Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
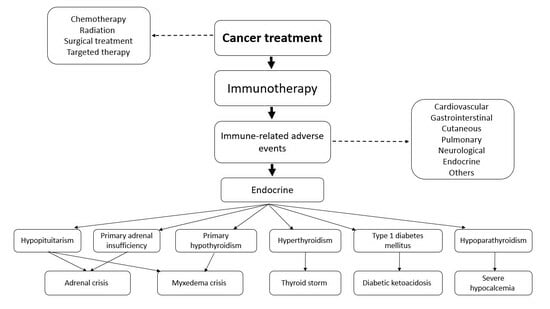
2. Cancer Immunotherapy
3. Adrenal Crisis Due to Central or Primary Adrenal Insufficiency
4. Thyroid Storm and Myxoedema Crisis
5. Diabetic Ketoacidosis
6. Severe Hypocalcaemia
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and clinical significance of in-stent restenosis in patients with diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial function assessment by flow-mediated dilation method: A valuable tool in the evaluation of the cardiovascular system. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Finocchario-Kessler, S.; Wexler, C.; Maloba, M.; Mabachi, N.; Ndikum-Moffor, F.; Bukusi, E. Cervical cancer prevention and treatment research in Africa: A systematic review from a public health perspective. BMC Womens Health 2016, 16, 29. [Google Scholar] [CrossRef]
- Weir, H.K.; Thompson, T.D.; Stewart, S.L.; White, M.C. Cancer incidence projections in the United States between 2015 and 2050. Prev. Chronic Dis. 2021, 18, E59. [Google Scholar] [CrossRef] [PubMed]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Krieghoff-Henning, E.; Kather, J.N.; Jutzi, T.; Höhn, J.; Kiehl, L.; Hekler, A.; Alwers, E.; von Kalle, C.; Fröhling, S.; et al. Gastrointestinal cancer classification and prognostication from histology using deep learning: Systematic review. Eur. J. Cancer 2021, 155, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Hao, J.; Wang, R.R.; Liu, X.; Gu, P.; Yu, H.; Yu, Y.; Wu, C.; Ou, B.; et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019. Cancer Med. 2022, 11, 1310–1323. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef]
- El Masri, J.; Phadke, S. Breast cancer epidemiology and contemporary breast cancer care: A review of the literature and clinical applications. Clin. Obstet. Gynecol. 2022, 65, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Vatseba, T.S. Cancer of the organs of the reproductive system in women with type 2 diabetes. effects of antidiabetic therapy. Wiad. Lek. 2020, 73, 967–971. [Google Scholar] [CrossRef]
- Siamof, C.M.; Goel, S.; Cai, W. Moving beyond the pillars of cancer treatment: Perspectives from nanotechnology. Front. Chem. 2020, 8, 598100. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Pansy, K.; Uhl, B.; Krstic, J.; Szmyra, M.; Fechter, K.; Santiso, A.; Thüminger, L.; Greinix, H.; Kargl, J.; Prochazka, K.; et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. Int. J. Mol. Sci. 2021, 22, 13311. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Fu, T.; Dai, L.J.; Wu, S.Y.; Xiao, Y.; Ma, D.; Jiang, Y.Z.; Shao, Z.M. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J. Hematol. Oncol. 2021, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the immune system: The history and background of immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- McDermott, D.; Haanen, J.; Chen, T.T.; Lorigan, P.; O’Day, S.; MDX010-20 investigators. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A.; Yang, J.C.; Sherry, R.M.; Hughes, M.S.; Kammula, U.S.; White, D.E.; Levy, C.L.; Rosenberg, S.A.; Phan, G.Q. CTLA-4 blockade with ipilimumab: Long-term follow-up of 177 patients with metastatic melanoma. Clin. Cancer Res. 2012, 18, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Ott, P.A. PD-1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. Oncotarget 2015, 6, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2023, 41, 715–723. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, N.Y. JAVELIN Head and Neck 100: A Phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. 2019, 15, 687–694. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ribatti, D. Update in TIGIT immune-checkpoint role in cancer. Front. Oncol. 2022, 12, 871085. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; You, X.; Han, S.; Sun, Y.; Zhang, J.; Zhang, Y. CD155/TIGIT, a novel immune checkpoint in human cancers (Review). Oncol. Rep. 2021, 45, 835–845. [Google Scholar] [CrossRef]
- Oncology (Cancer)/Hematologic Malignancies Approval Notifications. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications (accessed on 19 November 2023).
