Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search
2.2. Exclusion Criteria
2.3. Data Synthesis and Descriptive Analysis
3. Evidence from the Literature
3.1. Predisposing Factors
3.2. Symptomatology and Clinical Findings
3.3. Laboratory Findings
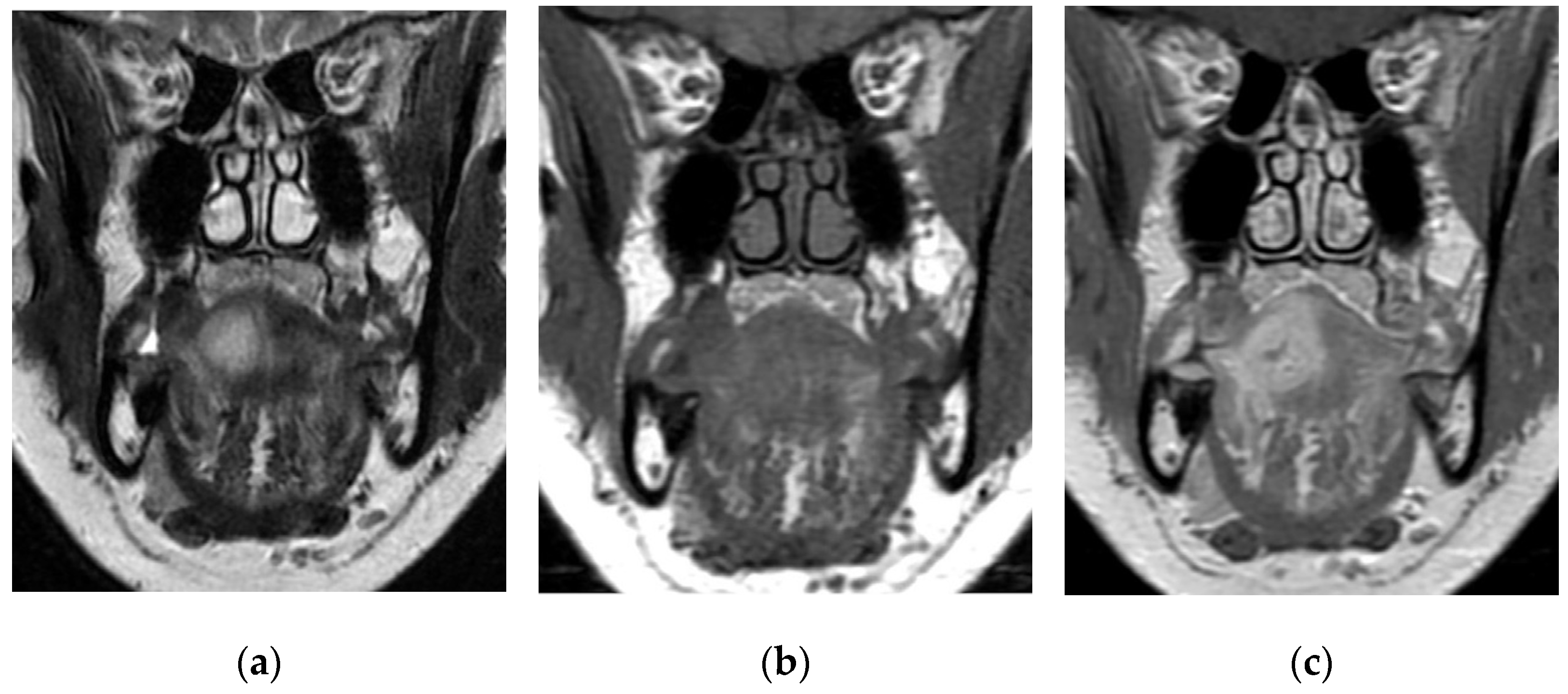
3.4. Radiological Findings
3.5. Management
| First Author | Pt | Symptoms (Days from Onset) | Clinical Findings | Laboratory Findings ^ | Imaging/Localization of the Abscess | Comorbidities | Local Predisposing Factors | Atypical Features *^ | Management ° |
|---|---|---|---|---|---|---|---|---|---|
| Lefler [3] | 1 | T, O, Sp | To elevation w/subglossal sw. | WBC 18.200 | CE CT/BOT | Renal Carcinoma | Periodontitis + tabagism | A | ATB(Clinda + Vanco) |
| Kettaneh [2] | 1 | Facial pain (5) | Submandibular sw.; distal To edema | WBC 11.000 | CE CT/anterior To | RA | NA | No T; No WBC | ATB(Ampicillin/Sulbactam + Vanco) |
| Vellin [12] | 1 | Aphagia (3) | To sw. | WBC 13.000; Gram + cocci and Anaerobes | CE CT/BOT | NA | Previous pharyngitis + tobacco | Light WBC | FNA(GA) +ATB (Cef + Metro) |
| Solomon [30] | 1 | T, O, Dy (7) | To firm mass w/limited mobility | NA | CE CT | NA | NA | Long Sy | Surgery + ATB |
| Veloo [14] | 1 | Pain, Sp (7) | To tender sw. | WBC 12.000; 12 bacteria species isolated | CE CT/central To | Asthma in tx w/budenoside | NA | No WBC; Long Sy | FNA + Surgery (drainage) + ATB (Amoxi/Clav) |
| Antoniades [8] | 3 | 1. Dys, Tr, Dy; 2. O, Dy, Sp, Tr; 3. T(38.9), Dy, Sp | 1. To sw. w/indurated margins; 2. To sw. w/debris; 3. Erythematous sw. | 1. WBC 4.600, S. Faecalis; 2. WBC 14.300, Strep + anaerobes; 3. WBC 15.000, Bacteroides species | 1;2;3:NA | 1. NA; 2. Chemotherapy for chronic leukaemia; 3. DMII | 1. Tooth extraction, alcohol abuse; 2. Poor oral Hy; 3. Oral trauma | No WBC | 1. FNA + ATB (Cefuroxime + Amikacin + Metro); 2. FNA + ATB (Ceforanide + Amikacin + Metro); 3. S urgery + ATB(Ticarcillin/Clav; + Amikacin) |
| Potigailo [17] | 1 | O, Dy, Sp | Cervical Lymp; BOT and epiglottic sw. | NA | CE CT/BOT | NA | NA | A | FNA + ATB |
| Little [27] | 1 | O (5) | Immobile To, Bilat. sublingual sw. | WBC 17.600, S. Anginosus+ Fusobacterium nucleate | CE CT/BOT | NA | NA | No response to double ATB | FNA + Surgery(transcervical) + ATB(Ampicillin/Sulbactam + Vanco) |
| Harrington [23] | 1 | O, Dy (5) | To and FOM sw. | WBC 21.500; S. Intermedius | CE CT/anterior To | NA | Recent tooth abscess | A | FNA + Surgery + ATB(Clindamycin) |
