Recurrence Patterns and Risk Factors after Curative Resection for Colorectal Cancer: Insights for Postoperative Surveillance Strategies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
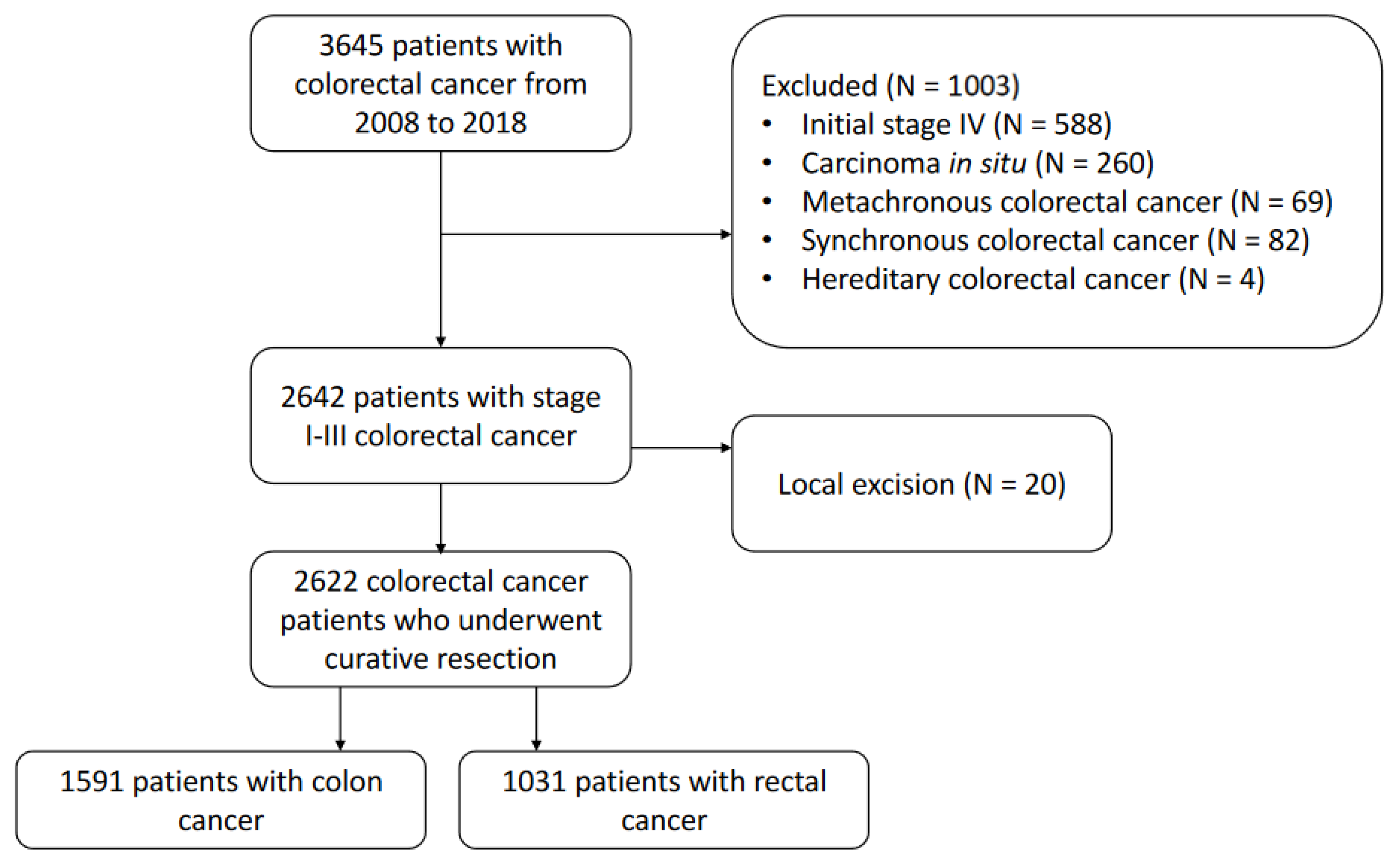
2.1. Study Population
2.2. Treatment and Postoperative Surveillance
2.3. Statistical Anayses
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes
3.3. Recurrence Patterns and Risk Factors Associated with Recurrence
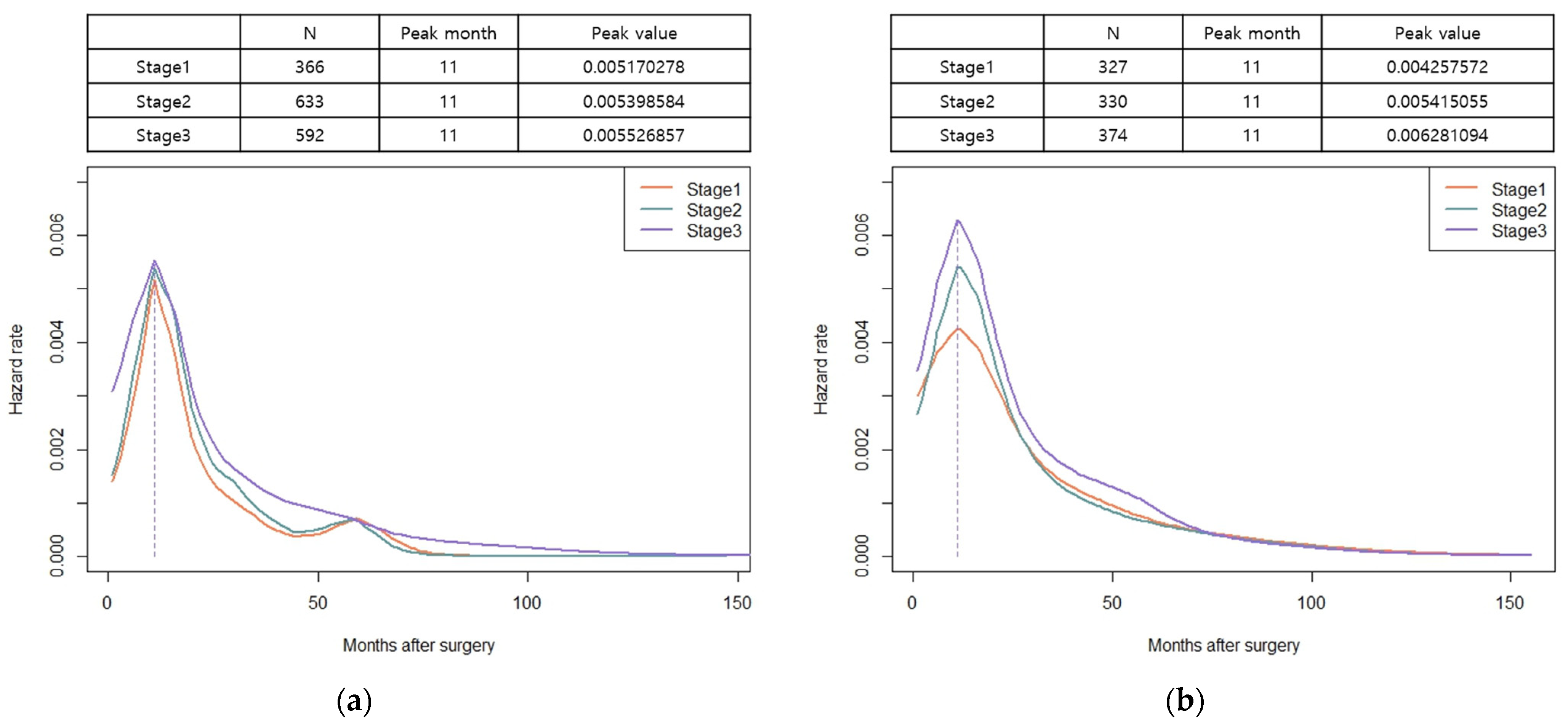
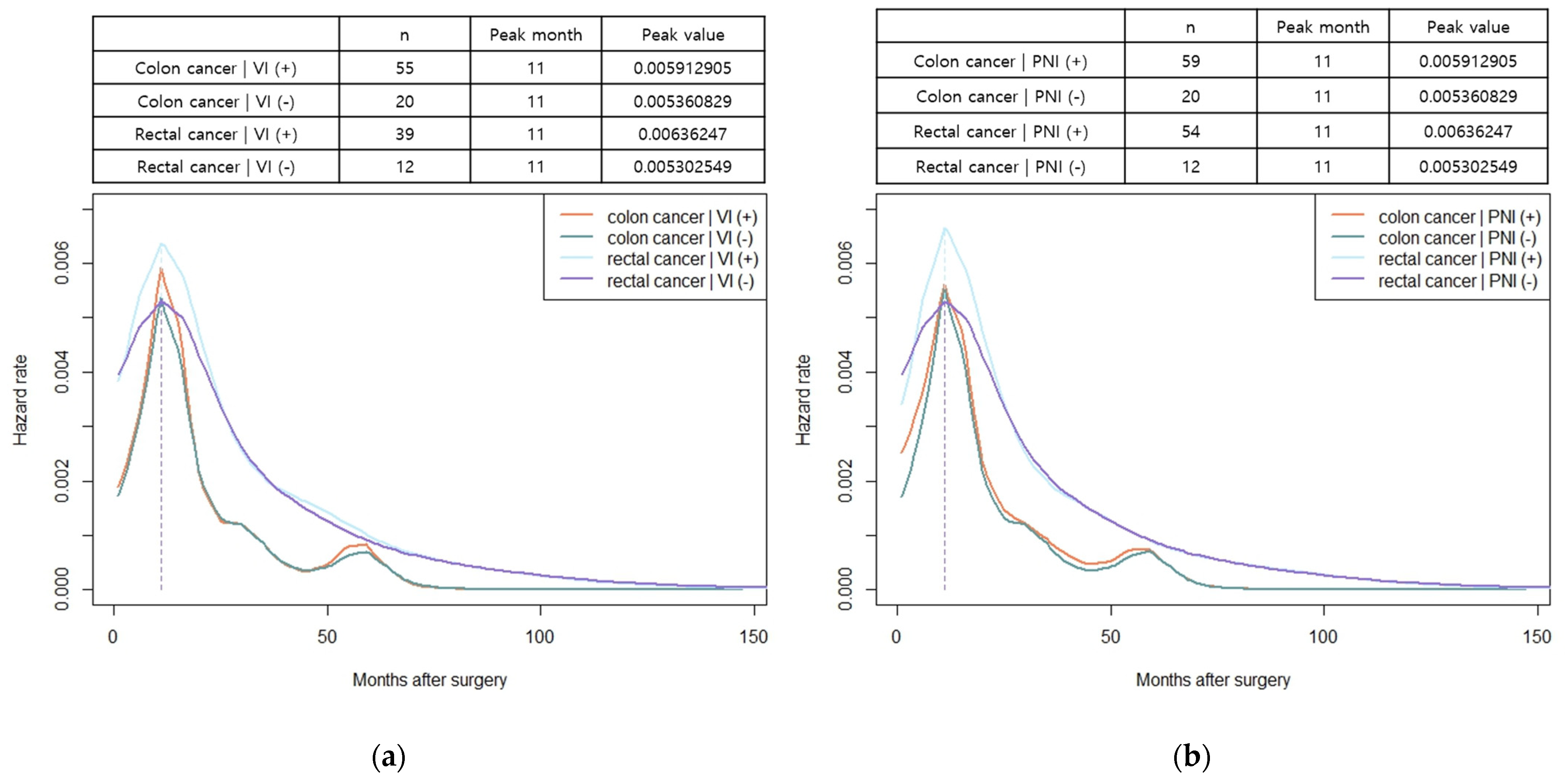
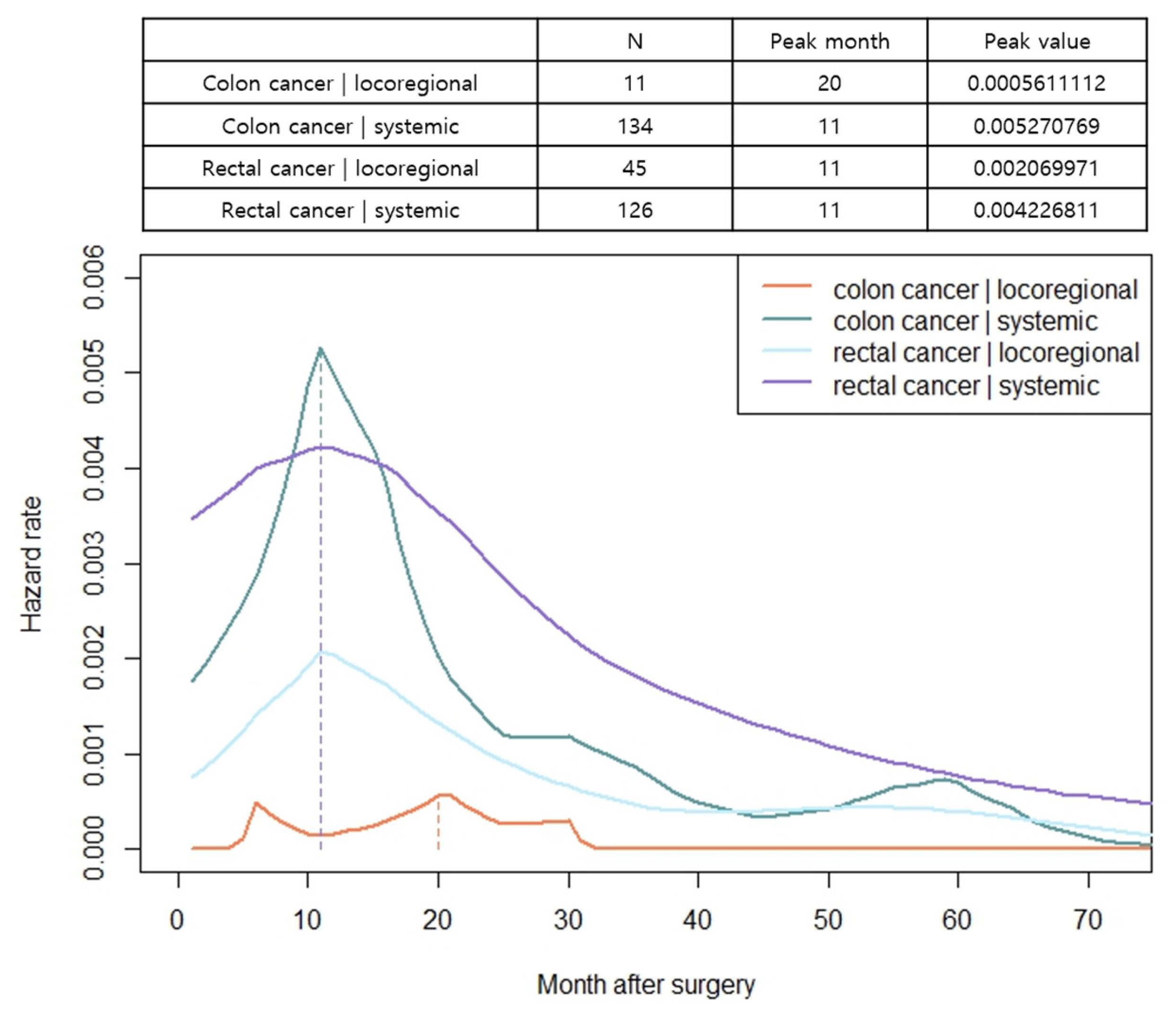
3.4. Hazard Functions for Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, E.J. Tailoring strategies for colorectal cancer screening and treatment based on age in colorectal cancer patients. Ann. Coloproctol. 2022, 38, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.Y. Advances in surgery for locally advanced rectal cancer. Ann. Coloproctol. 2022, 38, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Hemminki, A. Survival in colon and rectal cancers in Finland and Sweden through 50 years. BMJ Open Gastroenterol. 2021, 8, e000644. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, S.; Yi, N.; Kang, B.; Park, I.J. Colorectal cancer mortality trends in the era of cancer survivorship in Korea: 2000–2020. Ann. Coloproctol. 2022, 38, 343–352. [Google Scholar] [CrossRef]
- Pita-Fernández, S.; Alhayek-Aí, M.; González-Martín, C.; López-Calviño, B.; Seoane-Pillado, T.; Pértega-Díaz, S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 644–656. [Google Scholar] [CrossRef]
- Seo, S.I.; Lim, S.B.; Yoon, Y.S.; Kim, C.W.; Yu, C.S.; Kim, T.W.; Kim, J.H.; Kim, J.C. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J. Surg. Oncol. 2013, 108, 9–13. [Google Scholar] [CrossRef]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; Andre, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. 2009, 27, 872–877. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (vers 3.2023). 2023. Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 1 September 2023).
