Machine Learning-Based Mortality Prediction Model for Critically Ill Cancer Patients Admitted to the Intensive Care Unit (CanICU)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Source of Data
2.3. Outcomes
2.4. Predictor Variables
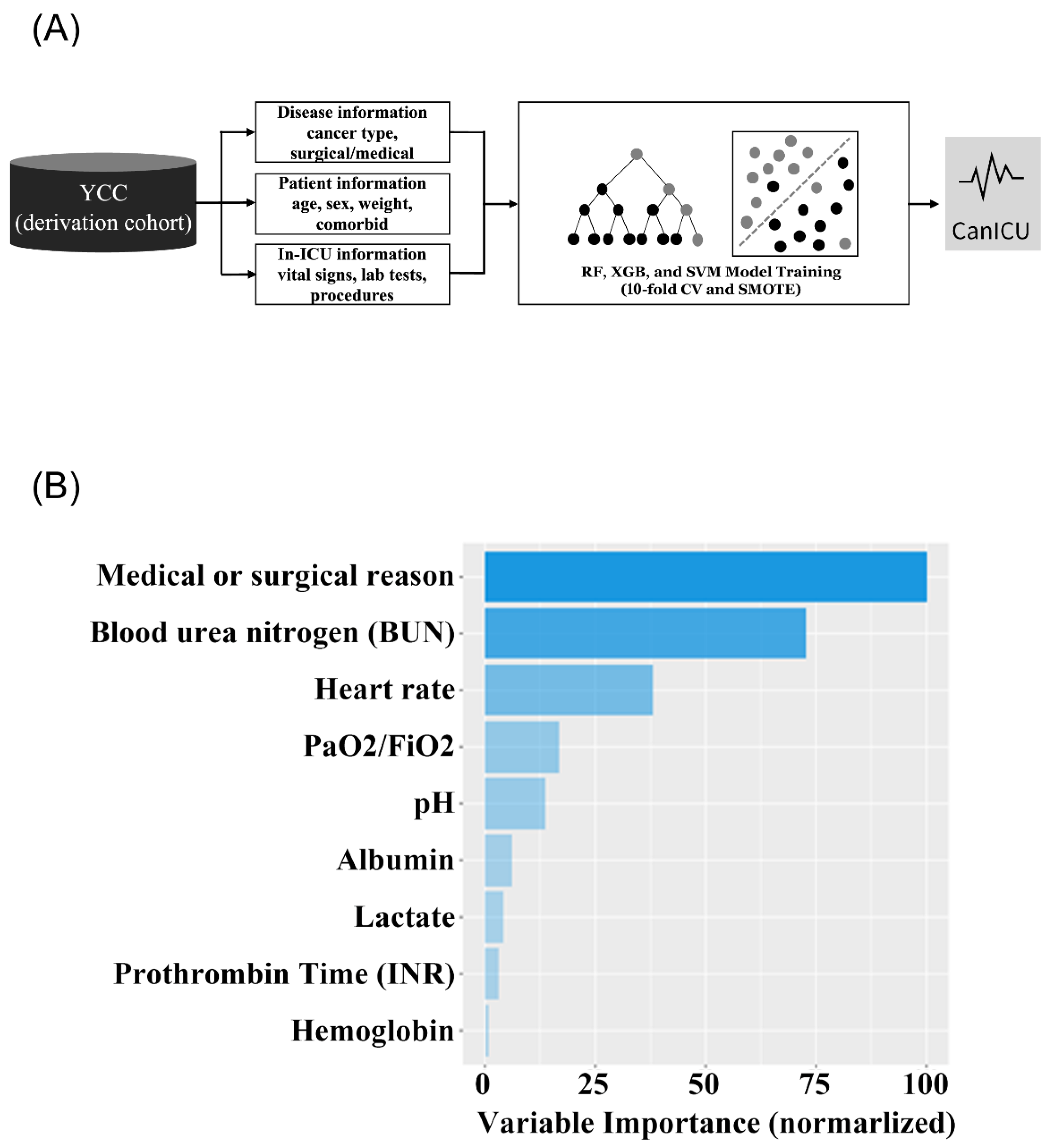
2.5. Modeling Strategies
2.6. Accuracy Comparisons
3. Results
3.1. Development of CanICU
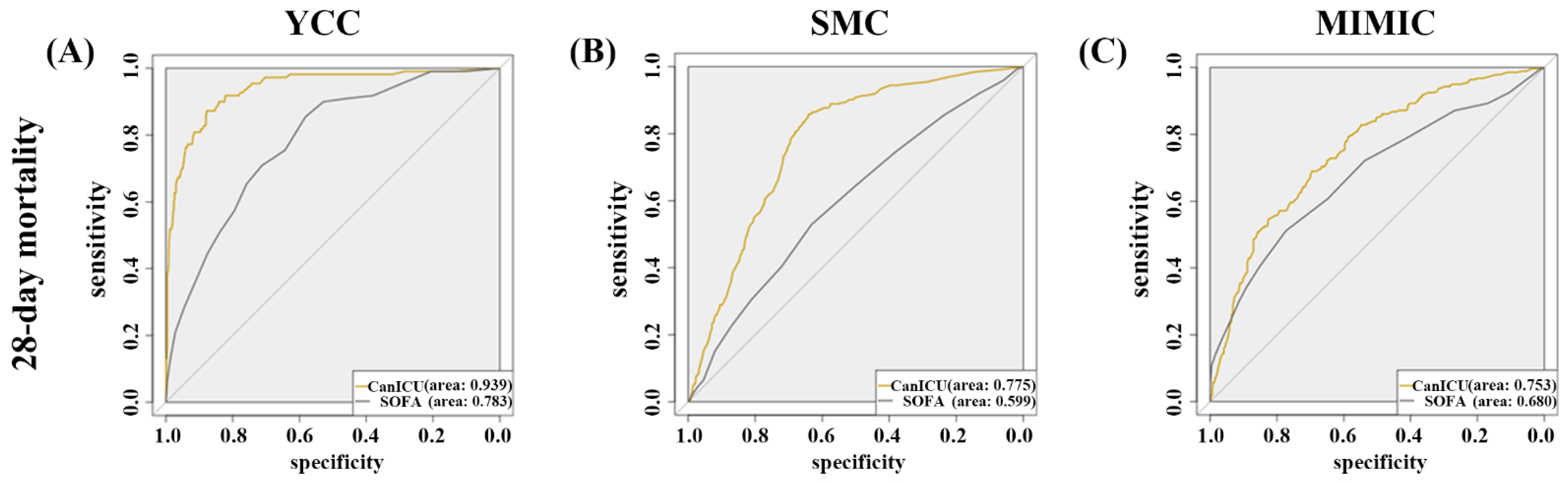
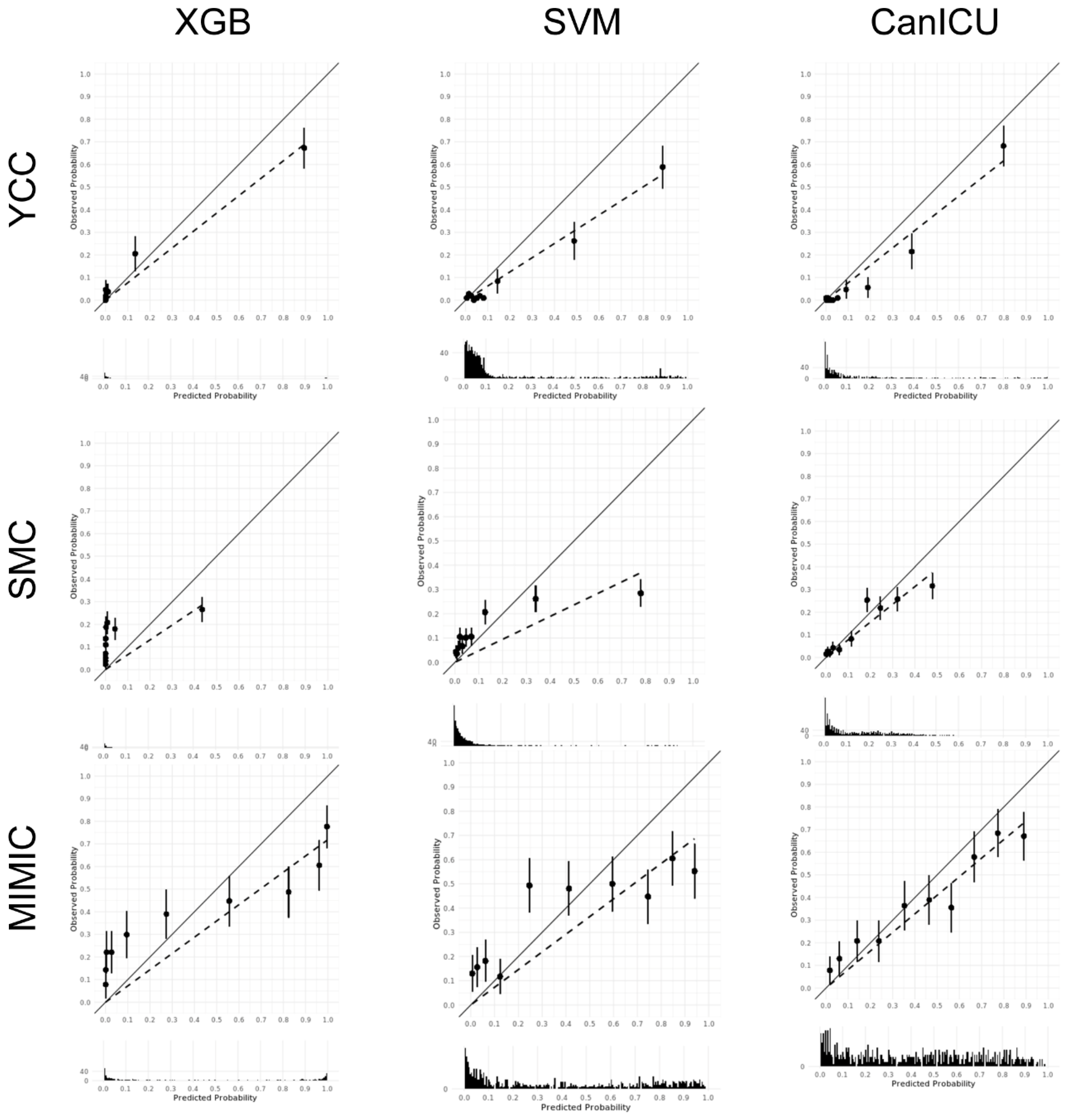
3.2. The Performance of CanICU in Terms of One-Year Mortality
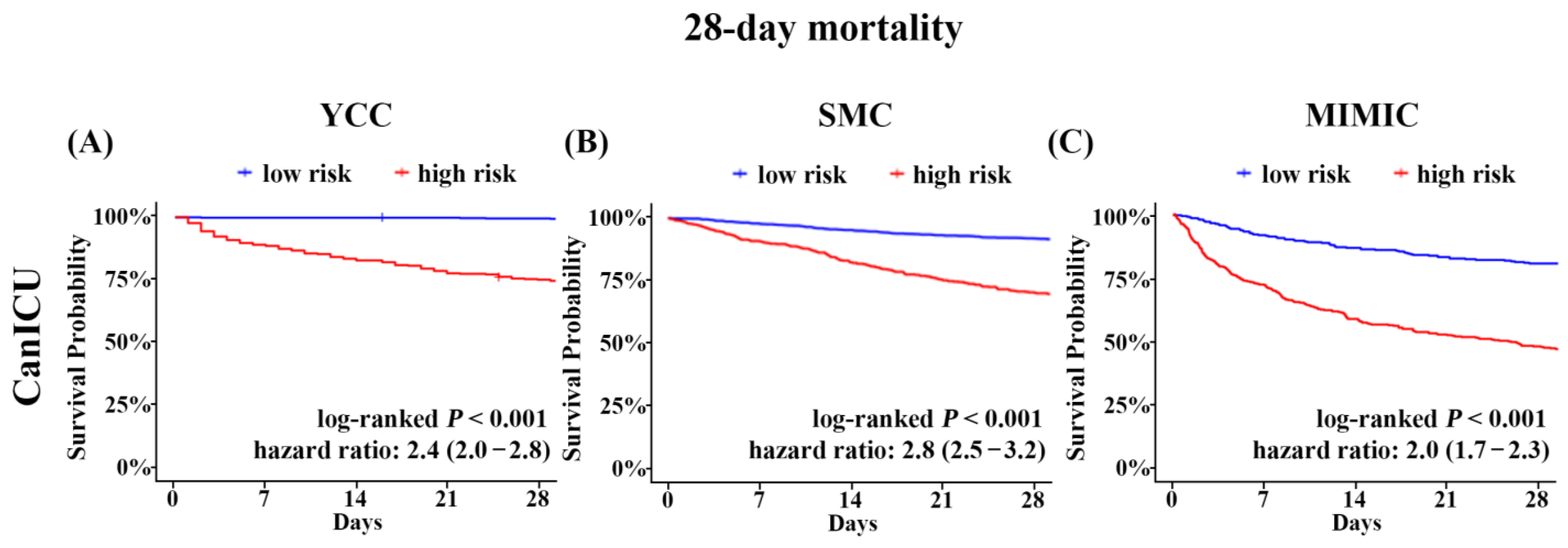
3.3. Risk Stratification of the Mortality Using CanICU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Risk of Critical Illness Among Patients With Solid Cancers: A Population-Based Observational Study. JAMA Oncol. 2015, 1, 1078–1085. [Google Scholar] [CrossRef]
- Azoulay, E.; Moreau, D.; Alberti, C.; Leleu, G.; Adrie, C.; Barboteu, M.; Cottu, P.; Levy, V.; Le Gall, J.R.; Schlemmer, B. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000, 26, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Artigas, A.A.; Sprung, C.L.; Moreno, R.; Sakr, Y.; Vincent, J.L. Characteristics and outcomes of cancer patients in European ICUs. Crit. Care 2009, 13, R15. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit. Care Med. 1999, 27, 633–638. [CrossRef]
- Garrouste-Orgeas, M.; Montuclard, L.; Timsit, J.F.; Reignier, J.; Desmettre, T.; Karoubi, P.; Moreau, D.; Montesino, L.; Duguet, A.; Boussat, S.; et al. Predictors of intensive care unit refusal in French intensive care units: A multiple-center study. Crit. Care Med. 2005, 33, 750–755. [Google Scholar] [CrossRef]
- Schapira, D.V.; Studnicki, J.; Bradham, D.D.; Wolff, P.; Jarrett, A. Intensive care, survival, and expense of treating critically ill cancer patients. JAMA 1993, 269, 783–786. [Google Scholar] [CrossRef]
