MicroRNAs in the Pathogenesis, Prognostication and Prediction of Treatment Resistance in Soft Tissue Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
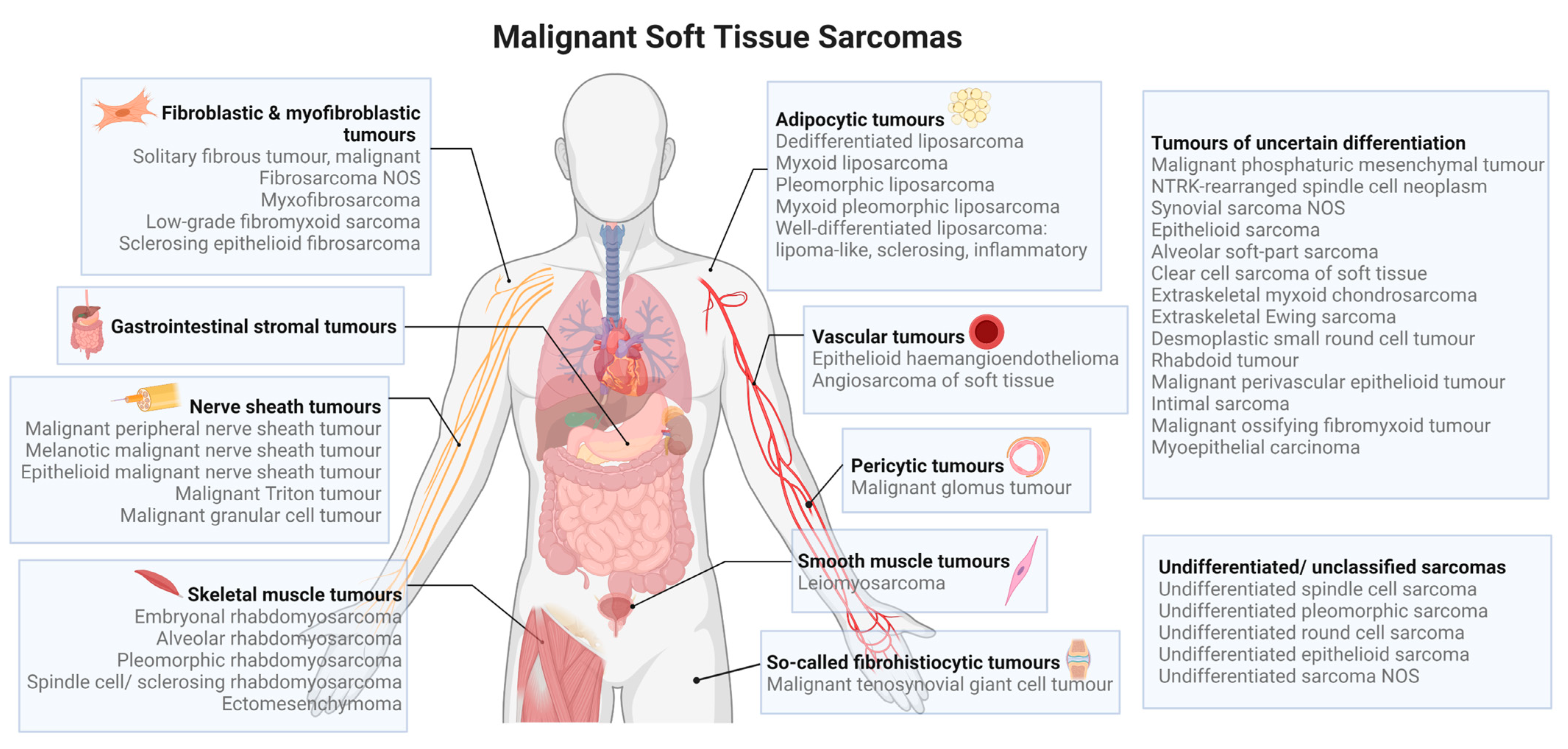
2. MicroRNAs in the Pathogenesis of Soft Tissue Sarcomas
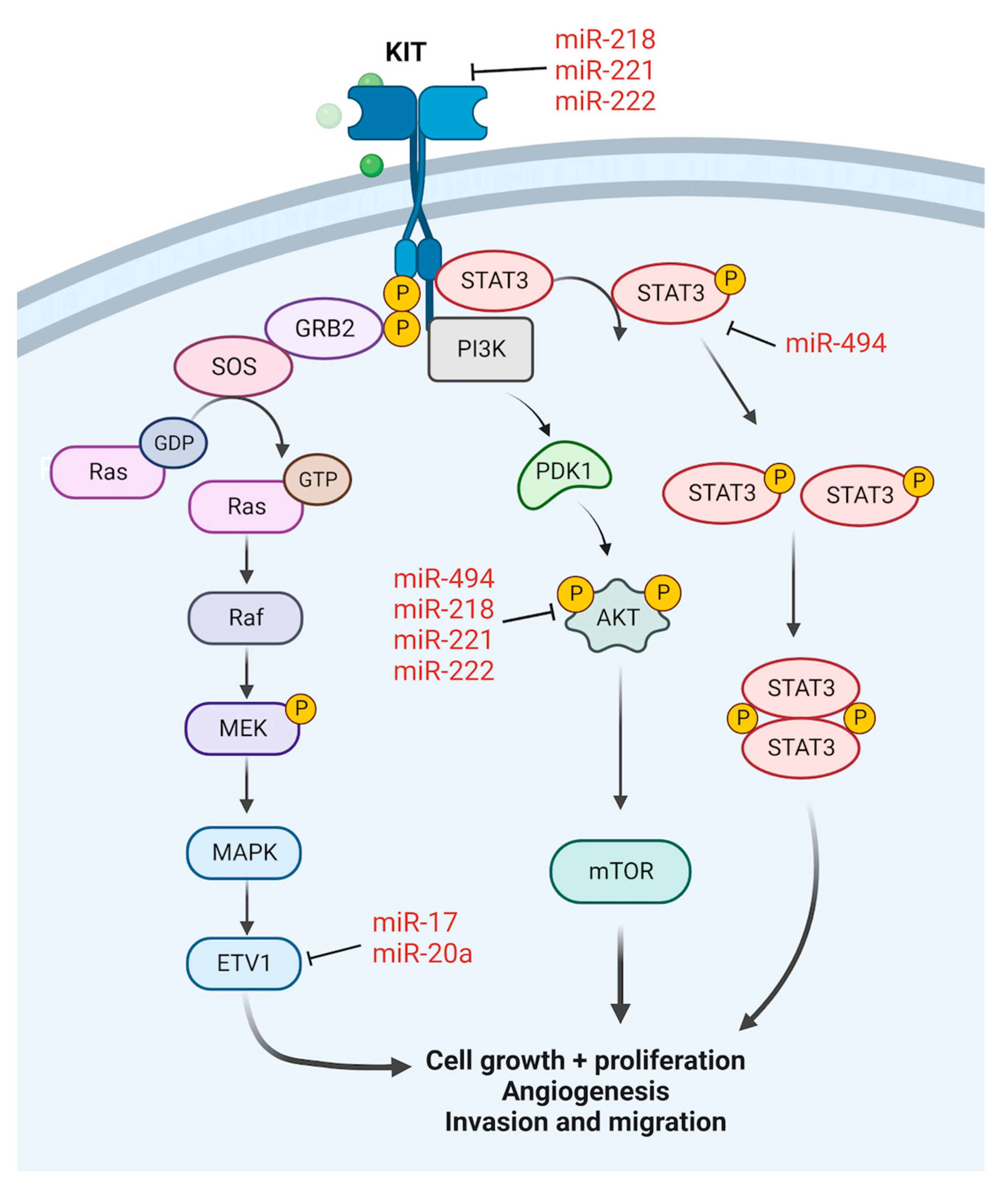
2.1. Gastrointestinal Stromal Tumour
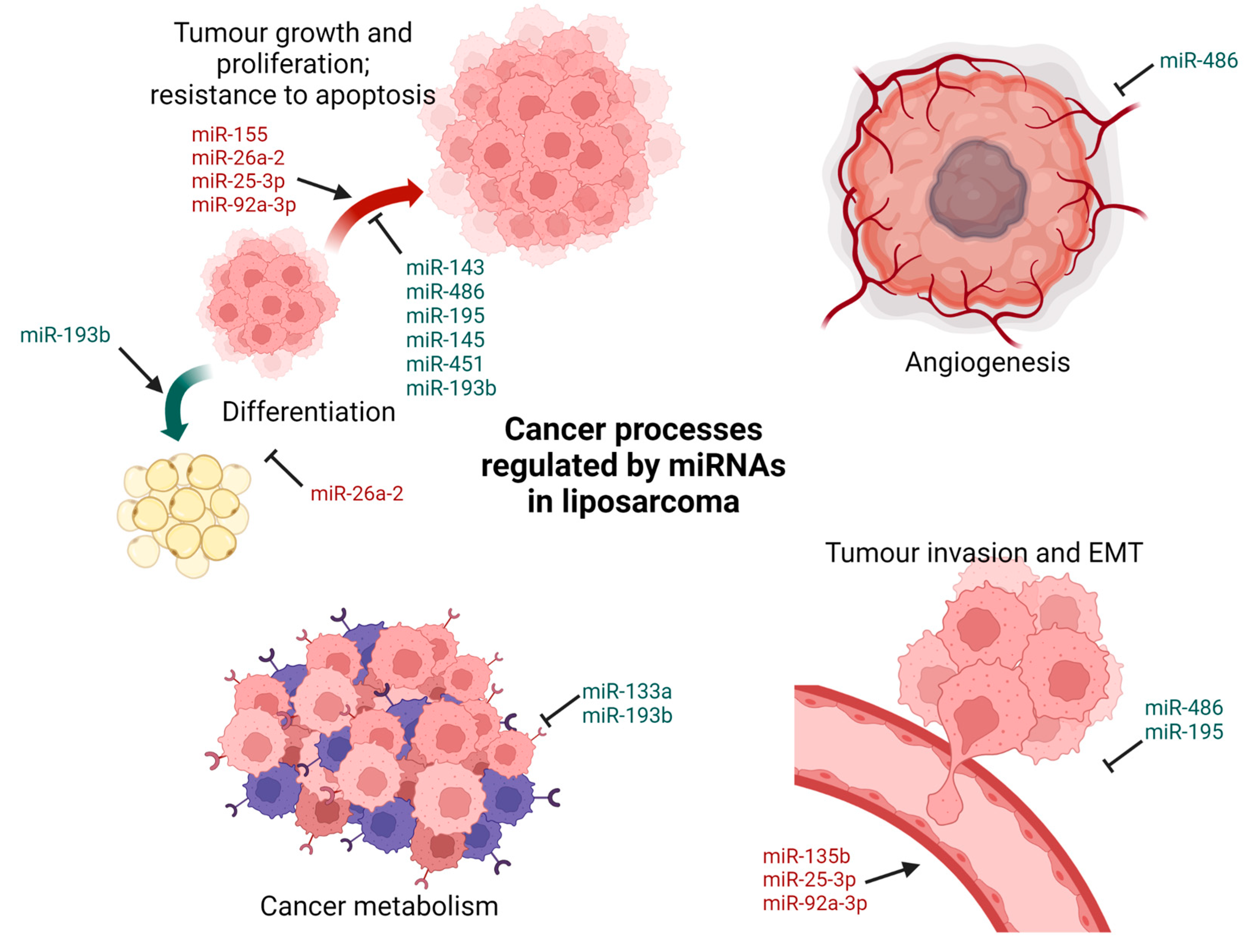
2.2. Liposarcoma
2.3. Rhabdomyosarcoma
2.4. Malignant Peripheral Nerve Sheath Tumour
2.5. Leiomyosarcoma
2.6. Synovial Sarcoma
2.7. Fibrosarcoma
2.8. Angiosarcoma
3. MicroRNAs in Prognostication of Soft Tissue Sarcomas
3.1. Gastrointestinal Stromal Tumour
3.2. Liposarcoma
3.3. Rhabdomyosarcoma
3.4. Leiomyosarcoma
3.5. Synovial Sarcoma
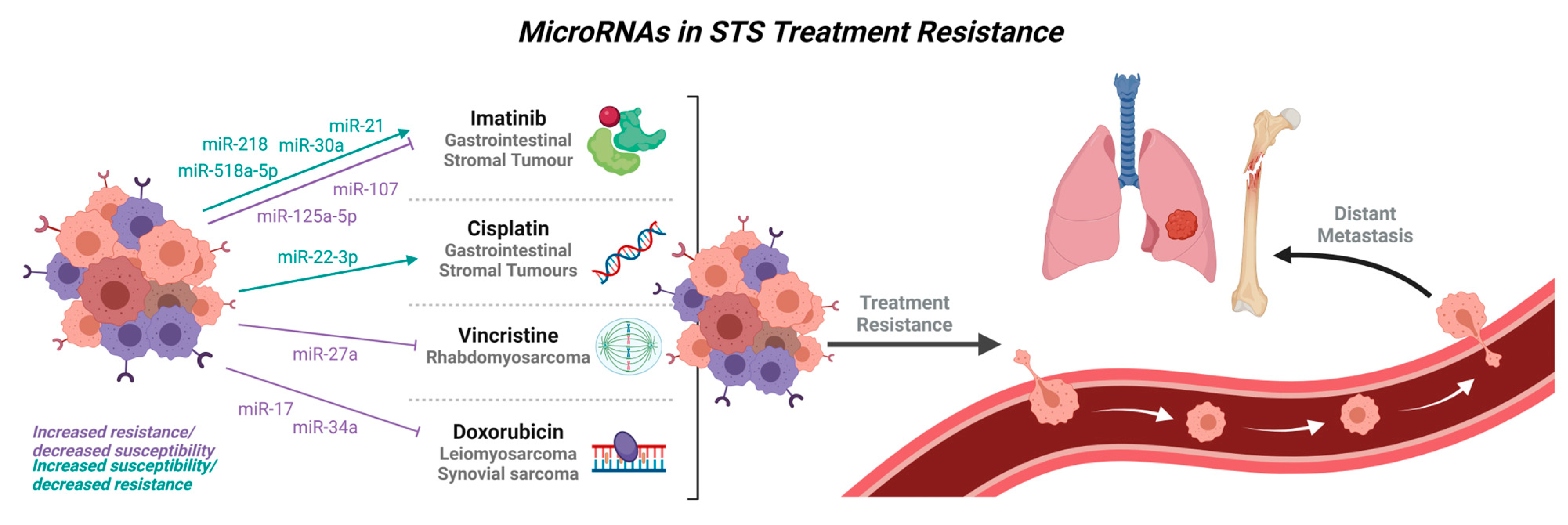
4. MicroRNAs in Treatment Resistance
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demetri, G.D.; Antonia, S.; Benjamin, R.S.; Bui, M.M.; Casper, E.S.; Conrad, E.U.; DeLaney, T.F.; Ganjoo, K.N.; Heslin, M.J.; Hutchinson, R.J. Soft tissue sarcoma. J. Natl. Compr. Cancer Netw. 2010, 8, 630–674. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- DeVita, V.T.; Hellman, S.; Rosenberg, S.A. Cancer, Principles and Practice of Oncology; Lippincott: New York, NY, USA, 1982; Volume 1. [Google Scholar]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.N.; Pollock, R.E. Soft tissue sarcomas. CA: A Cancer J. Clin. 2004, 54, 94–109. [Google Scholar] [CrossRef]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Velasco, J.; Zelefsky, M.J.; Woodruff, J.M.; Lewis, J.J.; Brennan, M.F. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Linch, M.; Miah, A.B.; Thway, K.; Judson, I.R.; Benson, C. Systemic treatment of soft-tissue sarcoma—Gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014, 11, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.W.; et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef]
- Demetri, G.D.; Le Cesne, A.; Chawla, S.P.; Brodowicz, T.; Maki, R.G.; Bach, B.A.; Smethurst, D.P.; Bray, S.; Hei, Y.-j.; Blay, J.-Y. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: A Phase I/II open-label and double-blind study. Eur. J. Cancer 2012, 48, 547–563. [Google Scholar] [CrossRef]
- Tap, W.D.; Papai, Z.; Van Tine, B.A.; Attia, S.; Ganjoo, K.N.; Jones, R.L.; Schuetze, S.; Reed, D.; Chawla, S.P.; Riedel, R.F.; et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): An international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1089–1103. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2017; Based on November 2019 SEER data submission, posted to the SEER web site; National Cancer Institute: Bethesda, MD, USA, April 2020. [Google Scholar]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Leva, G.D.; Garofalo, M.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef]
- Asano, N.; Matsuzaki, J.; Ichikawa, M.; Kawauchi, J.; Takizawa, S.; Aoki, Y.; Sakamoto, H.; Yoshida, A.; Kobayashi, E.; Tanzawa, Y.; et al. A serum microRNA classifier for the diagnosis of sarcomas of various histological subtypes. Nat. Commun. 2019, 10, 1299. [Google Scholar] [CrossRef]
- Smolle, M.A.; Leithner, A.; Posch, F.; Szkandera, J.; Liegl-Atzwanger, B.; Pichler, M. MicroRNAs in Different Histologies of Soft Tissue Sarcoma: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1960. [Google Scholar] [CrossRef]
- Kim, W.K.; Park, M.; Kim, Y.-K.; Tae, Y.K.; Yang, H.-K.; Lee, J.M.; Kim, H. MicroRNA-494 downregulates KIT and inhibits gastrointestinal stromal tumor cell proliferation. Clin. Cancer Res. 2011, 17, 7584–7594. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Kim, W.K.; Kwon, Y.; Jang, M.; Bauer, S.; Kim, H. Survivin is a novel transcription regulator of KIT and is downregulated by miRNA-494 in gastrointestinal stromal tumors. Int. J. Cancer 2018, 142, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhong, J.; Zheng, S.; Wang, Z.; Xu, Y.; Li, S.; Zhou, J.; Yuan, F. MicroRNA-218 inhibits gastrointestinal stromal tumor cell and invasion by targeting KIT. Tumor Biol. 2014, 35, 4209–4217. [Google Scholar] [CrossRef]
- Fan, R.; Zhong, J.; Zheng, S.; Wang, Z.; Xu, Y.; Li, S.; Zhou, J.; Yuan, F. microRNA-218 increase the sensitivity of gastrointestinal stromal tumor to imatinib through PI3K/AKT pathway. Clin. Exp. Med. 2015, 15, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Wang, M.; Zhao, W.-Y.; Zhang, Z.-Z.; Tang, D.-F.; Zhang, Y.-Q.; Cao, H.; Zhang, Z.-G. miRNA-218-loaded carboxymethyl chitosan-Tocopherol nanoparticle to suppress the proliferation of gastrointestinal stromal tumor growth. Mater. Sci. Eng. C 2017, 72, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Koelz, M.; Lense, J.; Wrba, F.; Scheffler, M.; Dienes, H.P.; Odenthal, M. Down-regulation of miR-221 and miR-222 correlates with pronounced Kit expression in gastrointestinal stromal tumors. Int. J. Oncol. 2011, 38, 503–511. [Google Scholar] [CrossRef]
- Ihle, M.A.; Trautmann, M.; Kuenstlinger, H.; Huss, S.; Heydt, C.; Fassunke, J.; Wardelmann, E.; Bauer, S.; Schildhaus, H.-U.; Buettner, R. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Mol. Oncol. 2015, 9, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Gits, C.M.; van Kuijk, P.F.; Jonkers, M.B.; Boersma, A.W.; Van Ijcken, W.; Wozniak, A.; Sciot, R.; Rutkowski, P.; Schöffski, P.; Taguchi, T. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br. J. Cancer 2013, 109, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, C.; Cui, X.; Geng, W.; Xian, G.; Wang, Z. miR-4510 acts as a tumor suppressor in gastrointestinal stromal tumor by targeting APOC2. J. Cell Physiol. 2020, 235, 5711–5721. [Google Scholar] [CrossRef]