| Kikidis [9] | 1 | Sp (7) | To sw. | NA | CE CT | NA | Poor oral Hy. | Long Sy | FNA + Surgery + ATB(Amoxi/clav + Metro) |
| Ozgur [13] | 1 | O, Dy (30) | BOT sw. | WBC 6.940, CRP 51, coagulase-negative Staph. | CE CT/BOT | NA | Recent tx for pharyngitis | Long Sy; Weight loss; No WBC | FNA + ATB(Cef) |
| Akin [15] | 3 | 1. Dy, O (2); 2. O, Dy, Dys (4); 3.O, Dy | 1. Painful To sw; 2. Painful To sw; 3. Multilobed abscess | 1. WBC 16.600, CRP 9.94, Group-D beta-haemolytic Strep; 2. WBC 10.900, CRP 26.18; 3.WBC 5.600, CRP 207, mixed oral flora | 1. CE CT/middle To; 2. MRI/middle To; 3. CE CT | 1. NA; 2. HT; 3. Acute lymphoblastic leukaemia under Chemo | 1. Previous dental intervention; 2. NA; 3. Poor oral Hy. | 1;2 No T 2;3 No WBC | 1. Surgery(GA) + ATB(Cef + Metro); 2. Surgery(GA) + tracheostomy + ATB(Piperacillin/Tazobactam + Teicoplanin) |
| Haydar [24] | 1 | O, Dy, Sp (5) | To sw. w/hyperemia | WBC 15.700, CRP 50 | CE MRI/anterior To | NA | NA | No T | Surgery + ATB (Cef + Clinda) |
| Mesolella [4] | 1 | Sp, Dy, O (15) | To sw., on nasofibroscopy no visualization of vocal cords | WBC elevated, Staph. species and anaerobes | CE CT + MRI/BOT | NA | Previous tonsillitis | Long Sy | Surgery(GA) + ATB(Cef) |
| Saro-Buendía [18] | 2 | 1. T, O, Sp; 2. O, Sp (3) | 1. BOT tumefaction w/erythema; 2. Vallecula tumefaction w/erythema | 1. WBC elevated, CPR 92.8; mixed flora w/Strep spp. and Fusobacterium spp.; 2. WBC elevated; CRP 30.4; mixed flora | 1. CE CT/BOT; 2. CE CT/vallecula and BOT | NA | NA | A | 1. Surgery + ATB(Cef + Clinda); 2. Surgery + ATB |
| Riccardi [20] | 1 | Dy, Sp (1) | To sw. w/papule on the dorsum | CRP 6.9 | US/right hemiTo | NA | NA | No T; slight CRP | Surgery |
| Binar [10] | 1 | O, Dys, Sp (3) | Neck sw., BOT sw., on nasofibroscopy no visualization of vocal cords | Hemophilus influenza type b | CE CT/BOT | NA | Poor oral Hy | No WBC | Surgery (transcervical) + ATB(Ampicillin/Sulbactam) |
| Bekele [19] | 1 | O, Dy, Sp (40) | To sw., pus oozing from the sw | Normal range; Gram + cocci | NA | NA | Poor oral Hy; previous tooth extraction | Long Sy; No WBC | Surgery + ATB(Amoxi/Clav. + Metro) |
| Varghese [22] | 1 | Dy, Dys (5) | Anterior 2/3 To sw. occluding oropharynx | WBC 11.500 | CE CT/anterior 2/3 To abscess | NA | Recent tooth extraction | No WBC | Surgery(GA) + tracheostomy + ATB |
| Burnham [16] | 1 | O (7) | To sw | WBC 11.340; PCR 24; Gram + and Gram-cocci | CE CT/midline and BOT | DMII | NA | Long Sy; No WBC | FNA + Surgery(GA) + (Amoxi-Clav) |