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-related adverse events (irAEs): Diagnosis, management, and clinical pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Stelmachowska-Banaś, M.; Czajka-Oraniec, I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis secondary to cytotoxic T-tymphocyte-associated protein 4 blockade: Insights into pathogenesis from an autopsy series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Del Rivero, J.; Cordes, L.M.; Klubo-Gwiezdzinska, J.; Madan, R.A.; Nieman, L.K.; Gulley, J.L. Endocrine-related adverse events related to immune checkpoint inhibitors: Proposed algorithms for management. Oncologist 2020, 25, 290–300. [Google Scholar] [CrossRef]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef]
- Torino, F.; Barnabei, A.; Paragliola, R.M.; Marchetti, P.; Salvatori, R.; Corsello, S.M. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: Clinical evidence and pathogenic hypotheses. Eur. J. Endocrinol. 2013, 169, R153–R164. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Min, L.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Luke, J.J.; Donahue, H.; Davis, M.; Carroll, R.S.; Kaiser, U.B. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: A retrospective cohort study. Clin. Cancer. Res. 2015, 21, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.S.; Long, G.V.; Guminski, A.; Clifton-Bligh, R.J.; Menzies, A.M.; Tsang, V.H. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur. J. Endocrinol. 2018, 178, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Grouthier, V.; Lebrun-Vignes, B.; Moey, M.; Johnson, D.B.; Moslehi, J.J.; Salem, J.E.; Bachelot, A. Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase report analysis. Oncologist 2020, 25, 696–701. [Google Scholar] [CrossRef]
- de Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bacanovic, S.; Burger, I.A.; Stolzmann, P.; Hafner, J.; Huellner, M.W. Ipilimumab-induced adrenalitis: A possible pitfall in 18F-FDG-PET/CT. Clin. Nucl. Med. 2015, 40, e518–e519. [Google Scholar] [CrossRef]
- Paepegaey, A.C.; Lheure, C.; Ratour, C.; Lethielleux, G.; Clerc, J.; Bertherat, J.; Kramkimel, N.; Groussin, L. Polyendocrinopathy resulting from pembrolizumab in a patient with a malignant melanoma. J. Endocr. Soc. 2017, 1, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Deligiorgi, M.V.; Trafalis, D.T. Reversible primary adrenal insufficiency related to anti-programmed cell-death 1 protein active immunotherapy: Insight into an unforeseen outcome of a rare immune-related adverse event. Int. Immunopharmacol. 2020, 89 Pt B, 107050. [Google Scholar] [CrossRef]
- Min, L.; Ibrahim, N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013, 1, e15. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv264–iv266. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl. Compr. Canc. Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, H.; Ahmed, S.F.; Bensing, S.; Beun, J.G.; Brösamle, M.; Chifu, I.; Claahsen van der Grinten, H.; Clemente, M.; Falhammar, H.; Hahner, S.; et al. Therapy options for adrenal insufficiency and recommendations for the management of adrenal crisis. Endocrine 2021, 71, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Galetta, F.; Citi, E.; Benvenga, S.; Antonelli, A. Thyroid disorders induced by checkpoint inhibitors. Rev. Endocr. Metab. Disord. 2018, 19, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Chera, A.; Stancu, A.L.; Bucur, O. Thyroid-related adverse events induced by immune checkpoint inhibitors. Front. Endocrinol. 2022, 13, 1010279. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Orlov, S.; Salari, F.; Kashat, L.; Walfish, P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J. Clin. Endocrinol. Metab. 2015, 100, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 2018, 28, 1243–1251. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, M.P.; Bornschlegl, S.; Merten, M.M.; Kottschade, L.; Withers, S.; Dietz, A.B.; Ryder, M. Pembrolizumab-induced thyroiditis: Comprehensive clinical review and insights into underlying involved mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Kottschade, L.; Ryder, M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 2020, 30, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, I.; Yasoda, A.; Matsumoto, S.; Sakamori, Y.; Kim, Y.H.; Nomura, M.; Otsuka, A.; Yamasaki, T.; Saito, R.; Kitamura, M.; et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 2019, 14, e0216954. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, E.; Rodríguez-Abreu, D.; Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune checkpoint inhibitors: Review and management of endocrine adverse events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, O.; Kandl, T.J.; Thakar, S.D.; Thuro, B.A.; Busaidy, N.L.; Cabanillas, M.; Jimenez, C.; Dadu, R.; Graham, P.H.; Debnam, J.M.; et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 50–52. [Google Scholar] [CrossRef] [PubMed]
- McElnea, E.; Ní Mhéalóid, A.; Moran, S.; Kelly, R.; Fulcher, T. Thyroid-like ophthalmopathy in a euthyroid patient receiving ipilimumab. Orbit 2014, 33, 424–427. [Google Scholar] [CrossRef]
- Campredon, P.; Imbert, P.; Mouly, C.; Grunenwald, S.; Mazières, J.; Caron, P. Severe inflammatory ophthalmopathy in a euthyroid patient during nivolumab treatment. Eur. Thyroid J. 2018, 7, 84–87. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Sagredou, S.; Vakkas, L.; Trafalis, D.T. The continuum of thyroid disorders related to immune checkpoint inhibitors: Still many pending queries. Cancers 2021, 13, 5277. [Google Scholar] [CrossRef]
- Yu, C.; Chopra, I.J.; Ha, E. A novel melanoma therapy stirs up a storm: Ipilimumab-induced thyrotoxicosis. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 140092. [Google Scholar] [CrossRef] [PubMed]
- Yonezaki, K.; Kobayashi, T.; Imachi, H.; Yoshimoto, T.; Kikuchi, F.; Fukunaga, K.; Sato, S.; Ibata, T.; Yamaji, N.; Lyu, J.; et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: A case report. J. Med. Case Rep. 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- McMillen, B.; Dhillon, M.S.; Yong-Yow, S. A rare case of thyroid storm. BMJ Case Rep. 2016, 2016, bcr2016214603. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Rizvi, H.; Sano, D.; Chiu, J.; Hadid, T. Nivolumab induced myxedema crisis. J. Immunother. Cancer. 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Goulden, E.; Cullen, G.; Crown, J.; Crowley, R.K. Myxoedema coma caused by immunotherapy-related thyroiditis and enteritis. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 21–0130. [Google Scholar] [CrossRef]
- Gummalla, S.; Manjunath, M.; Phillips, B. Myxedema coma: A life-threatening condition in patients using pembrolizumab. Case Rep. Endocrinol. 2020, 2020, 8855943. [Google Scholar] [CrossRef]
- Johnson, E.D.; Kerrigan, K.; Butler, K.; Patel, S.B. Nivolumab-induced hypothyoidism with consequent hypothyroid related myopathy. J. Oncol. Pharm. Pract. 2020, 26, 224–227. [Google Scholar] [CrossRef]
- Abushalha, K.; Abulaimoun, S.; Silberstein, P.T. So slow, so fast, a case of nivolumab-induced hypothyroidism with subsequent rhabdomyolysis. Immunotherapy 2020, 12, 625–628. [Google Scholar] [CrossRef]
- Badovinac, S.; Korsic, M.; Zarkovic, K.; Mursic, D.; Roglic, M.; Jakopovic, M.; Samarzija, M. Nivolumab-induced synchronous occurrence of myositis and hypothyroidism in a patient with squamous cell lung cancer. Immunotherapy 2018, 10, 427–431. [Google Scholar] [CrossRef]
- Min, L.; Hodi, F.S. Anti-PD1 following ipilimumab for mucosal melanoma: Durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol. Res. 2014, 2, 15–18. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Yang, J.; Tan, C.; Zhou, L.; Wang, X.; Xiao, L.; Zhang, S.; Chen, Y.; Liu, X. Diabetes mellitus induced by immune checkpoint inhibitors. Diabetes Metab. Res. Rev. 2021, 37, e3366. [Google Scholar] [CrossRef]
- de Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Ansari, M.J.; Salama, A.D.; Chitnis, T.; Smith, R.N.; Yagita, H.; Akiba, H.; Yamazaki, T.; Azuma, M.; Iwai, H.; Khoury, S.J.; et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003, 198, 63–69. [Google Scholar] [CrossRef]