- Lu, H.-J.; Yan, J.; Jin, P.-Y.; Zheng, G.-H.; Qin, S.-M.; Wu, D.-M.; Lu, J.; Zheng, Y.-L. MicroRNA-152 inhibits tumor cell growth while inducing apoptosis via the transcriptional repression of cathepsin L in gastrointestinal stromal tumor. Cancer Biomark. 2018, 21, 711–722. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kohashi, K.; Fujita, A.; Oda, Y. Fascin-1 overexpression and miR-133b downregulation in the progression of gastrointestinal stromal tumor. Mod. Pathol. 2013, 26, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, X.; Hu, Q.; Li, X.; Xu, J.; Lu, S.; Liu, Y.; Xu, C.; Jiang, D.; Lin, J. PIK3C2A is a gene-specific target of microRNA-518a-5p in imatinib mesylate-resistant gastrointestinal stromal tumor. Lab. Investig. 2016, 96, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, J.; Liao, G.; Zhang, Y.; Ye, K.; Lu, T.; Qi, J.; Wan, G. MiR-137 regulates epithelial-mesenchymal transition in gastrointestinal stromal tumor. Tumor Biol. 2014, 35, 9131–9138. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.-W.; Wu, J.-H.; Cai-Hong, Y.-N.W.; Zhou, Y. MiR-374b promotes proliferation and inhibits apoptosis of human GIST cells by inhibiting PTEN through activation of the PI3K/Akt pathway. Mol. Cells 2018, 41, 532. [Google Scholar] [PubMed]
- Niinuma, T.; Suzuki, H.; Nojima, M.; Nosho, K.; Yamamoto, H.; Takamaru, H.; Yamamoto, E.; Maruyama, R.; Nobuoka, T.; Miyazaki, Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012, 72, 1126–1136. [Google Scholar] [CrossRef]
- Ugras, S.; Brill, E.; Jacobsen, A.; Hafner, M.; Socci, N.D.; DeCarolis, P.L.; Khanin, R.; O’Connor, R.; Mihailovic, A.; Taylor, B.S. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Res. 2011, 71, 5659–5669. [Google Scholar] [CrossRef]
- Kapodistrias, N.; Mavridis, K.; Batistatou, A.; Gogou, P.; Karavasilis, V.; Sainis, I.; Briasoulis, E.; Scorilas, A. Assessing the clinical value of microRNAs in formalin-fixed paraffin-embedded liposarcoma tissues: Overexpressed miR-155 is an indicator of poor prognosis. Oncotarget 2017, 8, 6896. [Google Scholar] [CrossRef]
- Borjigin, N.; Ohno, S.; Wu, W.; Tanaka, M.; Suzuki, R.; Fujita, K.; Takanashi, M.; Oikawa, K.; Goto, T.; Motoi, T. TLS-CHOP represses miR-486 expression, inducing upregulation of a metastasis regulator PAI-1 in human myxoid liposarcoma. Biochem. Biophys. Res. Commun. 2012, 427, 355–360. [Google Scholar] [CrossRef]
- Gits, C.M.; van Kuijk, P.F.; Jonkers, M.B.; Boersma, A.W.; Smid, M.; van Ijcken, W.F.; Coindre, J.M.; Chibon, F.; Verhoef, C.; Mathijssen, R.H. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int. J. Cancer 2014, 135, 348–361. [Google Scholar] [CrossRef]
- Mazzu, Y.Z.; Hu, Y.; Soni, R.K.; Mojica, K.M.; Qin, L.-X.; Agius, P.; Waxman, Z.M.; Mihailovic, A.; Socci, N.D.; Hendrickson, R.C. miR-193b–Regulated Signaling Networks Serve as Tumor Suppressors in Liposarcoma and Promote Adipogenesis in Adipose-Derived Stem Cells. Cancer Res. 2017, 77, 5728–5740. [Google Scholar] [CrossRef]
- Mazzu, Y.Z.; Hu, Y.; Shen, Y.; Tuschl, T.; Singer, S. miR-193b regulates tumorigenesis in liposarcoma cells via PDGFR, TGFβ, and Wnt signaling. Sci. Rep. 2019, 9, 3197. [Google Scholar] [CrossRef] [PubMed]
- Peter, Y.Y.; Lopez, G.; Braggio, D.; Koller, D.; Bill, K.L.J.; Prudner, B.C.; Zewdu, A.; Chen, J.L.; Iwenofu, O.H.; Lev, D. miR-133a function in the pathogenesis of dedifferentiated liposarcoma. Cancer Cell Int. 2018, 18, 89. [Google Scholar]
- Cao, Y.; Li, L.; Han, L.; Zheng, J.; Lv, C. miR-195 Serves as a Tumor Suppressor in the Progression of Liposarcoma by Targeting OSBP. OncoTargets Ther. 2020, 13, 6465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bill, K.; Liu, J.; Young, E.; Peng, T.; Bolshakov, S.; Hoffman, A.; Song, Y.; Demicco, E.G.; Terrada, D.L. MiR-155 is a liposarcoma oncogene that targets casein kinase-1α and enhances β-catenin signaling. Cancer Res. 2012, 72, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Iuliani, M.; Zoccoli, A.; Pantano, F.; Fioramonti, M.; De Lisi, D.; Frezza, A.M.; Rabitti, C.; Perrone, G.; Muda, A.O. Deregulation of dicer and mir-155 expression in liposarcoma. Oncotarget 2015, 6, 10586. [Google Scholar] [CrossRef]
- Boro, A.; Bauer, D.; Born, W.; Fuchs, B. Plasma levels of miRNA-155 as a powerful diagnostic marker for dedifferentiated liposarcoma. Am. J. Cancer Res. 2016, 6, 544. [Google Scholar]
- Lee, D.; Amanat, S.; Goff, C.; Weiss, L.; Said, J.; Doan, N.; Sato-Otsubo, A.; Ogawa, S.; Forscher, C.; Koeffler, H. Overexpression of miR-26a-2 in human liposarcoma is correlated with poor patient survival. Oncogenesis 2013, 2, e47. [Google Scholar] [CrossRef]
- Lee, D.H.; Forscher, C.; Di Vizio, D.; Koeffler, H.P. Induction of p53-independent apoptosis by ectopic expression of HOXA5 in human liposarcomas. Sci. Rep. 2015, 5, 12580. [Google Scholar] [CrossRef]
- Nezu, Y.; Hagiwara, K.; Yamamoto, Y.; Fujiwara, T.; Matsuo, K.; Yoshida, A.; Kawai, A.; Saito, T.; Ochiya, T. miR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene 2016, 35, 6177–6188. [Google Scholar] [CrossRef]