| Eviatar [21] | 1 | T, O, Dy (4) | BOT sw., white exudate at palatine tonsils | WBC 10.800, Prevotella | CE CT/BOT | NA | NA | No WBC | FNA + ATB (Amoxi-Clav) |
| Mesfin [25] | 1 | T, Sp, O | Tender To, erythema and aphthous ulcers | NA | NA | NA | Khat chewer, poor oral Hy. | A | Surgery(GA) + ATB Cef + Metro) |
| Pallagatti [7] | 1 | O, Dy (4) | Anterior 2/3 To sw. | Normal range, Fusobacterium nucleatum, Prevotella and Strep spp. | US/anterior 2/3 To | NA | NA | Normal range | FNA + ATB(Amoxi + cloxacillin) |
| Stofferahn [31] | 1 | O, Dy, Sp (5) | To sw., erythema | WBC 17.400, Group B strep, oral flora | CE CT/Posterior 1/3 To | HT | NA | A | FNA + ATB(Ampicillin/Sulbactam + Linezolid) |
| Srivanitchapoom [29] | 6 | 1. T, O, Dy, Dys; 2. O; 3. O, Dy; 4.O, Dy, Dys; 5. Otalgia, O; 6.O | 1. BOT Marked sw.; 2. Antero-lateral To sw; 3. FOM and BOT Sw.; 4. FOM Sw.; 5.BOT Sw.; 6. Antero.midline To mass | 1. WBC 9300; S. Viridans; 2. WBC 4500; 3. WBC 14.500, Acitenobacter lwoffii; 4. WBC 12.100, Strep spp.; 5.WBC 5500; S. Viridans; 6. WBC 5800 | 1;3;4;5: CE CT 2;6: None | 1;3;5;6: None; 2;4:DM + HT; | 1;2: Poor oral Hy; 3;4;6:NA; 5. Poor oral hy + Thyroglossal duct cyst | 2;3;4;5;6 No T 1;2;4;5;6 No WBC | 1. Surgery + tracheostomy + ATB(Amoxi/Clav + Cef); 2. Surgery(LA) + ATB(Amoxi/Clav+ Cef); 3.Surgery(AG) + ATB therapy (Clinda; Cef); 4.Surgery(GA) + tracheostomy + ATB(clindamycin; Cef); 5. Surgery(GA) + ATB(Amoxi/Clav + Cef); 6. Surgery(GA) + ATB(Amoxi/Clav) |
| Balatsouras [11] | 4 | 1. Otalgia, O; 2. O, Sp; 3. Sp, O; 4. O, otalgia | 1. Sw. BOT 2. sw middle To and BOT, on nasopharyngoscopy edema of the epiglottis and left aryepiglottic fold 3. sw. of the middle portion of To 4. abscess at BOT and edema of epiglottis | 1. Strep. | 1;2;3: CE CT 4. None | 1; 4 DMII 2; 3 None | 1; 2; 3; Poor oral hy. 4. None | A | 1. FN +ATB(Penicillin + Gentamycin + Metro); 2. FNA + ATB(Penicillin+ Gentamycin + Metro); 3. FNA + ATB(Amoxi/Clav); 4. FNA + ATB(Amoxi/Clav) |
| Schweigert [1] | 1 | Sp; Dys; Dy | To sw. | NA | NA | CAD | NA | A | Emergent tracheotomy (patient deceased) |
| Ozturk [6] | 7 | 1. T, O; 2. T, O; 3. O; 4. O, Dys; 5. O; 6. Dys; 7. O | 1; 2; 3; 4; 5; 6; 7 To mass | 1; 2 Leukocytosis; anaerobic bacteria; 3. Normal range, S. Viridans; 4; 5; 6; 7 Normal range | 1; 2; 3; 4; 5; 6; 7 CE MRI | 1. alcohol abuse | 1; 2; 3 Poor oral Hy | 3;4;5;6;7 Normal range | 1; 2; 3; 4: Surgery(GA) 5; 6; 7: FNA |