- Peigne, V.; Rusinova, K.; Karlin, L.; Darmon, M.; Fermand, J.P.; Schlemmer, B.; Azoulay, E. Continued survival gains in recent years among critically ill myeloma patients. Intensive Care Med. 2009, 35, 512–518. [Google Scholar] [CrossRef]
- Mokart, D.; Pastores, S.M.; Darmon, M. Has survival increased in cancer patients admitted to the ICU? Yes. Intensive Care Med. 2014, 40, 1570–1572. [Google Scholar] [CrossRef]
- Azoulay, E.; Mokart, D.; Pene, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.M.; et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en reanimation onco-hematologique study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef]
- Azoulay, E.; Afessa, B. The intensive care support of patients with malignancy: Do everything that can be done. Intensive Care Med. 2006, 32, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Lemiale, V.; Mokart, D.; Pene, F.; Kouatchet, A.; Perez, P.; Vincent, F.; Mayaux, J.; Benoit, D.; Bruneel, F.; et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014, 40, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, P.; Staudinger, T.; Sperr, W.R.; Bojic, A.; Robak, O.; Hermann, A.; Laczika, K.; Carlstrom, A.; Riss, K.; Rabitsch, W.; et al. Prognostic factors, long-term survival, and outcome of cancer patients receiving chemotherapy in the intensive care unit. Ann. Hematol. 2014, 93, 1629–1636. [Google Scholar] [CrossRef]
- Staudinger, T.; Stoiser, B.; Mullner, M.; Locker, G.J.; Laczika, K.; Knapp, S.; Burgmann, H.; Wilfing, A.; Kofler, J.; Thalhammer, F.; et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit. Care Med. 2000, 28, 1322–1328. [Google Scholar] [CrossRef]
- den Boer, S.; de Keizer, N.F.; de Jonge, E. Performance of prognostic models in critically ill cancer patients—A review. Crit. Care 2005, 9, R458–R463. [Google Scholar] [CrossRef] [PubMed]
