Disparities in Cancer Incidence across Income Levels in South Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Participants
2.2. Identification of Cancer Incidence
2.3. Income Classification
2.4. Covariates
2.5. Analytical Approach and Statistics
3. Results
3.1. Baseline Characteristics
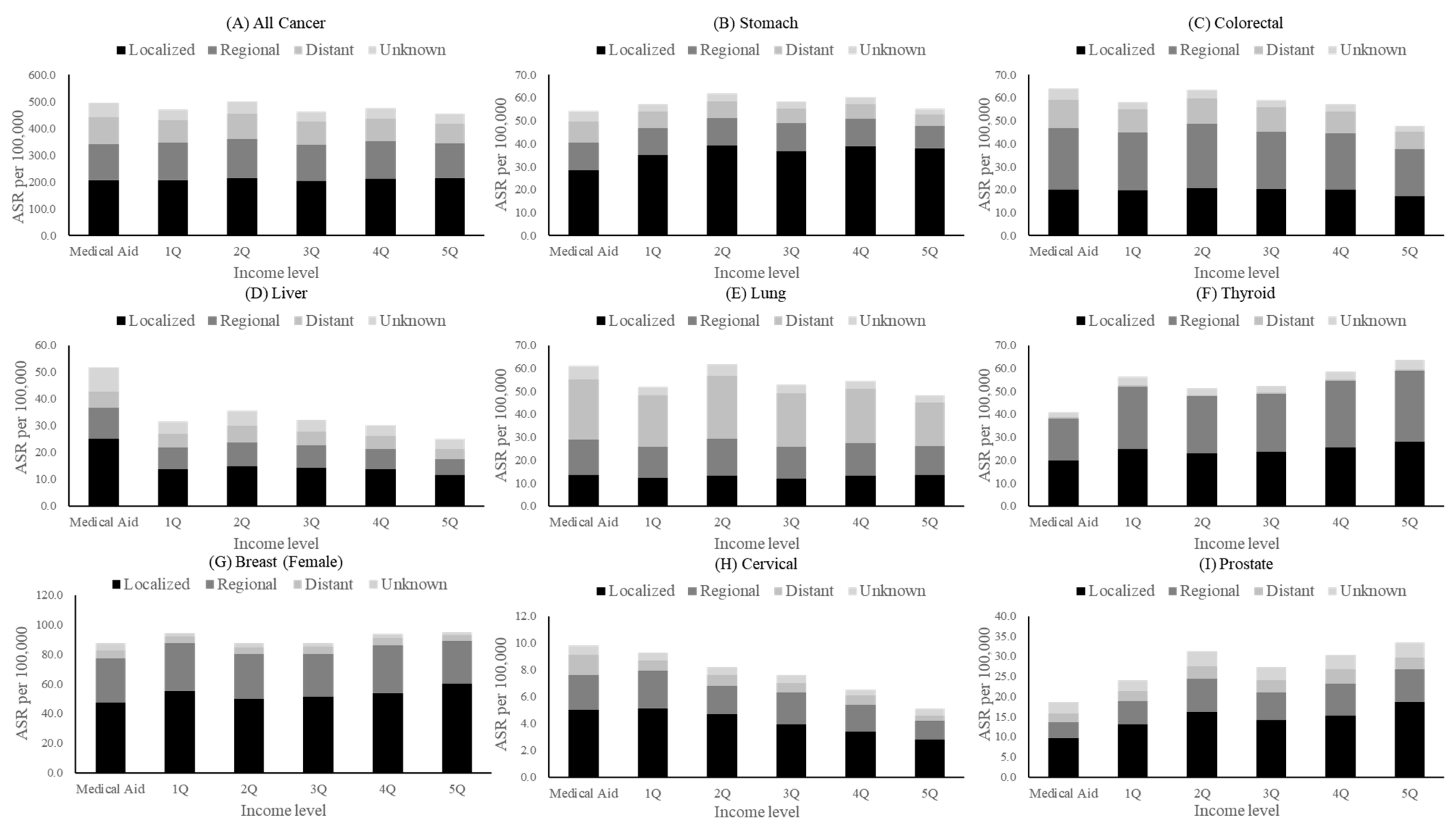
3.2. Incidence of Cancer and Disparities by Income
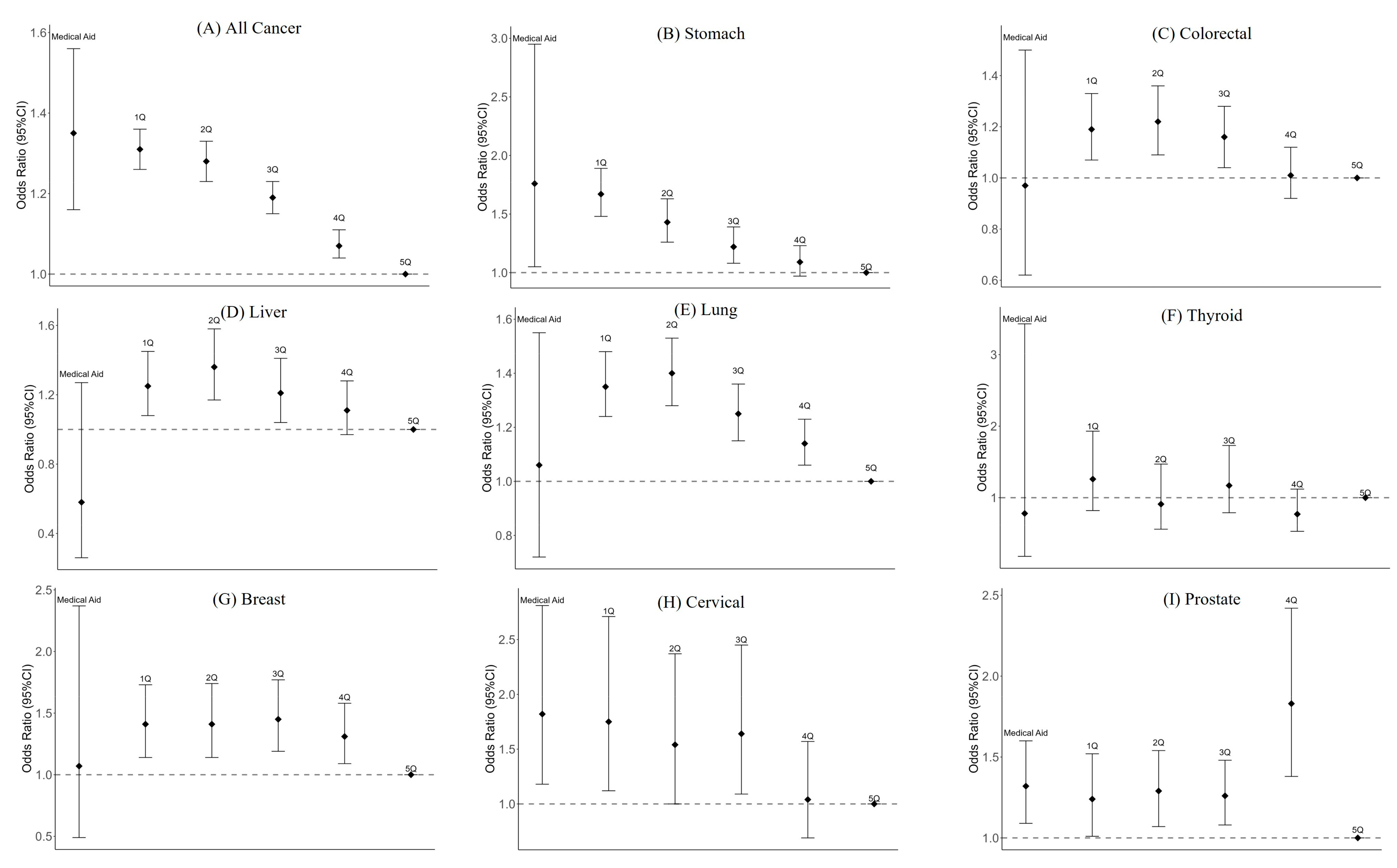
3.3. Association between Income and Distant Stage at Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res. Treat. 2022, 54, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Nédó, E.; Paulik, E. Association of smoking, physical activity, and dietary habits with socioeconomic variables: A cross-sectional study in adults on both sides of the Hungarian-Romanian border. BMC Public Health 2012, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, H.M.; Jung, B.Y.; Park, E.C.; Cho, W.H.; Lee, S.G. The association between cancer incidence and family income: Analysis of Korean National Health Insurance cancer registration data. Asian Pac. J. Cancer Prev. 2012, 13, 1371–1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tope, P.; Morais, S.; El-Zein, M.; Franco, E.L.; Malagón, T. Differences in site-specific cancer incidence by individual- and area-level income in Canada from 2006 to 2015. Int. J. Cancer 2023, 153, 1766–1783. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009, 20, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, R.T.; Howe, H.L. County-level poverty and distant stage cancer in the United States. Cancer Causes Control 2009, 20, 989–1000. [Google Scholar] [CrossRef]
- Bolormaa, E.; Choe, S.A.; Son, M.; Ki, M.; Paek, D. Income-based disparities in the risk of distant-stage cervical cancer and 5-year mortality after the introduction of a National Cancer Screening Program in Korea. Epidemiol. Health 2022, 44, e2022066. [Google Scholar] [CrossRef]
- Choe, S.A.; Roh, M.; Kim, H.R.; Lee, S.; Ki, M.; Paek, D.; Son, M. Income Disparity in Breast Cancer Incidence and Stage at Presentation: A National Population Study of South Korea. J. Breast Cancer 2022, 25, 415–424. [Google Scholar] [CrossRef]