- Casadei, L.; Calore, F.; Creighton, C.J.; Guescini, M.; Batte, K.; Iwenofu, O.H.; Zewdu, A.; Braggio, D.A.; Bill, K.L.; Fadda, P. Exosome-derived miR-25-3p and miR-92a-3p stimulate liposarcoma progression. Cancer Res. 2017, 77, 3846–3856. [Google Scholar] [CrossRef]
- Fricke, A.; Cimniak, A.; Ullrich, P.; Becherer, C.; Bickert, C.; Pfeifer, D.; Heinz, J.; Stark, G.; Bannasch, H.; Braig, D. Whole blood miRNA expression analysis reveals miR-3613-3p as a potential biomarker for dedifferentiated liposarcoma. Cancer Biomark. 2018, 22, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Taulli, R.; Bersani, F.; Foglizzo, V.; Linari, A.; Vigna, E.; Ladanyi, M.; Tuschl, T.; Ponzetto, C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J. Clin. Investig. 2009, 119, 2366–2378. [Google Scholar] [CrossRef]
- Yan, D.; Da Dong, X.; Chen, X.; Wang, L.; Lu, C.; Wang, J.; Qu, J.; Tu, L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J. Biol. Chem. 2009, 284, 29596–29604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sarver, A.L.; Alamgir, S.; Subramanian, S. Downregulation of microRNAs miR-1,-206 and-29 stabilizes PAX3 and CCND2 expression in rhabdomyosarcoma. Lab. Investig. 2012, 92, 571–583. [Google Scholar] [CrossRef]
- MacQuarrie, K.L.; Yao, Z.; Young, J.M.; Cao, Y.; Tapscott, S.J. miR-206 integrates multiple components of differentiation pathways to control the transition from growth to differentiation in rhabdomyosarcoma cells. Skelet. Muscle 2012, 2, 7. [Google Scholar] [CrossRef]
- Hanna, J.; Garcia, M.; Go, J.; Finkelstein, D.; Kodali, K.; Pagala, V.; Wang, X.; Peng, J.; Hatley, M. PAX7 is a required target for microRNA-206-induced differentiation of fusion-negative rhabdomyosarcoma. Cell Death Dis. 2016, 7, e2256. [Google Scholar] [CrossRef] [PubMed]
- Coda, D.M.; Lingua, M.F.; Morena, D.; Foglizzo, V.; Bersani, F.; Ala, U.; Ponzetto, C.; Taulli, R. SMYD1 and G6PD modulation are critical events for miR-206-mediated differentiation of rhabdomyosarcoma. Cell Cycle 2015, 14, 1389–1402. [Google Scholar] [CrossRef]
- Ciesla, M.; Marona, P.; Kozakowska, M.; Jez, M.; Seczynska, M.; Loboda, A.; Bukowska-Strakova, K.; Szade, A.; Walawender, M.; Kusior, M. Heme oxygenase-1 controls an HDAC4-miR-206 pathway of oxidative stress in rhabdomyosarcoma. Cancer Res. 2016, 76, 5707–5718. [Google Scholar] [CrossRef]
- Missiaglia, E.; Shepherd, C.J.; Patel, S.; Thway, K.; Pierron, G.; Pritchard-Jones, K.; Renard, M.; Sciot, R.; Rao, P.; Oberlin, O.; et al. MicroRNA-206 expression levels correlate with clinical behaviour of rhabdomyosarcomas. Br. J. Cancer 2010, 102, 1769–1777. [Google Scholar] [CrossRef]
- Rao, P.K.; Missiaglia, E.; Shields, L.; Hyde, G.; Yuan, B.; Shepherd, C.J.; Shipley, J.; Lodish, H.F. Distinct roles for miR-1 and miR-133a in the proliferation and differentiation of rhabdomyosarcoma cells. FASEB J. 2010, 24, 3427–3437. [Google Scholar] [CrossRef]
- Sugito, N.; Taniguchi, K.; Kuranaga, Y.; Ohishi, M.; Soga, T.; Ito, Y.; Miyachi, M.; Kikuchi, K.; Hosoi, H.; Akao, Y. Cancer-specific energy metabolism in rhabdomyosarcoma cells is regulated by microRNA. Nucleic Acid Ther. 2017, 27, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Garzon, R.; Sun, H.; Ladner, K.J.; Singh, R.; Dahlman, J.; Cheng, A.; Hall, B.M.; Qualman, S.J.; Chandler, D.S. NF-κB–YY1–miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 2008, 14, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Pang, Y.; Song, L.; Shang, H.; Li, Z.; Liu, Q.; Zhang, Y.; Wang, X.; Li, Q. MicroRNA-29 family inhibits rhabdomyosarcoma formation and progression by regulating GEFT function. Am. J. Transl. Res. 2020, 12, 1136. [Google Scholar] [PubMed]
- Ciarapica, R.; Russo, G.; Verginelli, F.; Raimondi, L.; Donfrancesco, A.; Rota, R.; Giordano, A. Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle 2009, 8, 172–175. [Google Scholar] [CrossRef]
- Tombolan, L.; Millino, C.; Pacchioni, B.; Cattelan, M.; Zin, A.; Bonvini, P.; Bisogno, G. Circulating miR-26a as potential prognostic biomarkers in pediatric rhabdomyosarcoma. Front. Genet. 2020, 11, 606274. [Google Scholar] [CrossRef]
- Yang, L.; Kong, D.; He, M.; Gong, J.; Nie, Y.; Tai, S.; Teng, C.-B. MiR-7 mediates mitochondrial impairment to trigger apoptosis and necroptosis in Rhabdomyosarcoma. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118826. [Google Scholar] [CrossRef]
- Molist, C.; Navarro, N.; Giralt, I.; Zarzosa, P.; Gallo-Oller, G.; Pons, G.; Magdaleno, A.; Moreno, L.; Guillén, G.; Hladun, R. miRNA-7 and miRNA-324-5p regulate alpha9-Integrin expression and exert anti-oncogenic effects in rhabdomyosarcoma. Cancer Lett. 2020, 477, 49–59. [Google Scholar] [CrossRef]
- Megiorni, F.; Cialfi, S.; McDowell, H.P.; Felsani, A.; Camero, S.; Guffanti, A.; Pizer, B.; Clerico, A.; De Grazia, A.; Pizzuti, A. Deep Sequencing the microRNA profile in rhabdomyosarcoma reveals down-regulation of miR-378 family members. BMC Cancer 2014, 14, 880. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Guo, L.; Xiao, H.; Dong, L.; Wang, F.; Huang, F.; Cao, D.; Qin, T.; Yin, X. TGF-β1 suppression of microRNA-450b-5p expression: A novel mechanism for blocking myogenic differentiation of rhabdomyosarcoma. Oncogene 2014, 33, 2075–2086. [Google Scholar] [CrossRef]
- Diao, Y.; Guo, X.; Jiang, L.; Wang, G.; Zhang, C.; Wan, J.; Jin, Y.; Wu, Z. miR-203, a tumor suppressor frequently down-regulated by promoter hypermethylation in rhabdomyosarcoma. J. Biol. Chem. 2014, 289, 529–539. [Google Scholar] [CrossRef]