- Kopterides, P.; Liberopoulos, P.; Ilias, I.; Anthi, A.; Pragkastis, D.; Tsangaris, I.; Tsaknis, G.; Armaganidis, A.; Dimopoulou, I. General prognostic scores in outcome prediction for cancer patients admitted to the intensive care unit. Am. J. Crit. Care 2011, 20, 56–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schellongowski, P.; Benesch, M.; Lang, T.; Traunmuller, F.; Zauner, C.; Laczika, K.; Locker, G.J.; Frass, M.; Staudinger, T. Comparison of three severity scores for critically ill cancer patients. Intensive Care Med. 2004, 30, 430–436. [Google Scholar] [CrossRef]
- Shillan, D.; Sterne, J.A.C.; Champneys, A.; Gibbison, B. Use of machine learning to analyse routinely collected intensive care unit data: A systematic review. Crit. Care 2019, 23, 284. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vellido, A.; Ribas, V.; Morales, C.; Ruiz Sanmartin, A.; Ruiz Rodriguez, J.C. Machine learning in critical care: State-of-the-art and a sepsis case study. Biomed. Eng. Online 2018, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, A.L.; Schmid, M. Machine learning versus statistical modeling. Biom. Journal. Biom. Z. 2014, 56, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Pirracchio, R.; Petersen, M.L.; Carone, M.; Rigon, M.R.; Chevret, S.; van der Laan, M.J. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): A population-based study. Lancet Respir. Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Thorsen-Meyer, H.-C.; Belling, K.; Nielsen, A.P.; Thomas, C.E.; Chmura, P.J.; Lademann, M.; Moseley, P.L.; Heimann, M.; Dybdahl, L.; et al. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: A retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit. Health 2019, 1, e78–e89. [Google Scholar] [CrossRef] [PubMed]