- Paterson, A.M.; Brown, K.E.; Keir, M.E.; Vanguri, V.K.; Riella, L.V.; Chandraker, A.; Sayegh, M.H.; Blazar, B.R.; Freeman, G.J.; Sharpe, A.H. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J. Immunol. 2011, 187, 1097–1105. [Google Scholar] [CrossRef]
- Colli, M.L.; Hill, J.L.E.; Marroquí, L.; Chaffey, J.; Dos Santos, R.S.; Leete, P.; Coomans de Brachène, A.; Paula, F.M.M.; Op de Beeck, A.; Castela, A.; et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction. EBioMedicine 2018, 36, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.L.; Laly, P.; Vidal-Trecan, T.; Baroudjian, B.; Gottlieb, J.; Madjlessi-Ezra, N.; Da Meda, L.; Madelaine-Chambrin, I.; Bagot, M.; Basset-Seguin, N.; et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: A case report and literature review. Cancer Immunol. Immunother. 2017, 66, 1399–1410. [Google Scholar] [CrossRef]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral damage: Insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.H.M.; McGrath, R.T.; Clifton-Bligh, R.J.; Scolyer, R.A.; Jakrot, V.; Guminski, A.D.; Long, G.V.; Menzies, A.M. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 5499–5506. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults—An updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet. Med. 2022, 39, e14788. [Google Scholar] [CrossRef] [PubMed]
- Nalluru, S.S.; Piranavan, P.; Ning, Y.; Ackula, H.; Siddiqui, A.D.; Trivedi, N. Hypocalcemia with immune checkpoint inhibitors: The disparity among various reports. Int. J. Endocrinol. 2020, 2020, 7459268. [Google Scholar] [CrossRef]
- Bai, X.; Lin, X.; Zheng, K.; Chen, X.; Wu, X.; Huang, Y.; Zhuang, Y. Mapping endocrine toxicity spectrum of immune checkpoint inhibitors: A disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine 2020, 69, 670–681. [Google Scholar] [CrossRef]
- Zhai, Y.; Ye, X.; Hu, F.; Xu, J.; Guo, X.; Zhuang, Y.; He, J. Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US Food and Drug Administration adverse events reporting system. J. Immunother. Cancer. 2019, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Deligiannis, N.G.; Sosa, S.; Danilowicz, K.; Rizzo, L.F.L. Endocrine dysfunction induced by immune checkpoint inhibitors. Medicina 2021, 81, 269–278. [Google Scholar]
- Lupi, I.; Brancatella, A.; Cetani, F.; Latrofa, F.; Kemp, E.H.; Marcocci, C. Activating antibodies to the calcium-sensing receptor in immunotherapy-induced hypoparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, dgaa092. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Kuhadiya, N.D.; Gonzalaes, M. Pembrolizumab-associated hypoparathyroidism: A single case report. AACE Clin. Case Rep. 2020, 7, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Umeguchi, H.; Takenoshita, H.; Inoue, H.; Kurihara, Y.; Sakaguchi, C.; Yano, S.; Hasuzawa, N.; Sakamoto, S.; Sakamoto, R.; Ashida, K. Autoimmune-related primary hypoparathyroidism possibly induced by the administration of pembrolizumab: A case report. J. Oncol. Pract. 2018, 14, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kreze, A.; Homer, M.; Barešová, T.; Klemperová, K. Hypoparathyroidism: An uncommon adverse effect of treatment with durvalumab. Endocr. Oncol. 2022, 2, K21–K24. [Google Scholar] [CrossRef] [PubMed]
- Piranavan, P.; Li, Y.; Brown, E.; Kemp, E.H.; Trivedi, N. Immune checkpoint inhibitor-induced hypoparathyroidism associated with calcium-sensing receptor-activating autoantibodies. J. Clin. Endocrinol. Metab. 2019, 104, 550–556. [Google Scholar] [CrossRef]
- Dadu, R.; Rodgers, T.E.; Trinh, V.A.; Kemp, E.H.; Cubb, T.D.; Patel, S.; Simon, J.M.; Burton, E.M.; Tawbi, H. Calcium-sensing receptor autoantibody-mediated hypoparathyroidism associated with immune checkpoint inhibitor therapy: Diagnosis and long-term follow-up. J. Immunother. Cancer 2020, 8, e000687. [Google Scholar] [CrossRef] [PubMed]