- National Comprehensive Cancer Network. Rectal Cancer (vers 5.2023). 2023. Available online: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 1 September 2023).
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Costas-Chavarri, A.; Nandakumar, G.; Temin, S.; Lopes, G.; Cervantes, A.; Cruz Correa, M.C.; Engineer, R.; Hamashima, C.; Ho, G.F.; Huitzil, F.D.; et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Colorectal Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Warren, E. Total Mesorectal Excision—The New Golden Standard of Surgery for Rectal Cancer. Ann. Med. 1997, 29, 127–133. [Google Scholar] [CrossRef]
- Muller, H.G.; Wang, J.-L. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics 1994, 50, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Kudose, Y.; Shida, D.; Ahiko, Y.; Nakamura, Y.; Sakamoto, R.; Moritani, K.; Tsukamoto, S.; Kanemitsu, Y. Evaluation of recurrence risk after curative resection for patients with stage I to III colorectal cancer using the hazard function: Retrospective analysis of a single-institution large cohort. Ann. Surg. 2022, 275, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Kawai, K.; Nozawa, H.; Sasaki, K.; Murono, K.; Emoto, S.; Ishii, H.; Anzai, H.; Sonoda, H.; Yamauchi, S.; et al. Hazard function analysis of metastatic recurrence after colorectal cancer surgery-A nationwide retrospective study. J. Surg. Oncol. 2021, 123, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Koedam, T.W.A.; Bootsma, B.T.; Deijen, C.L.; van de Brug, T.; Kazemier, G.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Haglind, E.; Tuynman, J.B.; et al. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: Increased risk of local recurrence. Ann. Surg. 2022, 275, e420–e427. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Q.; Jindou, L.; Cheng, Y. The influence of anastomotic leakage for rectal cancer oncologic outcome: A systematic review and meta-analysis. J. Surg. Oncol. 2020, 121, 1283–1297. [Google Scholar] [CrossRef]
- Costi, R.; Santi, C.; Bottarelli, L.; Azzoni, C.; Zarzavadjian Le Bian, A.; Riccó, M.; Sarli, L.; Silini, E.M.; Violi, V. Anastomotic recurrence of colon cancer: Genetic analysis challenges the widely held theories of cancerous cells’ intraluminal implantation and metachronous carcinogenesis. J. Surg. Oncol. 2016, 114, 228–236. [Google Scholar] [CrossRef]
- Ahlquist, T.; Lind, G.E.; Costa, V.L.; Meling, G.I.; Vatn, M.; Hoff, G.S.; Rognum, T.O.; Skotheim, R.I.; Thiis-Evensen, E.; Lothe, R.A. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol. Cancer 2008, 7, 94. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Detering, R.; Rutgers, M.L.W.; Bemelman, W.A.; Hompes, R.; Tanis, P.J. Prognostic importance of circumferential resection margin in the era of evolving surgical and multidisciplinary treatment of rectal cancer: A systematic review and meta-analysis. Surgery 2021, 170, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Krasna, M.J.; Flancbaum, L.; Cody, R.P.; Shneibaum, S.; Ben Ari, G.B. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer 1988, 61, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.L.; Cheng, K.I.; Lu, C.Y.; Kuo, C.H.; Ma, C.J.; Wu, J.Y.; Chai, C.Y.; Hsieh, J.S.; Wang, J.Y. Prognostic significance of depth of invasion, vascular invasion and numbers of lymph node retrievals in combination for patients with stage II colorectal cancer undergoing radical resection. J. Surg. Oncol. 2008, 97, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural invasion is a strong prognostic factor in colorectal cancer. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, S.; Yan, L.; Gu, J.; Li, J.; Wu, K.; Cai, K. Perineural invasion is associated with poor prognosis of colorectal cancer: A retrospective cohort study. Int. J. Colorectal Dis. 2020, 35, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi, T.R.; Magge, D.; Choudry, H.; Ramalingam, L.; Zhu, B.; Pingpank, J.; Ahrendt, S.; Holtzman, M.; Zeh, H.; Bartlett, D.; et al. Mucinous and signet ring cell differentiation affect patterns of metastasis in colorectal carcinoma and influence survival. Int. J. Surg. Pathol. 2017, 25, 108–117. [Google Scholar] [CrossRef]