- Danilatou, V.; Nikolakakis, S.; Antonakaki, D.; Tzagkarakis, C.; Mavroidis, D.; Kostoulas, T.; Ioannidis, S. Outcome Prediction in Critically-Ill Patients with Venous Thromboembolism and/or Cancer Using Machine Learning Algorithms: External Validation and Comparison with Scoring Systems. Int. J. Mol. Sci. 2022, 23, 7132. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Gao, Y.; Wang, H.; Huang, C.; Qu, S.; Zhang, H.; Wang, H.; Sun, K. Performance of three prognostic models in patients with cancer in need of intensive care in a medical center in China. PLoS ONE 2015, 10, e0131329. [Google Scholar] [CrossRef]
- Johnson, A.E.; Pollard, T.J.; Shen, L.; Lehman, L.W.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.D.; Vandewoude, K.H.; Decruyenaere, J.M.; Hoste, E.A.; Colardyn, F.A. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit. Care Med. 2003, 31, 104–112. [Google Scholar] [CrossRef]
- Vandijck, D.M.; Depuydt, P.O.; Offner, F.C.; Nollet, J.; Peleman, R.A.; Steel, E.; Noens, L.A.; Decruyenaere, J.M.; Benoit, D.D. Impact of organ dysfunction on mortality in ICU patients with hematologic malignancies. Intensive Care Med. 2010, 36, 1744–1750. [Google Scholar] [CrossRef]
- Georges, Q.; Azoulay, E.; Mokart, D.; Soares, M.; Jeon, K.; Oeyen, S.; Rhee, C.K.; Gruber, P.; Ostermann, M.; Hill, Q.A.; et al. Influence of neutropenia on mortality of critically ill cancer patients: Results of a meta-analysis on individual data. Crit. Care 2018, 22, 326. [Google Scholar] [CrossRef]
- Puxty, K.; McLoone, P.; Quasim, T.; Kinsella, J.; Morrison, D. Survival in solid cancer patients following intensive care unit admission. Intensive Care Med. 2014, 40, 1409–1428. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Breiman, L. “Random Forests” Machine Learning; Springer: Berlin/Heidelberg, Germany, 2001; Volume 45, pp. 5–32. [Google Scholar]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.H.; Kelm, B.M.; Masuch, R.; Himmelreich, U.; Bachert, P.; Petrich, W.; Hamprecht, F.A. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinform. 2009, 10, 213. [Google Scholar] [CrossRef]