- El Kawkgi, O.M.; Li, D.; Kotwal, A.; Wermers, R.A. Hypoparathyroidism: An uncommon complication associated with immune checkpoint inhibitor therapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.; Sanchez, G.O.; Herzig, P.; Läubli, H. Inflammation-induced hypoparathyroidism triggered by combination immune checkpoint blockade for melanoma. J. Immunother. Cancer 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Mytareli, C.; Ziogas, D.C.; Karampela, A.; Papalexis, P.; Siampanopoulou, V.; Lafioniatis, A.; Benopoulou, O.; Gogas, H.; Angelousi, A. The uncharted landscape of rare endocrine immune-related adverse events. Cancers 2023, 15, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gavalas, N.G.; Kemp, E.H.; Krohn, K.J.; Brown, E.M.; Watson, P.F.; Weetman, A.P. The calcium-sensing receptor is a target of autoantibodies in patients with autoimmune polyendocrine syndrome type 1. J. Clin. Endocrinol. Metab. 2007, 92, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Meager, A.; Visvalingam, K.; Peterson, P.; Möll, K.; Murumägi, A.; Krohn, K.; Eskelin, P.; Perheentupa, J.; Husebye, E.; Kadota, Y.; et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006, 3, e289. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Björklund, P.; Hallgren, A.; Pöntynen, N.; Szinnai, G.; Shikama, N.; Keller, M.P.; Ekwall, O.; Kinkel, S.A.; Husebye, E.S.; et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N. Engl. J. Med. 2008, 358, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Win, M.A.; Thein, K.Z.; Qdaisat, A.; Yeung, S.J. Acute symptomatic hypocalcemia from immune checkpoint therapy-induced hypoparathyroidism. Am. J. Emerg. Med. 2017, 35, 1039.e5–1039.e7. [Google Scholar] [CrossRef] [PubMed]
- Iwama, S.; Kobayashi, T.; Arima, H. Clinical characteristics, management, and potential biomarkers of endocrine dysfunction induced by immune checkpoint inhibitors. Endocrinol. Metab. 2021, 36, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Iwama, S.; Inaba, H.; Ariyasu, H.; Makita, N.; Otsuki, M.; Kageyama, K.; Imagawa, A.; Akamizu, T. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: Clinical guidelines of the Japan Endocrine Society. Endocr. J. 2019, 66, 581–586. [Google Scholar] [CrossRef]
- Husebye, E.S.; Castinetti, F.; Criseno, S.; Curigliano, G.; Decallonne, B.; Fleseriu, M.; Higham, C.E.; Lupi, I.; Paschou, S.A.; Toth, M.; et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: An ESE clinical practice guideline. Eur. J. Endocrinol. 2022, 187, G1–G21. [Google Scholar] [CrossRef] [PubMed]


| Adverse Event | Main Signs and Symptoms | Basic Assessment | Management | References |
|---|---|---|---|---|
| Adrenal crisis | Hypotension, nausea, vomiting, confusion as well as signs and symptoms of hyponatremia or hyperkalaemia |
|
| [45,49,52,118] |
| Thyroid storm | Tachycardia, high body temperature, anxiety, high blood pressure, nausea, and vomiting |
|
| [45,49,52,61,62,118] |
| Myxoedema crisis | Swelling, bradycardia, weakness, fatigue, hypothermia, depression, constipation, dyspnoea, slow voice, dry skin |
|
| [45,49,52,61,62,118] |
| Diabetic ketoacidosis | Nausea or vomiting, abdominal pain, hyperventilation, coma |
|
| [45,49,52,118] |
| Severe hypocalcaemia | Nausea and vomiting, paraesthesia, dizziness, ataxia, abdominal cramps or disbalance, positive Chvostek and Trousseau signs |
|
| [45,108,111,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basek, A.; Jakubiak, G.K.; Cieślar, G.; Stanek, A. Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 5786. https://doi.org/10.3390/cancers15245786
Basek A, Jakubiak GK, Cieślar G, Stanek A. Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy. Cancers. 2023; 15(24):5786. https://doi.org/10.3390/cancers15245786
Chicago/Turabian StyleBasek, Aleksandra, Grzegorz K. Jakubiak, Grzegorz Cieślar, and Agata Stanek. 2023. "Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy" Cancers 15, no. 24: 5786. https://doi.org/10.3390/cancers15245786
APA StyleBasek, A., Jakubiak, G. K., Cieślar, G., & Stanek, A. (2023). Life-Threatening Endocrinological Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy. Cancers, 15(24), 5786. https://doi.org/10.3390/cancers15245786