- Tamas, K.; Walenkamp, A.M.; De Vries, E.G.; Van Vugt, M.A.; Beets-Tan, R.G.; Van Etten, B.; De Groot, D.J.; Hospers, G.A. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Doah, K.; Shin, U.S.; Jeon, B.H.; Cho, S.S.; Moon, S.M. The Impact of Primary Tumor Resection on Survival in Asymptomatic Colorectal Cancer Patients With Unresectable Metastases. Ann. Coloproctol. 2021, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Yamazaki, T.; Iwaya, A.; Kameyama, H.; Komatsu, M.; Hirai, M. Comparison of the oncological outcomes of stenting as a bridge to surgery and surgery alone in stages II to III obstructive colorectal cancer: A retrospective study. Ann. Coloproctol. 2022, 38, 235. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.F.; Salama, T.M.S.; Afifi, A.H.; Dabous, H.M.K. Effectiveness and early postoperative outcomes of palliative endoluminal stenting versus Hartmann’s procedure in acute malignant bowel obstruction in high-risk patients. Ann. Coloproctol. 2022, 38, 141. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Saada, J.; Kapur, S.; Tighe, R.; Stearns, A.; Hernon, J.; Speakman, C. Technical and clinical outcomes after colorectal stenting in malignant large bowel obstruction: A single-center experience. Ann. Coloproctol. 2021, 37, 85. [Google Scholar] [CrossRef]
- Yang, P.; Lin, X.F.; Lin, K.; Li, W. The role of stents as bridge to surgery for acute left-sided obstructive colorectal cancer: Meta-analysis of randomized controlled trials. Rev. Investig. Clin. 2018, 70, 269–278. [Google Scholar] [CrossRef]
- Yamashita, S.; Tanemura, M.; Sawada, G.; Moon, J.; Shimizu, Y.; Yamaguchi, T.; Kuwai, T.; Urata, Y.; Kuraoka, K.; Hatanaka, N.; et al. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol. Lett. 2018, 15, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Detering, R.; Karthaus, E.G.; Borstlap, W.A.A.; Marijnen, C.A.M.; van de Velde, C.J.H.; Bemelman, W.A.; Beets, G.L.; Tanis, P.J.; Aalbers, A.G.J.; Dutch Snapshot Research Group. Treatment and survival of locally recurrent rectal cancer: A cross-sectional population study 15 years after the Dutch TME trial. Eur. J. Surg. Oncol. 2019, 45, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Galjart, B.; Verhoef, C.; Slooter, G.D.; Koopman, M.; Verhoeven, R.H.A.; de Wilt, J.H.W.; van Erning, F.N. Disease recurrence after colorectal cancer surgery in the modern era: A population-based study. Int. J. Colorectal. Dis. 2021, 36, 2399–2410. [Google Scholar] [CrossRef]
- Lindskog, E.B.; Gunnarsdóttir, K.Á.; Derwinger, K.; Wettergren, Y.; Glimelius, B.; Kodeda, K. A population-based cohort study on adherence to practice guidelines for adjuvant chemotherapy in colorectal cancer. BMC Cancer 2014, 14, 948. [Google Scholar] [CrossRef]
- Mant, D.; Gray, A.; Pugh, S.; Campbell, H.; George, S.; Fuller, A.; Shinkins, B.; Corkhill, A.; Mellor, J.; Dixon, E. A randomised controlled trial to assess the cost-effectiveness of intensive versus no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health Technol. Assess. 2017, 21, 1–86. [Google Scholar] [CrossRef]