- Sun, M.; Huang, F.; Yu, D.; Zhang, Y.; Xu, H.; Zhang, L.; Li, L.; Dong, L.; Guo, L.; Wang, S. Autoregulatory loop between TGF-β 1/miR-411-5p/SPRY4 and MAPK pathway in rhabdomyosarcoma modulates proliferation and differentiation. Cell Death Dis. 2015, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.A.; Garcia, M.R.; Lardennois, A.; Leavey, P.J.; Maglic, D.; Fagnan, A.; Go, J.C.; Roach, J.; Wang, Y.-D.; Finkelstein, D.; et al. PAX3-FOXO1 drives miR-486-5p and represses miR-221 contributing to pathogenesis of alveolar rhabdomyosarcoma. Oncogene 2018, 37, 1991–2007. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Liu, J.; Hua, H.; Li, S.-E.; Zhao, J.; Yue, S.; Yu, T.-T.; Jin, Y.-C.; Cheng, S.Y. MiR-214 and N-ras regulatory loop suppresses rhabdomyosarcoma cell growth and xenograft tumorigenesis. Oncotarget 2014, 5, 2161. [Google Scholar] [CrossRef] [PubMed]
- Vella, S.; Pomella, S.; Leoncini, P.P.; Colletti, M.; Conti, B.; Marquez, V.E.; Strillacci, A.; Roma, J.; Gallego, S.; Milano, G.M. MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin. Epigenetics 2015, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Liu, Y.; Li, Z.; Liu, Q.; Cui, W.; Zhang, L.; Pang, Y.; Liu, C.; Li, F. MicroRNA-874 functions as a tumor suppressor in rhabdomyosarcoma by directly targeting GEFT. Am. J. Cancer Res. 2019, 9, 668. [Google Scholar]
- Zhang, L.; Pang, Y.; Cui, X.; Jia, W.; Cui, W.; Liu, Y.; Liu, C.; Li, F. MicroRNA-410-3p upregulation suppresses proliferation, invasion and migration, and promotes apoptosis in rhabdomyosarcoma cells. Oncol. Lett. 2019, 18, 936–943. [Google Scholar] [CrossRef]
- Tombolan, L.; Zampini, M.; Casara, S.; Boldrin, E.; Zin, A.; Bisogno, G.; Rosolen, A.; De Pittà, C.; Lanfranchi, G. MicroRNA-27a contributes to rhabdomyosarcoma cell proliferation by suppressing RARA and RXRA. PLoS ONE 2015, 10, e0125171. [Google Scholar] [CrossRef]
- Bharathy, N.; Berlow, N.E.; Wang, E.; Abraham, J.; Settelmeyer, T.P.; Hooper, J.E.; Svalina, M.N.; Ishikawa, Y.; Zientek, K.; Bajwa, Z. The HDAC3–SMARCA4–miR-27a axis promotes expression of the PAX3: FOXO1 fusion oncogene in rhabdomyosarcoma. Sci. Signal. 2018, 11, eaau7632. [Google Scholar] [CrossRef]
- Gong, M.; Ma, J.; Li, M.; Zhou, M.; Hock, J.M.; Yu, X. MicroRNA-204 critically regulates carcinogenesis in malignant peripheral nerve sheath tumors. Neuro-oncology 2012, 14, 1007–1017. [Google Scholar] [CrossRef]
- Zhang, P.; Garnett, J.; Creighton, C.J.; Al Sannaa, G.A.; Igram, D.R.; Lazar, A.; Liu, X.; Liu, C.; Pollock, R.E. EZH2–miR-30d–KPNB1 pathway regulates malignant peripheral nerve sheath tumour cell survival and tumourigenesis. J. Pathol. 2014, 232, 308–318. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Ma, X.; Ingram, D.R.; Lazar, A.J.; Torres, K.E.; Pollock, R.E. Antitumor effects of pharmacological EZH2 inhibition on malignant peripheral nerve sheath tumor through the miR-30a and KPNB1 pathway. Mol. Cancer 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Thayanithy, V.; West, R.B.; Lee, C.H.; Beck, A.H.; Zhu, S.; Downs-Kelly, E.; Montgomery, K.; Goldblum, J.R.; Hogendoorn, P.C. Genome-wide transcriptome analyses reveal p53 inactivation mediated loss of miR-34a expression in malignant peripheral nerve sheath tumours. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2010, 220, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Itani, S.; Kunisada, T.; Morimoto, Y.; Yoshida, A.; Sasaki, T.; Ito, S.; Ouchida, M.; Sugihara, S.; Shimizu, K.; Ozaki, T. MicroRNA-21 correlates with tumorigenesis in malignant peripheral nerve sheath tumor (MPNST) via programmed cell death protein 4 (PDCD4). J. Cancer Res. Clin. Oncol. 2012, 138, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, Y.; Chen, J.; Liu, Y.; Bao, T. Identification of serum microRNAs in genome-wide serum microRNA expression profiles as novel noninvasive biomarkers for malignant peripheral nerve sheath tumor diagnosis. Med. Oncol. 2013, 30, 531. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Tate, K.; Yoneoka, Y.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Takizawa, S.; Aoki, Y. Serum microRNA profile enables preoperative diagnosis of uterine leiomyosarcoma. Cancer Sci. 2019, 110, 3718. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, C.; Li, C.; Jiao, X.; Griffin, B.B.; Dongol, S.; Wu, H.; Zhang, C.; Cao, W.; Dong, R.; et al. Upregulated MELK Leads to Doxorubicin Chemoresistance and M2 Macrophage Polarization via the miR-34a/JAK2/STAT3 Pathway in Uterine Leiomyosarcoma. Front. Oncol. 2020, 10, 453. [Google Scholar] [CrossRef]
- Pazzaglia, L.; Novello, C.; Conti, A.; Pollino, S.; Picci, P.; Benassi, M.S. miR-152 down-regulation is associated with MET up-regulation in leiomyosarcoma and undifferentiated pleomorphic sarcoma. Cell Oncol. 2017, 40, 77–88. [Google Scholar] [CrossRef]
- Abeshouse, A.; Anderson, M.L.; Armenia, J.; Auman, J.T.; Bailey, M.H.; Baker, L.; Balasundaram, M.; Balu, S.; Behera, M.; Benz, C.; et al. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e928. [Google Scholar] [CrossRef]
- Guled, M.; Pazzaglia, L.; Borze, I.; Mosakhani, N.; Novello, C.; Benassi, M.S.; Knuutila, S. Differentiating soft tissue leiomyosarcoma and undifferentiated pleomorphic sarcoma: A miRNA analysis. Genes Chromosomes Cancer 2014, 53, 693–702. [Google Scholar] [CrossRef]