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Schweigert, J.; Christian, R.; Kemp, W.L. Challenges in the Diagnosis of a Posterior Lingual Abscess, a Potential Lethal Disorder. Am. J. Forensic Med. Pathol. 2020, 41, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Kettaneh, N.; Williamson, K. Spontaneous Lingual Abscess in an Immunocompromised Patient. Am. J. Emerg. Med. 2014, 32, 492.e1–492.e2. [Google Scholar] [CrossRef]
- Lefler, J.E.; Masullo, L.N. Lingual Abscess in the Setting of Recent Periodontal Antibiotic Injections. J. Emerg. Med. 2016, 51, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Mesolella, M.; Allosso, S.; Iorio, B.; Motta, G. Clinical and Diagnostic Aspect of Tongue Abscess. Ear Nose Throat. J. 2021, 100, 1012S–1014S. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, B.J.; Kim, S.J.; Shim, W.-Y.; Baik, S.K.; Sunwoo, M. Tongue Abscess Mimicking Neoplasia. AJNR Am. J. Neuroradiol. 2006, 27, 2202–2203. [Google Scholar] [PubMed]
- Ozturk, M.; Mavili, E.; Erdogan, N.; Cagli, S.; Guney, E. Tongue Abscesses: MR Imaging Findings. AJNR Am. J. Neuroradiol. 2006, 27, 1300–1303. [Google Scholar] [PubMed]
- Pallagatti, S.; Sheikh, S.; Kaur, A.; Puri, N.; Singh, R.; Arya, S. Tongue Abscess: A Rare Clinical Entity. J. Investig. Clin. Dent. 2012, 3, 240–243. [Google Scholar] [CrossRef]
- Antoniades, K.; Hadjipetrou, L.; Antoniades, V.; Antoniades, D. Acute Tongue Abscess. Report of Three Cases. Oral. Surg. Oral. Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 570–573. [Google Scholar] [CrossRef]
- Kikidis, D.; Marinakis, K.; Sengas, J.; Chrysovergis, A. Lingual Abscess in a Psychiatric Patient: A Case Report. Case Rep. Med. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Binar, M.; Arslan, F.; Aydin, U. Another Cause of Difficult Airway in an Elderly Patient: Tongue-base Abscess. Gerodontology 2018, 35, 155–158. [Google Scholar] [CrossRef]
- Balatsouras, D.G.; Eliopoulos, P.N.; Kaberos, A.C. Lingual Abscess: Diagnosis and Treatment. Head Neck 2004, 26, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Vellin, J.-F.; Crestani, S.; Saroul, N.; Bivahagumye, L.; Gabrillargues, J.; Gilain, L. Acute Abscess of the Base of the Tongue: A Rare But Important Emergency. J. Emerg. Med. 2011, 41, e107–e110. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, G.T.; Akdogan, M.V.; Unler, G.K.; Gokturk, H.S. A Rare Cause of Acute Dysphagia: Abscess of the Base of the Tongue. Case Rep. Gastrointest. Med. 2015, 2015, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Veloo, A.C.M.; Schepers, R.H.; Welling, G.W.; Degener, J.E. Assessment of the Microbiota of a Mixed Infection of the Tongue Using Phenotypic and Genotypic Methods Simultaneously and a Review of the Literature. Anaerobe 2011, 17, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Akin, V.; Sivrice, M.E.; Kumbul, Y.Ç. A Rare Surgical Emergency: Lingual Abscess. Kulak Burun Boğaz Ve Baş Boyun Cerrahisi Derg. 2020, 28, 317–320. [Google Scholar] [CrossRef]
