity of community fecal immunotesting screening for colorectal carcinoma in a high-risk canadian population. Arch. Pathol. Lab. Med. 2015, 139, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.H.; Liang, J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev. Mol. Diagn. 2022, 22, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Wong, S.H.; Szeto, C.H.; Chu, E.S.; Lau, H.C.; Chen, Y.; Fang, J.; Yu, J.; Sung, J.J. Fecal microbial DNA markers serve for screening colorectal neoplasm in asymptomatic subjects. J. Gastroenterol. Hepatol. 2021, 36, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Zeng, Y.; Kwok, G.; Cheung, C.P.; Suen, B.Y.; Ching, J.Y.L.; To, K.F.; Yu, J.; Chan, F.K.L.; Ng, S.C. Novel microbiome signatures for non-invasive diagnosis of adenoma recurrence after colonoscopic polypectomy. Aliment. Pharmacol. Ther. 2022, 55, 847–855. [Google Scholar] [CrossRef]
- Shah, R.; Jones, E.; Vidart, V.; Kuppen, P.J.; Conti, J.A.; Francis, N.K. Biomarkers for Early Detection of Colorectal Cancer and Polyps: Systematic ReviewBiomarkers for Early Detection of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1712–1728. [Google Scholar] [CrossRef]
- Zhu, X.; Parks, P.D.; Weiser, E.; Fischer, K.; Griffin, J.M.; Limburg, P.J.; Rutten, L.J.F. National survey of patient factors associated with colorectal cancer screening preferences. Cancer Prev. Res. 2021, 14, 603–614. [Google Scholar] [CrossRef]



| Rate | Estimate | Reference |
|---|---|---|
| Sensitivity of FIT (cutoff value = 20 µg/g) in detecting colorectal cancer | 73.0% | [16] |
| Specificity of FIT (cutoff value = 20 µg/g) in detecting colorectal cancer | 91.9% | [27] |
| Sensitivity of M3CRC in detecting a composite measure of NAA + AA + CRC | 66.5% | [12] |
| Specificity of M3CRC in detecting a composite measure of NAA + AA + CRC | 83.8% | [12] |
| Compliance rate of FIT | 60.0% | [16] |
| Compliance rate of M3CRC | 99.0% | [12] |
| Compliance of Colonoscopy | 98.9% | [22] |
| Compliance rate of Colonoscopy after a positive result | 100% | [28] |
| Rate of polypectomy of FIT | 73.0% | [29] |