- Kim, C.W.; Lee, S.Y.; Moon, O.R. Inequalities in cancer incidence and mortality across income groups and policy implications in South Korea. Public Health 2008, 122, 229–236. [Google Scholar] [CrossRef]
- Tanaka, R.; Matsuzaka, M.; Sasaki, Y. Influence of Income on Cancer Incidence and Death among Patients in Aomori, Japan. Asian Pac. J. Cancer Prev. 2018, 19, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T. Current Status of the National Health Screening Programs in South Korea. Korean J. Fam. Med. 2022, 43, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.P.; Kunst, A.E. Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Soc. Sci. Med. 1997, 44, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Nugent, Z.; Decker, K.; Deniers, A.; Samaddar, J.; Torabi, M. Geographic variation and factors associated with colorectal cancer incidence in Manitoba. Can. J. Public Health 2017, 108, e558–e564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brooke, H.L.; Talbäck, M.; Martling, A.; Feychting, M.; Ljung, R. Socioeconomic position and incidence of colorectal cancer in the Swedish population. Cancer Epidemiol. 2016, 40, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.Y.; Hsu, H.S.; Kung, P.T.; Tsai, W.C. Impact of Socioeconomic Status on Cancer Incidence Risk, Cancer Staging, and Survival of Patients with Colorectal Cancer under Universal Health Insurance Coverage in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 12164. [Google Scholar] [CrossRef] [PubMed]
- Warren Andersen, S.; Blot, W.J.; Lipworth, L.; Steinwandel, M.; Murff, H.J.; Zheng, W. Association of Race and Socioeconomic Status with Colorectal Cancer Screening, Colorectal Cancer Risk, and Mortality in Southern US Adults. JAMA Netw. Open 2019, 2, e1917995. [Google Scholar] [CrossRef]
- Park, B.; Lee, Y.Y.; Song, S.Y.; Shin, H.Y.; Suh, M.; Choi, K.S.; Jun, J.K. Trends of Colorectal Cancer Screening Rates in Korea: Korean National Cancer Screening Survey 2005–2020. Gut Liver 2022, 16, 930–941. [Google Scholar] [CrossRef]
- Luu, X.Q.; Lee, K.; Jun, J.K.; Suh, M.; Choi, K.S. Socioeconomic inequality in organized and opportunistic screening for colorectal cancer: Results from the Korean National Cancer Screening Survey, 2009–2021. Epidemiol. Health 2023, 45, e2023086. [Google Scholar] [CrossRef]