- Burnham, R.M.C.; Hanu-Cernat, L. Glossal Abscesses—A Rare Presentation in the Oral Surgery World. Oral Surg. 2013, 6, 22–24. [Google Scholar] [CrossRef]
- Potigailo, V.; Weinsheim, T. Base of the Tongue Abscess: An Uncommon Entity. Vis. J. Emerg. Med. 2018, 10, 7–8. [Google Scholar] [CrossRef]
- Saro-Buendía, M.; Suarez Urquiza, P.; Amigo González, J.; Lesmas Navarro, M.J.; Mazón, M.; Armengot Carceller, M. Posterior Lingual Abscess; Report of Two Cases. Arch. Acad. Emerg. Med. 2023, 11, e18. [Google Scholar] [CrossRef]
- Bekele, K.; Markos, D. Lingual Abscess: A Case Report. Int. Med. Case Rep. J. 2017, 10, 285–287. [Google Scholar] [CrossRef]
- Riccardi, A.; Dignetti, P.; Tasso, F.; Caiti, M.; Lerza, R. Tongue Abscess: A Rare Occurrence Possibly Mimicking Angioedema. Emerg. Care J. 2013, 9, 23. [Google Scholar] [CrossRef]
- Eviatar, E.; Pitaro, K.; Segal, S.; Kessler, A. Lingual Abscess: Secondary to Follicular Tonsillitis. Otolaryngol. Head Neck Surg. 2004, 131, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Varghese, L.; Agarwal, P.; Rupa, V. Unusual Complication of Dental Extraction: Lingual Abscess. Indian J. Dent. Res. 2013, 24, 772. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.T.; Hsia, J.C.; Mendez, E.; Clarridge, J.E. A Lingual Abscess Caused by Streptococcus Intermedius. J. Med. Microbiol. 2012, 61, 590–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koç, R.H.; Solak, N.; Gülüstan, F.; Abakay, M.A. Lingual Abscess in a Young, Healthy Woman Patient. Kulak Burun Boğaz Ve Baş Boyun Cerrahisi Derg. 2021, 29, 241–244. [Google Scholar] [CrossRef]
- Mesfin, T.; Debele, G.; Seyoum, K.; Dadi, S.; Tsegaye, M.; Gomora, D.; Kene, C.; Tolosa, G. Tongue Abscess: A Case Report. Int. Med. Case Rep. J. 2022, 15, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.D.; Verma, A.K.; Kanaujia, R. A Rare Case of Hemilingual Abscess in a 17-Year-Old Girl: The Ease of Ultrasound and the Advantage of MRI. Jpn. J. Radiol. 2013, 31, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Little, C.C.; Filimonov, A.; Schwam, Z.G. Lingual Abscess: A Case Report of a Rare Clinical Entity. Otolaryngol. Case Rep. 2022, 23, 100411. [Google Scholar] [CrossRef]