- Wille-Jørgensen, P.; Syk, I.; Smedh, K.; Laurberg, S.; Nielsen, D.T.; Petersen, S.H.; Renehan, A.G.; Horváth-Puhó, E.; Påhlman, L.; Sørensen, H.T.; et al. Effect of more vs less frequent follow-up testing on overall and colorectal cancer–specific mortality in patients with stage II or III colorectal cancer: The COLOFOL randomized clinical trial. JAMA 2018, 319, 2095–2103. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hokuto, D.; Koyama, F.; Matsuo, Y.; Nomi, T.; Yoshikawa, T.; Kamitani, N.; Sadamitsu, T.; Takei, T.; Matsumoto, Y.; et al. The Prognosis and Recurrence Pattern of Right- and Left-Sided Colon Cancer in Stage II, Stage III, and Liver Metastasis After Curative Resection. Ann. Coloproctol. 2021, 37, 326–336. [Google Scholar] [CrossRef]
- Murakawa, T. Past, present, and future perspectives of pulmonary metastasectomy for patients with advanced colorectal cancer. Surg. Today 2021, 51, 204–211. [Google Scholar] [CrossRef]
- Park, K.J. Against all odds: Why surgeons need to be more aggressive in the era of the multidisciplinary team approach to colorectal cancer. Ann. Coloproctol. 2022, 38, 393. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, C.H. Treatment for peritoneal metastasis of patients with colorectal cancer. Ann. Coloproctol. 2021, 37, 425. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Shin, J.K.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Heo, J.S.; Choi, G.S.; Kim, S.T.; et al. Clinical outcomes of neoadjuvant chemotherapy in colorectal cancer patients with synchronous resectable liver metastasis: A propensity score matching analysis. Ann. Coloproctol. 2021, 37, 244. [Google Scholar] [CrossRef]

| Colon (N = 1591) | Rectum (N = 1031) | p Value | |
|---|---|---|---|
| Age, years (Mean ± SD) | 64.2 ± 11.8 | 61.6 ± 12.1 | <0.001 |
| Sex, n (%) | <0.001 | ||
| Male | 918 (57.7) | 679 (65.9) | |
| Female | 673 (42.3) | 352 (34.1) | |
| Body mass index, kg/m2 (Mean ± SD) | 23.8 ± 3.3 | 23.6 ± 3.2 | 0.18 |
| ASA score, n (%) | <0.001 | ||
| 1 | 296 (18.6) | 240 (23.3) | |
| 2 | 1155 (72.6) | 733 (71.1) | |
| 3 | 130 (8.2) | 58 (5.6) | |
| 4 | 10 (0.6) | 0 | |
| Comorbidities, n (%) | |||
| Endocrine | 342 (21.5) | 205 (19.9) | 0.32 |
| Cardiovascular | 64 (48.0) | 419 (40.6) | <0.001 |
| Respiratory | 138 (8.7) | 75 (7.3) | 0.20 |
| Smoking, n (%) | 0.001 | ||
| Yes | 467 (29.4) | 368 (35.7) | |
| No | 1124 (70.6) | 663 (64.3) | |
| Alcohol, n (%) | 0.88 | ||
| Yes | 642 (40.4) | 419 (40.6) | |
| No | 949 (59.6) | 612 (59.4) | |
| Tumor location, n (%) | |||
| Ascending colon | 410 (25.8) | ||
| Transverse colon | 261 (16.4) | ||
| Descending colon | 85 (5.3) | ||
| Sigmoid colon | 835 (52.5) | ||
| Upper Rectum | 151 (14.6) | ||
| Mid/Low rectum | 880 (85.4) | ||
| CEA level ≥ 5 ng/mL, n (%) | 321 (20.2) | 189(18.3) | 0.18 |
| Obstruction, n (%) | 199 (12.5) | 32 (3.1) | <0.001 |
| Perforation, n (%) | 48 (3.0) | 7 (0.7) | <0.001 |
| Endoscopic stent insertion, n (%) | 83 (5.2) | 13 (1.3) | <0.001 |
| Neoadjuvant therapy, n (%) | 9 (0.6) | 311 (30.2) | <0.001 |
| Adjuvant chemotherapy, n (%) | 770 (48.4) | 546 (53.0) | 0.021 |
| Colon Cancer (N = 1591) | Rectal Cancer (N = 1031) | p Value | |
|---|---|---|---|
| Emergency operation, n (%) | 43 (2.7) | 1 (0.1) | <0.001 |
| Open/Laparoscopic/Robotic, n (%) | 64 (4.0)/1496 (94.0)/31 (2.0) | 10 (1.0)/524 (50.8)/497 (48.2) | 0.02 |
| Open conversion, n (%) | 98 (6.2) | 17 (1.6) | <0.001 |
| R2 resection, n (%) | 6 (0.4) | 5 (0.5) | 0.69 |
| Estimated blood loss ≥ 800 mL | 30 (1.9) | 20 (1.9) | 0.92 |
| Operation time ≥ 240 min | 224 (14.1) | 497 (48.2) | <0.001 |
| Transfusion, n (%) | 94 (5.9) | 62 (6.0) | 0.91 |
| Anastomotic leakage, n (%) | 49 (3.1) | 105 (10.2) | <0.001 |