| Rate of polypectomy of M3CRC | 83.9% | [12] |
| Polypectomy bleeding rate | 0.98% | [30] |
| Polypectomy perforation rate | 0.08% | [30] |
| Morality due to perforation | 0.0029% | [30] |
| Cancer prevented by FIT | 21.0% | [31] |
| Cancer prevented by Colonoscopy | 54.0% | [22] |
| Cancer prevented by M3CRC | 51.5% | [12] |
| Staging of CRC at diagnosis | ||
| I | 11.3% | [32] |
| II | 25.4% | [32] |
| III | 32.4% | [32] |
| IV | 31.0% | [32] |
| Annual mortality of CRC patients at various stages of disease (1 year) | ||
| I | 1.0% | [33] |
| II | 4.5% | [33] |
| III | 8.7% | [33] |
| IV | 43.0% | [33] |
| Cost Item | Baseline Value (USD) | Reference |
|---|---|---|
| One kit of FIT | 19 | [16] |
| M3CRC | 64 | Internal source |
| Colonoscopy | 1259 | [26,34] |
| Consultation fee | 96 | [26,34] |
| Bleeding | 3320 | [26,34] |
| Histopathological examination | 142 | [26,34] |
| Perforation | 10,790 | [26,34] |
| Treatment for the stage I of CRC | 17,071 | [26,34] |
| Diagnosis | 6091 | [26,34] |
| Treatment | 10,377 | [26,34] |
| Follow-up | 603 | [26,34] |
| Treatment for the stage II of CRC | 19,755 | [26,34] |
| Diagnosis | 6091 | [26,34] |
| Treatment | 13,061 | [26,34] |
| Follow-up | 603 | [26,34] |
| Treatment for the stage III of CRC | 26,883 | [26,34] |
| Diagnosis | 6091 | [26,34] |
| Treatment | 20,189 | [26,34] |
| Follow-up | 603 | [26,34] |
| Treatment for the stage IV of CRC | 45,115 | [26,34] |
| Diagnosis | 6091 | [26,34] |
| Treatment | 38,422 | [26,34] |
| Follow-up | 603 | [26,34] |
| Screening Method | No Screening | FIT | M3CRC | Colonoscopy |
|---|---|---|---|---|
| Total number CRC cases | 3233 | 3087 | 1611 | 1575 |
| Total loss of cancer-related life years | 5635 | 5297 | 2783 | 2719 |
| Cases of CRC prevented | 0 | 146 | 1622 | 1657 |
| Proportion of CRC case prevented (%) | 0 | 4.5 | 50.2 | 51.3 |
| Life-years saved | 0 | 339 | 2852 | 2917 |
| Number of procedures | ||||
| FIT | 0 | 236,913 | 0 | 0 |
| M3CRC | 0 | 0 | 712,584 | 0 |
| Colonoscopy | 0 | 36,670 | 419,173 | 509,496 |