- Cherruault, M.; Le Goff, M.; Tamburini, J.; Pene, F. Urgent Chemotherapy in Sepsis-Like Shock Related to Hematologic Malignancies. Crit. Care Med. 2018, 46, e465–e468. [Google Scholar] [CrossRef]
- Awad, A.; Bader-El-Den, M.; McNicholas, J.; Briggs, J. Early hospital mortality prediction of intensive care unit patients using an ensemble learning approach. Int. J. Med. Inform. 2017, 108, 185–195. [Google Scholar] [CrossRef]
- Anand, R.S.; Stey, P.; Jain, S.; Biron, D.R.; Bhatt, H.; Monteiro, K.; Feller, E.; Ranney, M.L.; Sarkar, I.N.; Chen, E.S. Predicting Mortality in Diabetic ICU Patients Using Machine Learning and Severity Indices. AMIA Jt. Summits Transl. Sci. Proc. 2018, 2017, 310–319. [Google Scholar]
- Verplancke, T.; Van Looy, S.; Benoit, D.; Vansteelandt, S.; Depuydt, P.; De Turck, F.; Decruyenaere, J. Support vector machine versus logistic regression modeling for prediction of hospital mortality in critically ill patients with haematological malignancies. BMC Med. Inform. Decis. Mak. 2008, 8, 56. [Google Scholar] [CrossRef]
- Huynh, T.N.; Kleerup, E.C.; Wiley, J.F.; Savitsky, T.D.; Guse, D.; Garber, B.J.; Wenger, N.S. The frequency and cost of treatment perceived to be futile in critical care. JAMA Intern. Med. 2013, 173, 1887–1894. [Google Scholar] [CrossRef]
- Meltzer, L.S.; Huckabay, L.M. Critical care nurses’ perceptions of futile care and its effect on burnout. Am. J. Crit. Care 2004, 13, 202–208. [Google Scholar] [CrossRef]
- Soares, M.; Caruso, P.; Silva, E.; Teles, J.M.; Lobo, S.M.; Friedman, G.; Dal Pizzol, F.; Mello, P.V.; Bozza, F.A.; Silva, U.V.; et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit. Care Med. 2010, 38, 9–15. [Google Scholar] [CrossRef] [PubMed]