- Tewari, N.; Prakash Mathur, V.; Rajwar, A.; Mridha, A.; Mishra, D. 940-Nm Diode Laser-Assisted Management of Tongue Abscess. J. Dent. Child. 2018, 85, 147–150. [Google Scholar]
- Srivanitchapoom, C.; Yata, K. Lingual Abscess: Predisposing Factors, Pathophysiology, Clinical Manifestations, Diagnosis, and Management. Int. J. Otolaryngol. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Solomon, D.M.; Hahn, B. Lingual Abscess. J. Emerg. Med. 2012, 43, e53–e54. [Google Scholar] [CrossRef]
- Stofferahn, M.E.; Donnelly, J.A. Lingual Abscess With Associated Abiotrophia/Granulicatella Sp. Bacteremia in a Healthy Patient. Infect. Dis. Clin. Pract. 2011, 19, 48–50. [Google Scholar] [CrossRef]
- Rampi, A.; Lanzillotta, M.; Mancuso, G.; Vinciguerra, A.; Dagna, L. IgG4-Related Disease of the Oral Cavity. Case Series from a Large Single-Center Cohort of Italian Patients. Int. J. Environ. Res. Public Health 2020, 17, 8179. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, M.H.; Park, S.W.; Chang, K.H. Radiologic-Pathologic Correlation of Unusual Lingual Masses: Part I: Congenital Lesions. Korean J. Radiol. 2001, 2, 37–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bomfin, L.E.; de Abreu Alves, F.; de Almeida, O.P.; Kowalski, L.P.; Perez, D.E. da C. Multiple Granular Cell Tumors of the Tongue and Parotid Gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e10–e13. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.B.; Sant’Ana, M.S.P.; Silva, A.M.B.; Agostini, M.; Silva Canedo, N.H.; de Andrade, B.A.B.; Romañach, M.J.; Corrêa, D.L.; Tomasi, R.A.; Radhakrishnan, R.; et al. Solitary Fibrous Tumour of the Oral Cavity: An Update. J. Oral Pathol. Med. 2020, 49, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.; Gomes, G.A.; Tomita, S.; Fonseca Júnior, A.; El Hadj Miranda, L.A.; Arruda, A.M. Painful Tongue Leiomyoma. Braz J. Otorhinolaryngol. 2006, 72, 715. [Google Scholar] [CrossRef] [PubMed]
- Rozza-de-Menezes, R.E.; Andrade, R.M.; Israel, M.S.; Gonçalves Cunha, K.S. Intraoral Nerve Sheath Myxoma: Case Report and Systematic Review of the Literature. Head Neck 2013, 35, E397–E404. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, M.H.; Park, S.W.; Chang, K.H. Radiologic-Pathologic Correlation of Unusual Lingual Masses: Part II: Benign and Malignant Tumors. Korean J. Radiol. 2001, 2, 42–51. [Google Scholar] [CrossRef]
- Lombardi, N.; Varoni, E.M.; Moneghini, L.; Lodi, G. Submucosal Oral Squamous Cell Carcinoma of the Tongue. Oral Oncol 2021, 115, 105121. [Google Scholar] [CrossRef]