- Rubin, L.; Okitondo, C.; Haines, L.; Ebell, M. Interventions to increase colorectal cancer screening adherence in low-income settings within the United States: A systematic review and meta-analysis. Prev. Med. 2023, 172, 107522. [Google Scholar] [CrossRef]
- Parikh, S.; Brennan, P.; Boffetta, P. Meta-analysis of social inequality and the risk of cervical cancer. Int. J. Cancer 2003, 105, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Shields, T.S.; Brinton, L.A.; Burk, R.D.; Wang, S.S.; Weinstein, S.J.; Ziegler, R.G.; Studentsov, Y.Y.; McAdams, M.; Schiffman, M. A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1574–1582. [Google Scholar] [CrossRef]
- Jeudin, P.; Liveright, E.; del Carmen, M.G.; Perkins, R.B. Race, Ethnicity, and Income Factors Impacting Human Papillomavirus Vaccination rates. Clin. Ther. 2014, 36, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Fisher, E.S. Income and Cancer Overdiagnosis—When Too Much Care Is Harmful. N. Engl. J. Med. 2017, 376, 2208–2209. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Han, K.; Shin, D.W.; Kim, D.; Jeong, S.M.; Chun, S.; Choi, I.Y.; Jeon, K.H.; Kim, T.H. Changes in Smoking, Alcohol Consumption, and the Risk of Thyroid Cancer: A Population-Based Korean Cohort Study. Cancers 2021, 13, 2343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Witte, J.S.; McClure, L.A.; Shema, S.J.; Cockburn, M.G.; John, E.M.; Clarke, C.A. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020, 8, 49–54. [Google Scholar] [CrossRef]
- Shin, D.W.; Park, J.; Yeob, K.E.; Yoon, S.J.; Jang, S.N.; Kim, S.Y.; Park, J.H.; Park, J.H.; Kawachi, I. Disparities in prostate cancer diagnosis, treatment, and survival among men with disabilities: Retrospective cohort study in South Korea. Disabil. Health J. 2021, 14, 101125. [Google Scholar] [CrossRef]
- Uthman, O.A.; Jadidi, E.; Moradi, T. Socioeconomic position and incidence of gastric cancer: A systematic review and meta-analysis. J. Epidemiol. Community Health 2013, 67, 854–860. [Google Scholar] [CrossRef]