| Variables | YCC (2008–2017) (n = 3571) | SMC (2011–2017) (n = 2563) | MIMIC (2001–2012) (n = 766) | p Value | |||
|---|---|---|---|---|---|---|---|
| Age, years | 64 | (55–72) | 63 | (54–72) | 66 | (56–75) | <0.001 |
| Sex, male | 2383 | (66.7) | 1668 | (65.1) | 474 | (61.9) | 0.030 |
| Primary cancer | |||||||
| Liver | 820 | (23.0) | 470 | (18.3) | 107 | (14.0) | <0.001 |
| Colorectal | 693 | (19.4) | 358 | (14.0) | 41 | (5.4) | <0.001 |
| Lung | 124 | (3.5) | 149 | (5.8) | 92 | (12.0) | <0.001 |
| Stomach | 414 | (11.6) | 428 | (16.7) | 15 | (2.0) | <0.001 |
| Hematologic malignancy | 99 | (2.8) | 409 | (16.0) | 170 | (22.2) | <0.001 |
| Others * | 1421 | (39.8) | 749 | (29.2) | 341 | (44.5) | <0.001 |
| Admission type | |||||||
| Medical | 595 | (16.7) | 898 | (35.0) | 448 | (58.5) | <0.001 |
| Surgical | 2976 | (83.3) | 1665 | (65.0) | 318 | (41.5) | <0.001 |
| SOFA at ICU admission | 6 | (2–10) | 6 | (4–8) | 6 | (4–8) | <0.001 |
| Organ support at ICU admission | |||||||
| Requirement of mechanical ventilation | 1494 | (41.8) | 603 | (23.5) | 698 | (91.1) | <0.001 |
| Vasopressor use | 1448 | (40.6) | 315 | (12.3) | 411 | (53.7) | <0.001 |
| Renal replacement therapy | 108 | (3.0) | 121 | (4.7) | 23 | (3.0) | 0.001 |
| Outcome | |||||||
| 28-day mortality | 363 | (10.2) | 325 | (12.7) | 280 | (36.6) | <0.001 |
| 1-year mortality | 1072 | (30.0) | 938 | (36.6) | 448 | (58.5) | <0.001 |
| YCC (n = 1072) | SMC (n = 2563) | MIMIC (n = 766) | ||||
|---|---|---|---|---|---|---|
| CanICU | SOFA Score | CanICU | SOFA Score | CanICU | SOFA Score | |
| Outcome | ||||||
| 28-Day Mortality | 105 | 325 | 280 | |||
| 1-Year Mortality | 311 | 938 | 448 | |||
| Model performance | ||||||
| AUC | 0.939 (0.914–0.964) | 0.783 (0.741–0.825) | 0.775 (0.751–0.799) | 0.599 (0.566–0.632) | 0.753 (0.718–0.788) | 0.680 (0.639–0.720) |