| Pathologic T stage, n (%) | <0.001 | ||
| T0 | 24 (2.3) | ||
| T1 | 234 (14.7) | 129 (12.5) | |
| T2 | 212 (13.3) | 253 (24.5) | |
| T3 | 1008 (63.4) | 592 (57.4) | |
| T4 | 137 (8.6) | 33 (3.2) | |
| Pathologic N stage, n (%) | 0.64 | ||
| N0 | 999 (62.8) | 657 (63.7) | |
| N1 | 442 (27.8) | 254 (24.6) | |
| N2 | 150 (9.4) | 120 (11.6) | |
| TNM Stage, n (%) | 0.002 | ||
| 0 | 20 (1.9) | ||
| I | 366 (23.0) | 307 (29.8) | |
| II | 633 (39.8) | 330 (32.0) | |
| III | 592 (37.2) | 374 (36.3) | |
| Harvested LN < 12, n (%) | 146 (9.2) | 168 (16.3) | <0.001 |
| Differentiation, n (%) | 0.15 | ||
| Well differentiated | 302 (19.0) | 194 (18.8) | |
| Moderately differentiated | 1168 (73.4) | 784 (76.0) | |
| Poorly differentiated | 45 (2.8) | 16 (1.6) | |
| Mucinous | 70 (4.4) | 34 (3.3) | |
| Signet ring cell | 3 (0.2) | 2 (0.2) | |
| Other types | 3 (0.2) | 1 (0.1) | |
| Lymphatic invasion, n (%) | 275 (17.3) | 137 (13.3) | 0.028 |
| Vascular invasion, n (%) | 55 (3.5) | 39 (3.8) | 0.90 |
| Perineural invasion, n (%) | 59 (3.7) | 54 (5.2) | 0.26 |
| DRM involvement, n (%) | 1 (0.1) | 7 (0.7) | 0.005 |
| CRM involvement, n (%) | 15 (0.9) | 28 (2.7) | <0.001 |
| Colon Cancer (N = 1591) | Rectal Cancer (N = 1031) | p Value | |
|---|---|---|---|
| Median time to recurrence (median, IQR) | 14 (9–20.5) | 15 (9–25.3) | 0.049 |
| Overall recurrence, n (%) | 141 (8.9) | 154 (14.9) | <0.001 |
| Locoregional recurrence, n (%) | 11 (0.7) | 45 (4.4) | <0.001 |
| Systemic recurrence, n (%) | 136 (8.5) | 127 (12.3) | <0.001 |
| Combined locoregional and systemic recurrence, n (%) | 6 (0.4) | 18 (1.7) | 0.008 |
| Systemic recurrence site | |||
| Liver | 39 (2.5) | 41 (4.0) | 0.027 |
| Lung | 39 (2.5) | 59 (5.7) | <0.001 |
| Peritoneal seeding | 36 (2.3) | 23 (2.2) | 0.96 |
| Ovary | 9 (0.6) | 3 (0.3) | 0.31 |
| Distant LNs | 21 (1.3) | 27 (2.6) | 0.015 |
| Bone | 5 (0.3) | 7 (0.7) | 0.18 |
| a Others | 6 (0.4) | 3 (0.3) | 0.71 |
| Locoregional | Liver | Lung | Distant LNs | Peritoneal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | |
| Rt colon vs. Lt colon | 3.24 | 0.70–14.98 | 0.13 | 1.04 | 0.55–1.97 | 0.90 | 0.69 | 0.34–1.29 | 0.25 | 0.80 | 0.34–1.89 | 0.61 | 0.54 | 0.28–1.06 | 0.073 |
| Colon vs. Rectum | 6.37 | 3.29–12.31 | <0.001 | 1.63 | 1.05–2.53 | 0.029 | 2.36 | 1.58–3.54 | <0.001 | 1.20 | 1.13–3.54 | 0.017 | 1.02 | 0.60–1.73 | 0.94 |
| Obstruction | 1.39 | 0.60–3.24 | 0.45 | 2.02 | 1.09–3.73 | 0.025 | 1.62 | 0.88–2.96 | 0.12 | 1.67 | 0.71–3.93 | 0.24 | 3.32 | 1.79–6.16 | <0.001 |
| Perforation | 1.91 | 0.47–7.82 | 0.37 | 1.32 | 0.33–5.38 | 0.70 | 1.07 | 0.26–4.34 | 0.93 | 1.11 | 0.15–8.02 | 0.92 | 0.90 | 0.12–6.49 | 0.92 |
| Stent insertion | 1.69 | 0.53–5.41 | 0.38 | 1.15 | 0.36–3.65 | 0.81 | 2.31 | 1.07–4.99 | 0.032 | 2.76 | 0.99–7.69 | 0.052 | 4.71 | 0.23–9.94 | <0.001 |
| Anastomotic leakage | 4.48 | 2.32–8.66 | <0.001 | 2.28 | 1.14–3.56 | 0.020 | 1.42 | 0.66–3.05 | 0.38 | 2.11 | 0.84–5.33 | 0.11 | 1.34 | 0.48–3.69 | 0.58 |
| pT1/T2 vs. pT3/T4 | 2.66 | 1.31–5.43 | 0.007 | 6.24 | 2.72–14.34 | <0.001 | 2.62 | 1.54–4.48 | <0.001 | 3.55 | 1.51–8.36 | 0.004 | 4.39 | 1.89–10.23 | 0.001 |
| N0 vs. N1/N2 | 2.29 | 1.35–3.88 | 0.002 | 2.34 | 1.52–3.67 | <0.001 | 2.93 | 1.95–4.40 | <0.001 | 2.58 | 1.45–4.58 | 0.001 | 2.80 | 1.66–4.84 | <0.001 |
| Differentiation | 2.12 | 0.96–4.68 | 0.063 | 1.42 | 0.65–3.08 | 0.38 | 0.47 | 0.15–1.48 | 0.20 | 1.35 | 0.49–3.76 | 0.57 | 3.09 | 1.56–6.11 | 0.001 |
| Vascular invasion | 3.24 | 1.29–8.12 | 0.012 | 4.08 | 2.04–8.16 | <0.001 | 3.40 | 1.71–6.74 | <0.001 | 5.64 | 2.53–12.58 | <0.001 | 3.05 | 1.22–7.63 | 0.017 |