- Jorge, J.; Pires, F.R.; Alves, F.A.; Perez, D.E.C.; Kowalski, L.P.; Lopes, M.A.; Almeida, O.P. Juvenile Intraoral Pleomorphic Adenoma: Report of Five Cases and Review of the Literature. Int. J. Oral Maxillofac. Surg. 2002, 31, 273–275. [Google Scholar] [CrossRef]
- Fronie, A.; Simionescu, C.; Vască, V.; Dumitrescu, D.; Fronie, A.S. Histopathological Aspects of Benign Mesenchymal Tumors Located in “High Risk Areas” of the Tongue. Rom. J. Morphol. Embryol. 2010, 51, 97–104. [Google Scholar] [PubMed]
- Mavili, E.; Ozturk, M.; Yucel, T.; Yuce, I.; Cagli, S. Tongue Metastasis Mimicking Abscess: A Case Report. Diagn. Interv. Radiol. 2008, 16, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Iiris, S.; Sara, D.; Hanna, A.; Tuula, S.; Matti, M. Tongue Metastasis of Malignant Melanoma: A Case Report and a Systemic Review of the Literature. Oral Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Medford, S.; Islam, S.; Ramsingh, C.; Christopher, M. Extranodal Lymphoma of the Tongue, a Very Rare Entity-Report of Two Cases with Literature Review. Int. J. Surg. Case Rep. 2019, 54, 70–74. [Google Scholar] [CrossRef]
- De Luca, P.; Tassone, D.; de Campora, L.; Simone, M.; Marra, P.; Colacurcio, V.; Scarpa, A.; Costarelli, L.; Camaioni, A. Cribriform Adenocarcinoma of the Tongue and Minor Salivary Glands: A Systematic Review of an Uncommon Clinicopathological Entity. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 2719–2725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampi, A.; Tettamanti, A.; Bertotto, I.; Comini, L.V.; Howardson, B.O.; Luparello, P.; Di Santo, D.; Bondi, S. Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges. Cancers 2023, 15, 5871. https://doi.org/10.3390/cancers15245871
Rampi A, Tettamanti A, Bertotto I, Comini LV, Howardson BO, Luparello P, Di Santo D, Bondi S. Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges. Cancers. 2023; 15(24):5871. https://doi.org/10.3390/cancers15245871
Chicago/Turabian StyleRampi, Andrea, Alberto Tettamanti, Ilaria Bertotto, Lara Valentina Comini, Bright Oworae Howardson, Paolo Luparello, Davide Di Santo, and Stefano Bondi. 2023. "Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges" Cancers 15, no. 24: 5871. https://doi.org/10.3390/cancers15245871
APA StyleRampi, A., Tettamanti, A., Bertotto, I., Comini, L. V., Howardson, B. O., Luparello, P., Di Santo, D., & Bondi, S. (2023). Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges. Cancers, 15(24), 5871. https://doi.org/10.3390/cancers15245871