| Diagnostic (without polypectomy) | 0 | 9901 | 67,487 | 141,655 |
| Therapeutic (with polypectomy) | 0 | 26,769 | 351,686 | 367,841 |
| Number of complications | ||||
| Bleeding | 0 | 73 | 838 | 1019 |
| Perforations | 0 | 29 | 335 | 403 |
| Costs (USD) | ||||
| FIT | 0 | 4,247,330 | 0 | 0 |
| M3CRC | 0 | 0 | 37,385,539 | 0 |
| Colonoscopy | 0 | 40,555,839 | 408,885,333 | 526,128,915 |
| Polypectomy | 0 | 3,100,114 | 35,922,369 | 39,807,084 |
| Bleeding | 0 | 973,819 | 9,818,076 | 12,633,307 |
| Perforations | 0 | 258,360 | 2,604,796 | 3,314,825 |
| Care of CRC | ||||
| Stage I | 4,050,827 | 3,839,170 | 2,010,230 | 1,964,971 |
| Stage II | 14,828,981 | 14,046,379 | 7,355,408 | 7,189,266 |
| Stage III | 36,148,483 | 34,219,225 | 17,920,737 | 17,514,640 |
| Stage IV | 170,486,718 | 160,919,746 | 84,332,191 | 82,394,622 |
| Total | 225,515,010 | 262,159,983 | 606,234,678 | 690,947,627 |
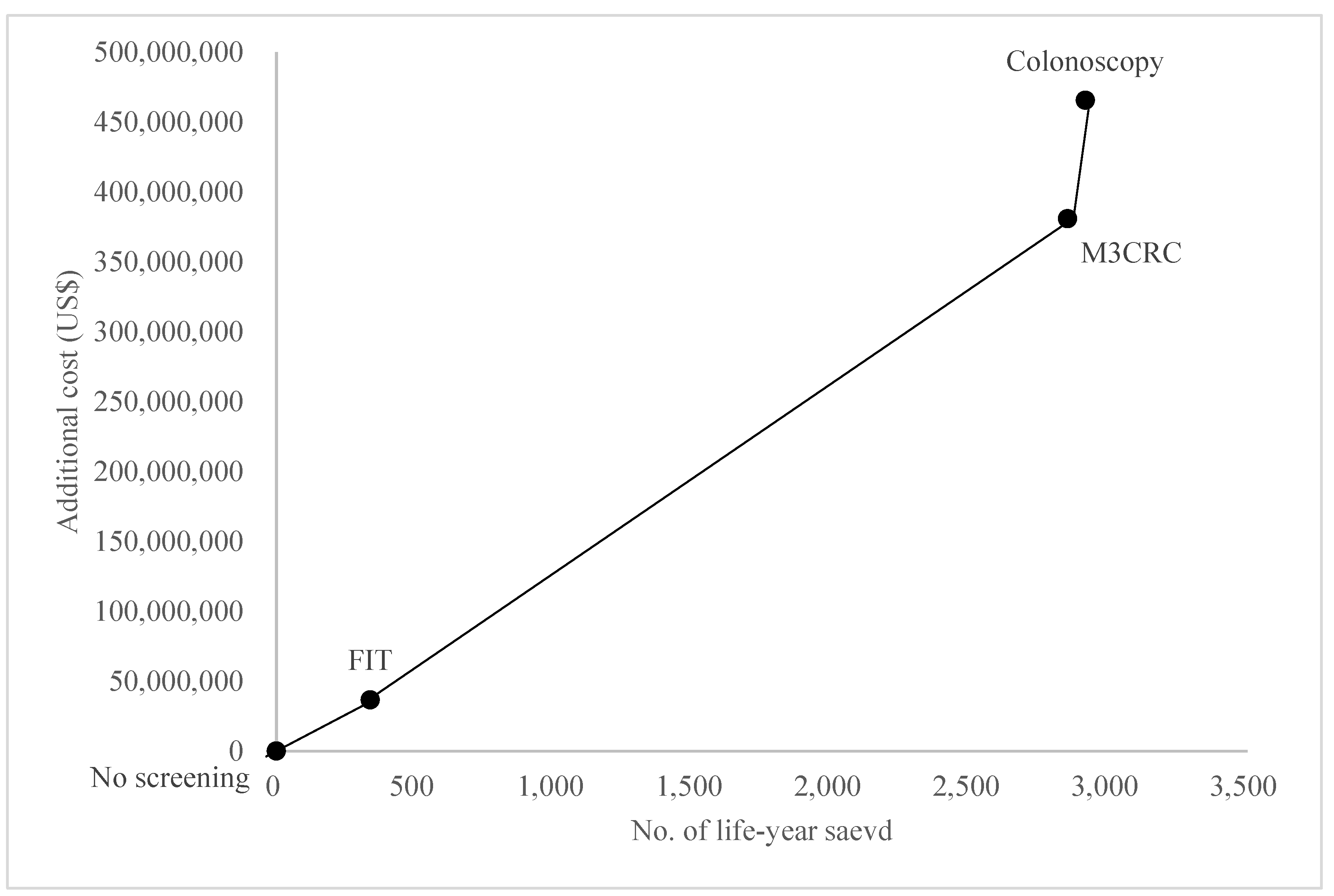
| Total costs per life-years saved | - | 773,894 | 212,553 | 236,909 |
| ICER vs. no screening | 108,176 | 133,485 | 159,586 | |
| ICER vs. FIT | 136,896 | 166,342 | ||
| ICER vs. M3CRC | 1,316,491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, M.C.S.; Huang, J.; Wong, Y.-Y.; Ko, S.; Chan, V.C.W.; Ng, S.C.; Chan, F.K.L. The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study. Cancers 2023, 15, 633. https://doi.org/10.3390/cancers15030633
Wong MCS, Huang J, Wong Y-Y, Ko S, Chan VCW, Ng SC, Chan FKL. The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study. Cancers. 2023; 15(3):633. https://doi.org/10.3390/cancers15030633
Chicago/Turabian StyleWong, Martin C. S., Junjie Huang, Yuet-Yan Wong, Samantha Ko, Victor C. W. Chan, Siew C. Ng, and Francis K. L. Chan. 2023. "The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study" Cancers 15, no. 3: 633. https://doi.org/10.3390/cancers15030633
APA StyleWong, M. C. S., Huang, J., Wong, Y.-Y., Ko, S., Chan, V. C. W., Ng, S. C., & Chan, F. K. L. (2023). The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study. Cancers, 15(3), 633. https://doi.org/10.3390/cancers15030633