- Sidorchuk, A.; Agardh, E.E.; Aremu, O.; Hallqvist, J.; Allebeck, P.; Moradi, T. Socioeconomic differences in lung cancer incidence: A systematic review and meta-analysis. Cancer Causes Control 2009, 20, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.; Lee, J.; Lee, J.-H.; Shin, S.; Tchoe, H.j.; Kwon, J.-W. Risk factors for developing liver cancer in people with and without liver disease. PLoS ONE 2018, 13, e0206374. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Lim, J.; Jung, Y.S.; Kim, Y.; Jung, K.W.; Hong, S.; Won, Y.J. Decreasing trends in thyroid cancer incidence in South Korea: What happened in South Korea? Cancer Med. 2021, 10, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, Y.Y.; Suh, M.; Choi, E.; Mai, T.T.X.; Cho, H.; Park, B.; Jun, J.K.; Kim, Y.; Oh, J.K.; et al. Socioeconomic Inequalities in Stomach Cancer Screening in Korea, 2005-2015: After the Introduction of the National Cancer Screening Program. Yonsei Med. J. 2018, 59, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.; Subramanian, S.V. Has the National Cancer Screening Program reduced income inequalities in screening attendance in South Korea? Cancer Causes Control 2015, 26, 1617–1625. [Google Scholar] [CrossRef]
- Finney Rutten, L.J.; Nelson, D.E.; Meissner, H.I. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987–2000). Prev. Med. 2004, 38, 258–268. [Google Scholar] [CrossRef]
- Forbes, L.J.L.; Warburton, F.; Richards, M.A.; Ramirez, A.J. Risk factors for delay in symptomatic presentation: A survey of cancer patients. Br. J. Cancer 2014, 111, 581–588. [Google Scholar] [CrossRef]
- Sandström, N.; Johansson, M.; Jekunen, A.; Andersén, H. Socioeconomic status and lifestyle patterns in the most common cancer types-community-based research. BMC Public Health 2023, 23, 1722. [Google Scholar] [CrossRef]
- Housten, A.J.; Gunn, C.M.; Paasche-Orlow, M.K.; Basen-Engquist, K.M. Health Literacy Interventions in Cancer: A Systematic Review. J. Cancer Educ. 2021, 36, 240–252. [Google Scholar] [CrossRef]
| Characteristics | Cancer Patients (All Cancers) | Gastric | Colorectal | Liver | Lung | Thyroid | Breast (Female) | Cervical (Female) | Prostate (Male) |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male | 104,069 (51.8) | 17,528 (68.3) | 15,439 (59.6) | 10,616 (74.9) | 16,503 (68.0) | 6506 (23.6) | NA | NA | 13,417 (100.0) |
| Female | 97,027 (48.2) | 8152 (31.7) | 10,470 (40.4) | 3559 (25.1) | 7752 (32.0) | 21,035 (76.4) | 22,209 (100.0) | 3295 (100.0) | NA |