- Pazzaglia, L.; Pollino, S.; Vitale, M.; Bientinesi, E.; Benini, S.; Ferrari, C.; Palmerini, E.; Gambarotti, M.; Picci, P.; Benassi, M.S. miR-494.3 p expression in synovial sarcoma: Role of CXCR4 as a potential target gene. Int. J. Oncol. 2019, 54, 361–369. [Google Scholar]
- Feng, Q.; Wang, D.; Guo, P.; Zhang, Z.; Feng, J. Long non-coding RNA HOTAIR promotes the progression of synovial sarcoma through microRNA-126/stromal cell-derived factor-1 regulation. Oncol. Lett. 2021, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka, M.; Matsuyama, A.; Nagao, Y.; Luan, L.; Kuroda, T.; Akiyama, H.; Kondo, S.; Hashimoto, H. Identification of altered MicroRNA expression patterns in synovial sarcoma. Genes Chromosomes Cancer 2011, 50, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Uotani, K.; Fujiwara, T.; Yoshida, A.; Iwata, S.; Morita, T.; Kiyono, M.; Yokoo, S.; Kunisada, T.; Takeda, K.; Hasei, J. Circulating MicroRNA-92b-3p as a novel biomarker for monitoring of synovial sarcoma. Sci. Rep. 2017, 7, 14634. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Homme, M.; Yamazaki, Y.; Ae, K.; Matsumoto, S.; Subramanian, S.; Nakamura, T. Cooperation between SS18-SSX1 and miR-214 in synovial sarcoma development and progression. Cancers 2020, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Z.; Li, X.-A.; Luo, Y.; Liu, J.-F.; Wu, H.-W.; Huang, G. MiR-9 promotes synovial sarcoma cell migration and invasion by directly targeting CDH1. Int. J. Biochem. Cell Biol. 2019, 112, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kohsaka, S.; Tsuda, M.; Yachi, K.; Hatori, N.; Tanino, M.; Kimura, T.; Nishihara, H.; Minami, A.; Iwasaki, N. SS 18-SSX-regulated miR-17 promotes tumor growth of synovial sarcoma by inhibiting p21 WAF 1/CIP 1. Cancer Sci. 2014, 105, 1152–1159. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, S.; Shin, B.A. MicroRNA-29 family suppresses the invasion of HT1080 human fibrosarcoma cells by regulating matrix metalloproteinase 2 expression. Chonnam Med. J. 2017, 53, 161. [Google Scholar] [CrossRef]
- Jain, N.; Das, B.; Mallick, B. Restoration of microRNA-197 expression suppresses oncogenicity in fibrosarcoma through negative regulation of RAN. IUBMB Life 2020, 72, 1034–1044. [Google Scholar] [CrossRef]
- Liu, P.; Wilson, M.J. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in human fibrosarcoma cells. J. Cell Physiol. 2012, 227, 867–876. [Google Scholar] [CrossRef]
- Chen, Y.; Kuang, D.; Zhao, X.; Chen, D.; Wang, X.; Yang, Q.; Wan, J.; Zhu, Y.; Wang, Y.; Zhang, S. miR-497-5p inhibits cell proliferation and invasion by targeting KCa3. 1 in angiosarcoma. Oncotarget 2016, 7, 58148. [Google Scholar] [CrossRef]
- Nakashima, S.; Jinnin, M.; Kanemaru, H.; Kajihara, I.; Igata, T.; Okamoto, S.; Tazaki, Y.; Harada, M.; Masuguchi, S.; Fukushima, S. The role of miR-210, E2F3 and ephrin A3 in angiosarcoma cell proliferation. Eur. J. Dermatol. 2017, 27, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y. MicroRNA-340 inhibits the growth and invasion of angiosarcoma cells by targeting SIRT7. Biomed. Pharmacother. 2018, 103, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Fletcher, J.A.; Heinrich, M.C. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 2004, 22, 3813–3825. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001, 438, 1–12. [Google Scholar] [CrossRef]
- Liegl, B.; Kepten, I.; Le, C.; Zhu, M.; Demetri, G.D.; Heinrich, M.C.; Fletcher, C.D.; Corless, C.L.; Fletcher, J.A. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J. Pathol. 2008, 216, 64–74. [Google Scholar] [CrossRef]
- Miller, T.E.; Ghoshal, K.; Ramaswamy, B.; Roy, S.; Datta, J.; Shapiro, C.L.; Jacob, S.; Majumder, S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008, 283, 29897–29903. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Zhang, J.-X.; Zhang, A.-L.; Shi, Z.-D.; Han, L.; Jia, Z.-F.; Yang, W.-D.; Wang, G.-X.; Jiang, T.; You, Y.-P. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, C.; Nagel, R.; Egan, D.A.; Schrier, M.; Mesman, E.; Mangiola, A.; Anile, C.; Maira, G.; Mercatelli, N.; Ciafrè, S.A. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007, 26, 3699–3708. [Google Scholar] [CrossRef]
- Chi, P.; Chen, Y.; Zhang, L.; Guo, X.; Wongvipat, J.; Shamu, T.; Fletcher, J.A.; Dewell, S.; Maki, R.G.; Zheng, D.; et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010, 467, 849–853. [Google Scholar] [CrossRef]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 2015, 155, 105–116. [Google Scholar] [CrossRef]
- Moreau, L.C.; Turcotte, R.; Ferguson, P.; Wunder, J.; Clarkson, P.; Masri, B.; Isler, M.; Dion, N.; Werier, J.; Ghert, M.; et al. Myxoid\round cell liposarcoma (MRCLS) revisited: An analysis of 418 primarily managed cases. Ann. Surg. Oncol. 2012, 19, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kohama, I.; Asano, N.; Matsuzaki, J.; Yamamoto, Y.; Yamamoto, T.; Takahashi, R.-U.; Kobayashi, E.; Takizawa, S.; Sakamoto, H.; Kato, K. Comprehensive serum and tissue microRNA profiling in dedifferentiated liposarcoma. Oncol. Lett. 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Maillard, C.; Jost, M.; Lijnen, R.H.; Gils, A.; Declerck, P.; Carmeliet, P.; Foidart, J.-M.; Noel, A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene 2004, 23, 6986–6990. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.N.; Sublett, J.E.; Li, B.; Downing, J.R.; Naeve, C.W. Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 1993, 53, 5108–5112. [Google Scholar] [PubMed]