| Lymphatic invasion | 0.82 | 0.37–1.80 | 0.62 | 2.02 | 1.23–3.32 | 0.006 | 1.12 | 0.66–1.92 | 0.67 | 1.91 | 0.995–3.68 | 0.052 | 1.64 | 0.89–3.04 | 0.12 |
| Perineural invasion | 2.71 | 1.08–6.81 | 0.033 | 5.29 | 2.92–9.60 | <0.001 | 2.50 | 1.21–5.14 | 0.013 | 3.30 | 1.30–8.34 | 0.012 | 3.16 | 1.36–7.36 | 0.008 |
| CRM | 5.24 | 1.90–14.50 | 0.001 | 2.68 | 0.85–8.49 | 0.094 | 3.71 | 1.51–9.12 | 0.004 | 1.47 | 0.20–10.66 | 0.70 | 1.21 | 0.17–8.71 | 0.85 |
| Neoadjuvant chemoradiotherapy | 5.33 | 3.13–9.07 | <0.001 | 2.05 | 1.20–3.51 | 0.008 | 3.26 | 2.11–5.03 | <0.001 | 2.04 | 1.02–4.09 | 0.045 | 1.04 | 0.47–2.30 | 0.92 |
| Adjuvant chemotherapy | 2.14 | 1.21–3.78 | 0.009 | 1.54 | 0.98–2.42 | 0.063 | 2.33 | 1.50–3.61 | <0.001 | 1.40 | 0.78–2.49 | 0.26 | 1.77 | 1.03–3.04 | 0.039 |
| Locoregional | Liver | Lung | Distant LNs | Peritoneal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | HRs | 95% CI | p Value | |
| Rt colon vs. Lt colon | |||||||||||||||
| Colon vs. Rectum | 4.31 | 2.08–8.94 | <0.001 | 1.54 | 0.91–2.59 | 0.11 | 1.93 | 1.20–3.13 | 0.007 | 1.92 | 1.01–3.63 | 0.046 | |||
| Obstruction | 1.64 | 0.86–3.13 | 0.14 | 1.85 | 0.73–4.69 | 0.20 | |||||||||
| Perforation | |||||||||||||||
| Stent insertion | 2.47 | 1.11–5.49 | 0.027 | 1.96 | 0.64–5.99 | 0.24 | |||||||||
| Anastomotic leakage | 2.86 | 1.45–5.62 | 0.002 | 1.91 | 0.93–3.89 | 0.076 | |||||||||
| pT1/T2 vs. pT3/T4 | 2.56 | 1.18–5.55 | 0.017 | 4.69 | 2.00–11.01 | <0.001 | 2.09 | 1.17–3.74 | 0.012 | 2.85 | 1.18–6.90 | 0.020 | 2.94 | 1.20–7.19 | 0.018 |
| N0 vs. N1/N2 | 2.06 | 1.12–3.81 | 0.020 | 1.56 | 0.97–2.50 | 0.066 | 2.57 | 1.59–4.15 | <0.001 | 1.91 | 1.05–3.48 | 0.034 | 2.49 | 1.34–4.66 | 0.004 |
| Differentiation | 2.77 | 1.39–5.51 | 0.004 | ||||||||||||
| Vascular invasion | 2.50 | 0.93–6.74 | 0.071 | 1.81 | 0.82–3.97 | 0.14 | 2.29 | 1.11–4.76 | 0.026 | 3.64 | 1.53–8.67 | 0.004 | 1.76 | 0.65–4.82 | 0.27 |
| Lymphatic invasion | 1.38 | 0.80–2.40 | 0.25 | ||||||||||||
| Perineural invasion | 1.12 | 0.41–3.01 | 0.83 | 2.49 | 1.28–4.86 | 0.007 | 1.08 | 0.50–2.35 | 0.84 | 1.37 | 0.50–3.74 | 0.54 | 1.57 | 0.62–3.99 | 0.34 |
| CRM | 2.83 | 1.01–7.94 | 0.048 | 2.22 | 0.90–5.49 | 0.085 | |||||||||
| Neoadjuvant chemoradiotherapy | 2.56 | 1.42–4.61 | 0.002 | 1.77 | 0.95–3.29 | 0.073 | 2.58 | 1.55–4.30 | <0.001 | 1.56 | 0.71–3.40 | 0.27 | |||
| Adjuvant chemotherapy | 0.82 | 0.42–1.63 | 0.58 | 0.90 | 0.53–1.53 | 0.69 | 0.65 | 0.34–1.24 | 0.19 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.S.; Kim, J.; Park, Y.R.; Cho, E.H.; Choo, J.M.; Kim, J.-S.; Baek, S.-J.; Kwak, J.-M. Recurrence Patterns and Risk Factors after Curative Resection for Colorectal Cancer: Insights for Postoperative Surveillance Strategies. Cancers 2023, 15, 5791. https://doi.org/10.3390/cancers15245791
Ryu HS, Kim J, Park YR, Cho EH, Choo JM, Kim J-S, Baek S-J, Kwak J-M. Recurrence Patterns and Risk Factors after Curative Resection for Colorectal Cancer: Insights for Postoperative Surveillance Strategies. Cancers. 2023; 15(24):5791. https://doi.org/10.3390/cancers15245791
Chicago/Turabian StyleRyu, Hyo Seon, Jin Kim, Ye Ryung Park, Eun Hae Cho, Jeong Min Choo, Ji-Seon Kim, Se-Jin Baek, and Jung-Myun Kwak. 2023. "Recurrence Patterns and Risk Factors after Curative Resection for Colorectal Cancer: Insights for Postoperative Surveillance Strategies" Cancers 15, no. 24: 5791. https://doi.org/10.3390/cancers15245791
APA StyleRyu, H. S., Kim, J., Park, Y. R., Cho, E. H., Choo, J. M., Kim, J.-S., Baek, S.-J., & Kwak, J.-M. (2023). Recurrence Patterns and Risk Factors after Curative Resection for Colorectal Cancer: Insights for Postoperative Surveillance Strategies. Cancers, 15(24), 5791. https://doi.org/10.3390/cancers15245791