| Age group | |||||||||
| 20–29 | 4468 (2.2) | 54 (0.2) | 113 (0.4) | 43 (0.3) | 35 (0.1) | 2081 (7.6) | 130 (0.6) | 71 (2.2) | 2 (0.0) |
| 30–39 | 11,242 (5.6) | 495 (1.9) | 622 (2.4) | 229 (1.6) | 175 (0.7) | 5675 (20.6) | 1724 (7.8) | 576 (17.5) | 5 (0.0) |
| 40–49 | 25,867 (12.9) | 2271 (8.8) | 2010 (7.8) | 1143 (8.1) | 814 (3.4) | 7505 (27.3) | 7096 (32.0) | 820 (24.9) | 78 (0.6) |
| 50–59 | 42,317 (21.0) | 5749 (22.4) | 5287 (20.4) | 3420 (24.1) | 3251 (13.4) | 6964 (25.3) | 6849 (30.8) | 783 (23.8) | 1091 (8.1) |
| 60–69 | 49,008 (24.4) | 7525 (29.3) | 6731 (26.0) | 4011 (28.3) | 6919 (28.5) | 3684 (13.4) | 3983 (17.9) | 490 (14.9) | 4309 (32.1) |
| ≥70 | 68,194 (33.9) | 9586 (37.3) | 11,146 (43.0) | 5329 (37.6) | 13,061 (53.8) | 1632 (5.9) | 2427 (10.9) | 555 (16.8) | 7932 (59.1) |
| Income | |||||||||
| Medicaid | 9754 (4.9) | 1176 (4.6) | 1583 (6.1) | 1118 (7.9) | 1688 (7.0) | 564 (2.0) | 738 (3.3) | 166 (5.0) | 540 (4.0) |
| 1Q | 31,440 (15.6) | 3993 (15.5) | 4254 (16.4) | 2311 (16.3) | 3786 (15.6) | 4026 (14.6) | 3964 (17.8) | 650 (19.7) | 1753 (13.1) |
| 2Q | 27,225 (13.5) | 3428 (13.3) | 3595 (13.9) | 2045 (14.4) | 3135 (12.9) | 3894 (14.1) | 3297 (14.8) | 570 (17.3) | 1536 (11.4) |
| 3Q | 31,443 (15.6) | 4054 (15.8) | 4166 (16.1) | 2242 (15.8) | 3529 (14.5) | 4657 (16.9) | 3516 (15.8) | 579 (17.6) | 1821 (13.6) |
| 4Q | 41,340 (20.6) | 5298 (20.6) | 5162 (19.9) | 2748 (19.4) | 4758 (19.6) | 6357 (23.1) | 4486 (20.2) | 666 (20.2) | 2684 (20.0) |
| 5Q | 59,894 (29.8) | 7731 (30.1) | 7149 (27.6) | 3711 (26.2) | 7359 (30.3) | 8043 (29.2) | 6208 (28.0) | 664 (20.2) | 5083 (37.9) |
| Employment type | |||||||||
| Employee | 58,356 (29.0) | 7682 (29.9) | 8047 (31.1) | 4631 (32.7) | 7193 (29.7) | 6433 (23.4) | 6553 (29.5) | 1118 (33.9) | 3794 (28.3) |
| Local | 132,986 (66.1) | 16,822 (65.5) | 16,279 (62.8) | 8426 (59.4) | 15,374 (63.4) | 20,544 (74.6) | 14,918 (67.2) | 2011 (61.0) | 9083 (67.7) |
| Medicaid | 9754 (4.9) | 1176 (4.6) | 1583 (6.1) | 1118 (7.9) | 1688 (7.0) | 564 (2.0) | 738 (3.3) | 166 (5.0) | 540 (4.0) |
| BMI | |||||||||
| <18.5 | 6700 (3.3) | 870 (3.4) | 842 (3.2) | 459 (3.2) | 822 (3.4) | 944 (3.4) | 741 (3.3) | 99 (3.0) | 460 (3.4) |