- Galili, N.; Davis, R.J.; Fredericks, W.J.; Mukhopadhyay, S.; Rauscher, F.J., 3rd; Emanuel, B.S.; Rovera, G.; Barr, F.G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 1993, 5, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; D’Cruz, C.M.; Lovell, M.A.; Biegel, J.A.; Barr, F.G. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994, 54, 2869–2872. [Google Scholar]
- Missiaglia, E.; Williamson, D.; Chisholm, J.; Wirapati, P.; Pierron, G.; Petel, F.; Concordet, J.P.; Thway, K.; Oberlin, O.; Pritchard-Jones, K.; et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J. Clin. Oncol. 2012, 30, 1670–1677. [Google Scholar] [CrossRef]
- Skapek, S.X.; Anderson, J.; Barr, F.G.; Bridge, J.A.; Gastier-Foster, J.M.; Parham, D.M.; Rudzinski, E.R.; Triche, T.; Hawkins, D.S. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: A children’s oncology group report. Pediatr. Blood Cancer 2013, 60, 1411–1417. [Google Scholar] [CrossRef]
- Sorensen, P.H.; Lynch, J.C.; Qualman, S.J.; Tirabosco, R.; Lim, J.F.; Maurer, H.M.; Bridge, J.A.; Crist, W.M.; Triche, T.J.; Barr, F.G. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the children’s oncology group. J. Clin. Oncol. 2002, 20, 2672–2679. [Google Scholar] [CrossRef]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-β regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Taulli, R.; Scuoppo, C.; Bersani, F.; Accornero, P.; Forni, P.E.; Miretti, S.; Grinza, A.; Allegra, P.; Schmitt-Ney, M.; Crepaldi, T. Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res. 2006, 66, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, M.; Tsuchiya, K.; Yoshida, H.; Yagyu, S.; Kikuchi, K.; Misawa, A.; Iehara, T.; Hosoi, H. Circulating muscle-specific microRNA, miR-206, as a potential diagnostic marker for rhabdomyosarcoma. Biochem. Biophys. Res. Commun. 2010, 400, 89–93. [Google Scholar] [CrossRef]
- Sun, C.; Liu, C.; Li, S.; Li, H.; Wang, Y.; Xie, Y.; Li, B.; Cui, X.; Chen, Y.; Zhang, W.; et al. Overexpression of GEFT, a Rho family guanine nucleotide exchange factor, predicts poor prognosis in patients with rhabdomyosarcoma. Int. J. Clin. Exp. Pathol. 2014, 7, 1606–1615. [Google Scholar]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Kim, K.H.; Roberts, C.W. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Ducatman, B.S.; Scheithauer, B.W.; Piepgras, D.G.; Reiman, H.M.; Ilstrup, D.M. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986, 57, 2006–2021. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Reith, J.D.; Shahlaee, A.; Bush, C.H.; Hochwald, S.N. Malignant peripheral nerve sheath tumor: Molecular pathogenesis and current management considerations. J. Surg. Oncol. 2008, 97, 340–349. [Google Scholar] [CrossRef]
- Widemann, B.C. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr. Oncol. Rep. 2009, 11, 322–328. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Yang, C.H.; Pfeffer, L.M. The role of miR-21 in cancer. Drug Dev. Res. 2015, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Lankat-Buttgereit, B.; Göke, R. The tumour suppressor Pdcd4: Recent advances in the elucidation of function and regulation. Biol. Cell 2009, 101, 309–317. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Serrano, C.; Hensley, M.L.; Ray-Coquard, I. Soft Tissue and Uterine Leiomyosarcoma. J. Clin. Oncol. 2018, 36, 144–150. [Google Scholar] [CrossRef]
- Brooks, S.E.; Zhan, M.; Cote, T.; Baquet, C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 2004, 93, 204–208. [Google Scholar] [CrossRef]
- Benna, C.; Rajendran, S.; Rastrelli, M.; Mocellin, S. miRNA deregulation targets specific pathways in leiomyosarcoma development: An in silico analysis. J. Transl. Med. 2019, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Ravid, Y.; Formanski, M.; Smith, Y.; Reich, R.; Davidson, B. Uterine leiomyosarcoma and endometrial stromal sarcoma have unique miRNA signatures. Gynecol. Oncol. 2016, 140, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-L.; Park, J.-H.; Nishidate, T.; Nakamura, Y.; Katagiri, T. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res. 2007, 9, R17. [Google Scholar] [CrossRef]
- Kuner, R.; Fälth, M.; Pressinotti, N.C.; Brase, J.C.; Puig, S.B.; Metzger, J.; Gade, S.; Schäfer, G.; Bartsch, G.; Steiner, E. The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J. Mol. Med. 2013, 91, 237–248. [Google Scholar] [CrossRef]
- Kransdorf, M.J. Malignant soft-tissue tumors in a large referral population: Distribution of diagnoses by age, sex, and location. AJR Am. J. Roentgenol. 1995, 164, 129–134. [Google Scholar] [CrossRef]
- Ladanyi, M.; Antonescu, C.R.; Leung, D.H.; Woodruff, J.M.; Kawai, A.; Healey, J.H.; Brennan, M.F.; Bridge, J.A.; Neff, J.R.; Barr, F.G.; et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: A multi-institutional retrospective study of 243 patients. Cancer Res. 2002, 62, 135–140. [Google Scholar]
- Schimanski, C.C.; Schwald, S.; Simiantonaki, N.; Jayasinghe, C.; Gönner, U.; Wilsberg, V.; Junginger, T.; Berger, M.R.; Galle, P.R.; Moehler, M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin. Cancer Res. 2005, 11, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Zeelenberg, I.S.; Ruuls-Van Stalle, L.; Roos, E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003, 63, 3833–3839. [Google Scholar] [PubMed]