| Kappa | 0.336 | 0.171 | 0.210 | 0.085 | 0.338 | 0.290 |
| F1 | 0.841 | 0.728 | 0.723 | 0.743 | 0.688 | 0.753 |
| Discrimination Indices | ||||||
| Sensitivity | 0.955 | 0.855 | 0.889 | 0.529 | 0.786 | 0.511 |
| Specificity | 0.729 | 0.582 | 0.575 | 0.632 | 0.588 | 0.774 |
| PPV | 0.287 | 0.190 | 0.233 | 0.173 | 0.524 | 0.565 |
| NPV | 0.993 | 0.972 | 0.973 | 0.902 | 0.827 | 0.733 |
| Brier score | 0.735 | 0.023 | 0.727 | 0.020 | 0.419 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, R.-E.; Cho, J.; Shin, M.-K.; Oh, S.W.; Seong, Y.; Jeon, J.; Jeon, K.; Paik, S.; Lim, J.S.; Shin, S.J.; et al. Machine Learning-Based Mortality Prediction Model for Critically Ill Cancer Patients Admitted to the Intensive Care Unit (CanICU). Cancers 2023, 15, 569. https://doi.org/10.3390/cancers15030569
Ko R-E, Cho J, Shin M-K, Oh SW, Seong Y, Jeon J, Jeon K, Paik S, Lim JS, Shin SJ, et al. Machine Learning-Based Mortality Prediction Model for Critically Ill Cancer Patients Admitted to the Intensive Care Unit (CanICU). Cancers. 2023; 15(3):569. https://doi.org/10.3390/cancers15030569
Chicago/Turabian StyleKo, Ryoung-Eun, Jaehyeong Cho, Min-Kyue Shin, Sung Woo Oh, Yeonchan Seong, Jeongseok Jeon, Kyeongman Jeon, Soonmyung Paik, Joon Seok Lim, Sang Joon Shin, and et al. 2023. "Machine Learning-Based Mortality Prediction Model for Critically Ill Cancer Patients Admitted to the Intensive Care Unit (CanICU)" Cancers 15, no. 3: 569. https://doi.org/10.3390/cancers15030569
APA StyleKo, R.-E., Cho, J., Shin, M.-K., Oh, S. W., Seong, Y., Jeon, J., Jeon, K., Paik, S., Lim, J. S., Shin, S. J., Ahn, J. B., Park, J. H., You, S. C., & Kim, H. S. (2023). Machine Learning-Based Mortality Prediction Model for Critically Ill Cancer Patients Admitted to the Intensive Care Unit (CanICU). Cancers, 15(3), 569. https://doi.org/10.3390/cancers15030569