| 18.5–25 | 120,740 (60.0) | 15,426 (60.1) | 15,661 (60.4) | 8357 (59.0) | 14,557 (60.0) | 16,574 (60.2) | 13,273 (59.8) | 2036 (61.8) | 8013 (59.7) |
| 25–30 | 63,520 (31.6) | 8040 (31.3) | 8137 (31.4) | 4626 (32.6) | 7670 (31.6) | 8663 (31.5) | 7045 (31.7) | 988 (30.0) | 4242 (31.6) |
| ≥30 | 10,136 (5.0) | 1344 (5.2) | 1269 (4.9) | 733 (5.2) | 1206 (5.0) | 1360 (4.9) | 1150 (5.2) | 172 (5.2) | 702 (5.2) |
| Smoking status | |||||||||
| Never | 138,892 (69.1) | 17,688 (68.9) | 17,979 (69.4) | 9775 (69.0) | 16,782 (69.2) | 18,971 (68.9) | 15,347 (69.1) | 2297 (69.7) | 9344 (69.6) |
| Past | 39,823 (19.8) | 5112 (19.9) | 5105 (19.7) | 2782 (19.6) | 4776 (19.7) | 5479 (19.9) | 4416 (19.9) | 643 (19.5) | 2595 (19.3) |
| Current | 22,381 (11.1) | 2880 (11.2) | 2825 (10.9) | 1618 (11.4) | 2697 (11.1) | 3091 (11.2) | 2446 (11.0) | 355 (10.8) | 1478 (11.0) |
| Alcohol consumption | |||||||||
| No | 118,335 (58.8) | 15,032 (58.5) | 15,434 (59.6) | 8344 (58.9) | 14,315 (59.0) | 16,168 (58.7) | 12,999 (58.5) | 1918 (58.2) | 7897 (58.9) |
| Yes | 82,761 (41.2) | 10,648 (41.5) | 10,475 (40.4) | 5831 (41.1) | 9940 (41.0) | 11,373 (41.3) | 9210 (41.5) | 1377 (41.8) | 5520 (41.1) |
| Cancer Type | Medical Aid | Health Insurance Subscribers | SII | SII 95% CI | RII | RII 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Q | 2Q | 3Q | 4Q | 5Q | ||||||||
| All cancers | 496.6 | 470.8 | 499.8 | 463.2 | 477.4 | 454.3 | −35.75 | −93.65 | 22.15 | −0.07 | −0.20 | 0.05 |
| Stomach | 54.4 | 57.1 | 62.0 | 58.6 | 60.3 | 55.4 | −3.29 | −14.38 | 7.79 | −0.06 | −0.25 | 0.13 |
| Colorectal | 64.0 | 58.0 | 63.7 | 59.2 | 57.1 | 47.8 | −17.23 | −31.64 | −2.82 | −0.30 | −0.54 | −0.05 |
| Liver | 51.8 | 31.6 | 35.7 | 32.2 | 30.2 | 25.1 | −14.43 | −29.94 | 1.09 | −0.42 | −0.87 | 0.03 |
| Lung | 61.3 | 52.0 | 61.7 | 53.0 | 54.4 | 48.2 | −10.55 | −27.10 | 5.99 | −0.19 | −0.49 | 0.11 |
| Thyroid | 41.0 | 56.3 | 51.2 | 52.4 | 58.7 | 63.6 | 15.97 | 1.14 | 30.81 | 0.30 | 0.02 | 0.57 |
| Breast (female) | 44.9 | 54.1 | 46.7 | 43.8 | 44.3 | 45.3 | 5.96 | −7.93 | 19.84 | 0.07 | −0.09 | 0.22 |
| Cervical | 9.8 | 9.3 | 8.2 | 7.5 | 6.6 | 5.0 | −5.49 | −6.10 | −4.87 | −0.71 | −0.79 | −0.63 |
| Prostate | 18.6 | 24.1 | 31.4 | 27.4 | 30.4 | 33.5 | 10.76 | 0.29 | 21.23 | 0.39 | 0.01 | 0.77 |