- Li, Y.-J.; Dai, Y.-L.; Zhang, W.-B.; Li, S.-J.; Tu, C.-Q. Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: A meta-analysis. Clin. Exp. Med. 2017, 17, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Armenteros-Monterroso, E.; East, P.; Chakravorty, P.; Matthews, N.; Winslow, M.M.; Downward, J. HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature 2014, 505, 212–217. [Google Scholar] [CrossRef]
- Park, S.-M.; Shell, S.; Radjabi, A.R.; Schickel, R.; Feig, C.; Boyerinas, B.; Dinulescu, D.M.; Lengyel, E.; Peter, M.E. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle 2007, 6, 2585–2590. [Google Scholar] [CrossRef]
- Jin, Q.; Mao, X.; Li, B.; Guan, S.; Yao, F.; Jin, F. Overexpression of SMARCA5 correlates with cell proliferation and migration in breast cancer. Tumor Biol. 2015, 36, 1895–1902. [Google Scholar] [CrossRef]
- Kong, Z.; Wan, X.; Zhang, Y.; Zhang, P.; Zhang, Y.; Zhang, X.; Qi, X.; Wu, H.; Huang, J.; Li, Y. Androgen-responsive circular RNA circSMARCA5 is up-regulated and promotes cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1217–1223. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Folpe, A.L. Fibrosarcoma: A review and update. Histopathology 2014, 64, 12–25. [Google Scholar] [CrossRef]
- Abe, H.; Kamai, T.; Shirataki, H.; Oyama, T.; Arai, K.; Yoshida, K.I. High expression of Ran GTPase is associated with local invasion and metastasis of human clear cell renal cell carcinoma. Int. J. Cancer 2008, 122, 2391–2397. [Google Scholar] [CrossRef]
- Kurisetty, V.; Johnston, P.G.; Johnston, N.; Erwin, P.; Crowe, P.; Fernig, D.; Campbell, F.C.; Anderson, I.; Rudland, P.; El-Tanani, M. RAN GTPase is an effector of the invasive/metastatic phenotype induced by osteopontin. Oncogene 2008, 27, 7139–7149. [Google Scholar] [CrossRef] [PubMed]
- Barrès, V.; Ouellet, V.; Lafontaine, J.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.-M. An essential role for Ran GTPase in epithelial ovarian cancer cell survival. Mol. Cancer 2010, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, RA32–RA40. [Google Scholar] [PubMed]
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 2000, 85, 14–20. [Google Scholar] [CrossRef]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, M.; Wang, W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem. Biophys. Res. Commun. 2013, 437, 653–658. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Zhou, Y.; Hu, Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol. Rep. 2012, 28, 1346–1352. [Google Scholar] [CrossRef]
- Fernandez, S.; Risolino, M.; Mandia, N.; Talotta, F.; Soini, Y.; Incoronato, M.; Condorelli, G.; Banfi, S.; Verde, P. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene 2015, 34, 3240–3250. [Google Scholar] [CrossRef]
- Akcakaya, P.; Caramuta, S.; Åhlen, J.; Ghaderi, M.; Berglund, E.; Östman, A.; Bränström, R.; Larsson, C.; Lui, W. microRNA expression signatures of gastrointestinal stromal tumours: Associations with imatinib resistance and patient outcome. Br. J. Cancer 2014, 111, 2091–2102. [Google Scholar] [CrossRef]
- Fernandez-Serra, A.; Moura, D.S.; Sanchez-Izquierdo, M.D.; Calabuig-Fariñas, S.; Lopez-Alvarez, M.; Martínez-Martínez, A.; Carrasco-Garcia, I.; Ramírez-Calvo, M.; Blanco-Alcaina, E.; López-Reig, R. Prognostic Impact of let-7e MicroRNA and Its Target Genes in Localized High-Risk Intestinal GIST: A Spanish Group for Research on Sarcoma (GEIS) Study. Cancers 2020, 12, 2979. [Google Scholar] [CrossRef]
- Niinuma, T.; Kai, M.; Kitajima, H.; Yamamoto, E.; Harada, T.; Maruyama, R.; Nobuoka, T.; Nishida, T.; Kanda, T.; Hasegawa, T. Downregulation of miR-186 is associated with metastatic recurrence of gastrointestinal stromal tumors. Oncol. Lett. 2017, 14, 5703–5710. [Google Scholar] [CrossRef] [PubMed]
- Gyvyte, U.; Juzenas, S.; Salteniene, V.; Kupcinskas, J.; Poskiene, L.; Kucinskas, L.; Jarmalaite, S.; Stuopelyte, K.; Steponaitiene, R.; Hemmrich-Stanisak, G. MiRNA profiling of gastrointestinal stromal tumors by next-generation sequencing. Oncotarget 2017, 8, 37225. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Cao, C.; Niu, H.; Kang, S.; Cong, C.; Kang, S. miRNA-21 sensitizes gastrointestinal stromal tumors (GISTs) cells to Imatinib via targeting B-cell lymphoma 2 (Bcl-2). Eur. Rev. Med. Pharm. Sci. 2016, 20, 3574–3581. [Google Scholar]
- Chen, W.; Li, Z.; Liu, H.; Jiang, S.; Wang, G.; Sun, L.; Li, J.; Wang, X.; Yu, S.; Huang, J. MicroRNA-30a targets BECLIN-1 to inactivate autophagy and sensitizes gastrointestinal stromal tumor cells to imatinib. Cell Death Dis. 2020, 11, 198. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, K.; Tang, Y.; Luan, X.; Zheng, X.; Lu, X.; Mao, J.; Hu, L.; Zhang, S.; Zhang, X. LncRNA-HOTAIR activates autophagy and promotes the imatinib resistance of gastrointestinal stromal tumor cells through a mechanism involving the miR-130a/ATG2B pathway. Cell Death Dis. 2021, 12, 367. [Google Scholar] [CrossRef]
- Gao, X.; Shen, K.; Wang, C.; Ling, J.; Wang, H.; Fang, Y.; Shi, Y.; Hou, Y.; Qin, J.; Sun, Y. MiR-320a downregulation is associated with imatinib resistance in gastrointestinal stromal tumors. Acta Biochim. Biophys Sin. 2014, 46, 72–75. [Google Scholar] [CrossRef]