| Type of Cancer | Medical Aid | 1Q | 2Q | 3Q | 4Q | 5Q |
|---|---|---|---|---|---|---|
| All cancers | 1.35 | 1.31 | 1.28 | 1.19 | 1.07 | Reference |
| (1.16–1.56) | (1.26–1.36) | (1.23–1.33) | (1.15–1.23) | (1.04–1.11) | ||
| Stomach | 1.76 | 1.67 | 1.43 | 1.22 | 1.09 | Reference |
| (1.05–2.95) | (1.48–1.89) | (1.26–1.63) | (1.08–1.39) | (0.97–1.23) | ||
| Colorectal | 0.97 | 1.19 | 1.22 | 1.16 | 1.01 | Reference |
| (0.62–1.50) | (1.07–1.33) | (1.09–1.36) | (1.04–1.28) | (0.92–1.12) | ||
| Liver | 0.58 | 1.25 | 1.36 | 1.21 | 1.11 | Reference |
| (0.26–1.27) | (1.08–1.45) | (1.17–1.58) | (1.04–1.41) | (0.97–1.28) | ||
| Lung | 1.06 | 1.35 | 1.4 | 1.25 | 1.14 | Reference |
| (0.72–1.55) | (1.24–1.48) | (1.28–1.53) | (1.15–1.36) | (1.06–1.23) | ||
| Thyroid | 0.78 | 1.26 | 0.91 | 1.17 | 0.77 | Reference |
| (0.18–3.43) | (0.82–1.93) | (0.56–1.47) | (0.79–1.73) | (0.53–1.12) | ||
| Breast | 1.07 | 1.41 | 1.41 | 1.45 | 1.31 | Reference |
| (0.49–2.37) | (1.14–1.73) | (1.14–1.74) | (1.19–1.77) | (1.09–1.58) | ||
| Cervical | 1.82 | 1.75 | 1.54 | 1.64 | 1.04 | Reference |
| (1.18–2.81) | (1.12–2.71) | (1.00–2.37) | (1.09–2.45) | (0.69–1.57) | ||
| Prostate | 1.32 | 1.24 | 1.29 | 1.26 | 1.83 | Reference |
| (1.09–1.60) | (1.01–1.52) | (1.07–1.54) | (1.08–1.48) | (1.38–2.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-M.; Jung, K.-W.; Park, J.; Kim, N.; Shin, D.W.; Suh, M. Disparities in Cancer Incidence across Income Levels in South Korea. Cancers 2023, 15, 5898. https://doi.org/10.3390/cancers15245898
Jeong S-M, Jung K-W, Park J, Kim N, Shin DW, Suh M. Disparities in Cancer Incidence across Income Levels in South Korea. Cancers. 2023; 15(24):5898. https://doi.org/10.3390/cancers15245898
Chicago/Turabian StyleJeong, Su-Min, Kyu-Won Jung, Juwon Park, Nayeon Kim, Dong Wook Shin, and Mina Suh. 2023. "Disparities in Cancer Incidence across Income Levels in South Korea" Cancers 15, no. 24: 5898. https://doi.org/10.3390/cancers15245898
APA StyleJeong, S.-M., Jung, K.-W., Park, J., Kim, N., Shin, D. W., & Suh, M. (2023). Disparities in Cancer Incidence across Income Levels in South Korea. Cancers, 15(24), 5898. https://doi.org/10.3390/cancers15245898