- Huang, W.-K.; Akçakaya, P.; Gangaev, A.; Lee, L.; Zeljic, K.; Hajeri, P.; Berglund, E.; Ghaderi, M.; Åhlén, J.; Bränström, R. miR-125a-5p regulation increases phosphorylation of FAK that contributes to imatinib resistance in gastrointestinal stromal tumors. Exp. Cell Res. 2018, 371, 287–296. [Google Scholar] [CrossRef]
- Kou, Y.; Yang, R.; Wang, Q. Serum miR-518e-5p is a potential biomarker for secondary imatinib-resistant gastrointestinal stromal tumor. J. Biosci. 2018, 43, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. MicroRNAs in Cancer: A Historical Perspective on the Path from Discovery to Therapy. Cancers 2015, 7, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
| Soft Tissue Sarcoma | Effect on Cancer Development | microRNA |
|---|---|---|
| GIST | Inhibit | miR-494 [23,24] |
| miR-218 [25,26,27] | ||
| miR-221/222 [28,29,30] | ||
| miR-17 [30] | ||
| miR-20a [30] | ||
| miR-4510 [31] | ||
| miR-152 [32] | ||
| miR-133b [33] | ||
| miR-518a-5p [34] | ||
| miR-137 [35] | ||
| Promote | miR-374b [36] | |
| miR-196a [37] | ||
| Liposarcoma | Inhibit | miR-143 [38,39] |
| miR-486 [40] | ||
| miR-145 [38,41] | ||
| miR-451 [39,41] | ||
| miR-193b [42,43] | ||
| miR-133a [44] | ||
| miR-195 [45] | ||
| Promote | miR-155 [39,46,47,48] | |
| miR-26a-2 [38,49,50] | ||
| miR-135b [51] | ||
| miR-25-3p [52] | ||
| miR-92a-3p [52] | ||
| miR-3613-3p [53] | ||
| Rhabdomyosarcoma | Inhibit | miR-206 [54,55,56,57,58,59,60,61] |
| miR-1 [55,56,62,63] | ||
| miR-29 [56,64,65] | ||
| miR-26a [66,67] | ||
| miR-7 [68,69] | ||
| miR-324-5p [69] | ||
| miR-378 family [70] | ||
| miR-133a [62] | ||
| miR-133b [63] | ||
| miR-450b-5p [71] | ||
| miR-203 [72] | ||
| miR-411-5p [73] | ||
| miR-221/222 [74] | ||
| miR-214 [75] | ||
| miR-101 [76] | ||
| miR-874 [77] | ||
| miR-410-3p [78] | ||
| Promote | miR-27a [79,80] | |
| miR-486-5p [74] | ||
| Malignant peripheral nerve sheath tumour | Inhibit | miR-204 [81] |
| miR-30d [82] | ||
| miR-30a [83] | ||
| miR-200b [83] | ||
| miR-34a [84] | ||
| Promote | miR-21 [85] | |
| miR-801 [86] | ||
| miR-214 [86] | ||
| Leiomyosarcoma | Inhibit | miR-1246 [87] |
| miR-191-5p [87] | ||
| miR-34a [88] | ||
| miR-152 [89] | ||
| Promote | miR-181b [90] | |
| miR-320a [91] | ||
| Synovial sarcoma | Inhibit | miR-494-3p [92] |
| miR-126 [93] | ||
| Promote | Let-7e [94] | |
| miR-99b [94] | ||
| miR-92b-3p [95] | ||
| miR-214 [96] | ||
| miR-9 [97] | ||
| miR-17 [98] | ||
| Fibrosarcoma | Inhibit | miR-29 [99] |
| miR-197 [100] | ||
| Promote | miR-520c [101] | |
| miR-373 [101] | ||
| Angiosarcoma | Inhibit | miR-497-5p [102] |
| miR-210 [103] | ||
| miR-340 [104] | ||
| Promote | - |
| GIST | Poor patient survival | miR-494 (downregulation) [24] |
| miR-133b (downregulation) [33] | ||
| miR-1915 (downregulation) [161] | ||
| miR-196a (overexpression) [37] | ||
| let-7e (downregulation) [162] | ||
| Increased metastatic risk | miR-494 (downregulation) [24] | |
| miR-133b (downregulation) [33] | ||
| miR-1915 (downregulation) [161] | ||
| miR-186 (downregulation) [163] | ||
| miR-196a (overexpression) [37] | ||
| miR-215-5p (downregulation) [164] | ||
| Liposarcoma | Poor patient survival | miR-26a-2 (overexpression) [49] |
| miR-135b (overexpression) [51] | ||
| miR-155 (overexpression) [39] | ||
| Increased metastatic risk | miR-135b (overexpression) [51] | |
| Rhabdomyosarcoma | Poor patient survival | miR-206 (downregulation) [61] miR-26a (downregulation) [67] |
| Increased metastatic risk | miR-206 (downregulation) [61] miR-486-5p (overexpression) [74] | |
| Leiomyosarcoma | Poor patient survival | miR-181b (downregulation) [90] |
| Increased metastatic risk | miR-15a (overexpression) [138] | |
| miR-92a (overexpression) [138] | ||
| miR-31 (downregulation) [138] | ||
| Synovial sarcoma | Poor patient survival | miR-214 (overexpression) [96] |
| Increased metastatic risk | miR-494-3p (downregulation) [92] |
| Type of Treatment | Soft Tissue Sarcoma | microRNA Involvement |
|---|---|---|
| Imatinib | GIST | miR-218 [26] |
| miR-518a-5p [34] | ||
| miR-130a [170] | ||
| miR-320a [171] | ||
| miR-21 [168] | ||
| miR-30a [169] | ||
| miR-125a-5p [161,172] | ||
| miR-107 [161] | ||
| miR-518e-5p [173] | ||
| Vincristine | Rhabdomyosarcoma | miR-27a [80] |
| Doxorubicin resistance | Leiomyosarcoma | miR-34a [88] |
| Synovial sarcoma | miR-17 [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, A.Y.T.; Lim, V.Y.; Yang, V.S. MicroRNAs in the Pathogenesis, Prognostication and Prediction of Treatment Resistance in Soft Tissue Sarcomas. Cancers 2023, 15, 577. https://doi.org/10.3390/cancers15030577
Teo AYT, Lim VY, Yang VS. MicroRNAs in the Pathogenesis, Prognostication and Prediction of Treatment Resistance in Soft Tissue Sarcomas. Cancers. 2023; 15(3):577. https://doi.org/10.3390/cancers15030577
Chicago/Turabian StyleTeo, Andrea York Tiang, Vivian Yujing Lim, and Valerie Shiwen Yang. 2023. "MicroRNAs in the Pathogenesis, Prognostication and Prediction of Treatment Resistance in Soft Tissue Sarcomas" Cancers 15, no. 3: 577. https://doi.org/10.3390/cancers15030577
APA StyleTeo, A. Y. T., Lim, V. Y., & Yang, V. S. (2023). MicroRNAs in the Pathogenesis, Prognostication and Prediction of Treatment Resistance in Soft Tissue Sarcomas. Cancers, 15(3), 577. https://doi.org/10.3390/cancers15030577






