Dynamic Chest Radiograph Simulation Technique with Deep Convolutional Neural Networks: A Proof-of-Concept Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Datasets
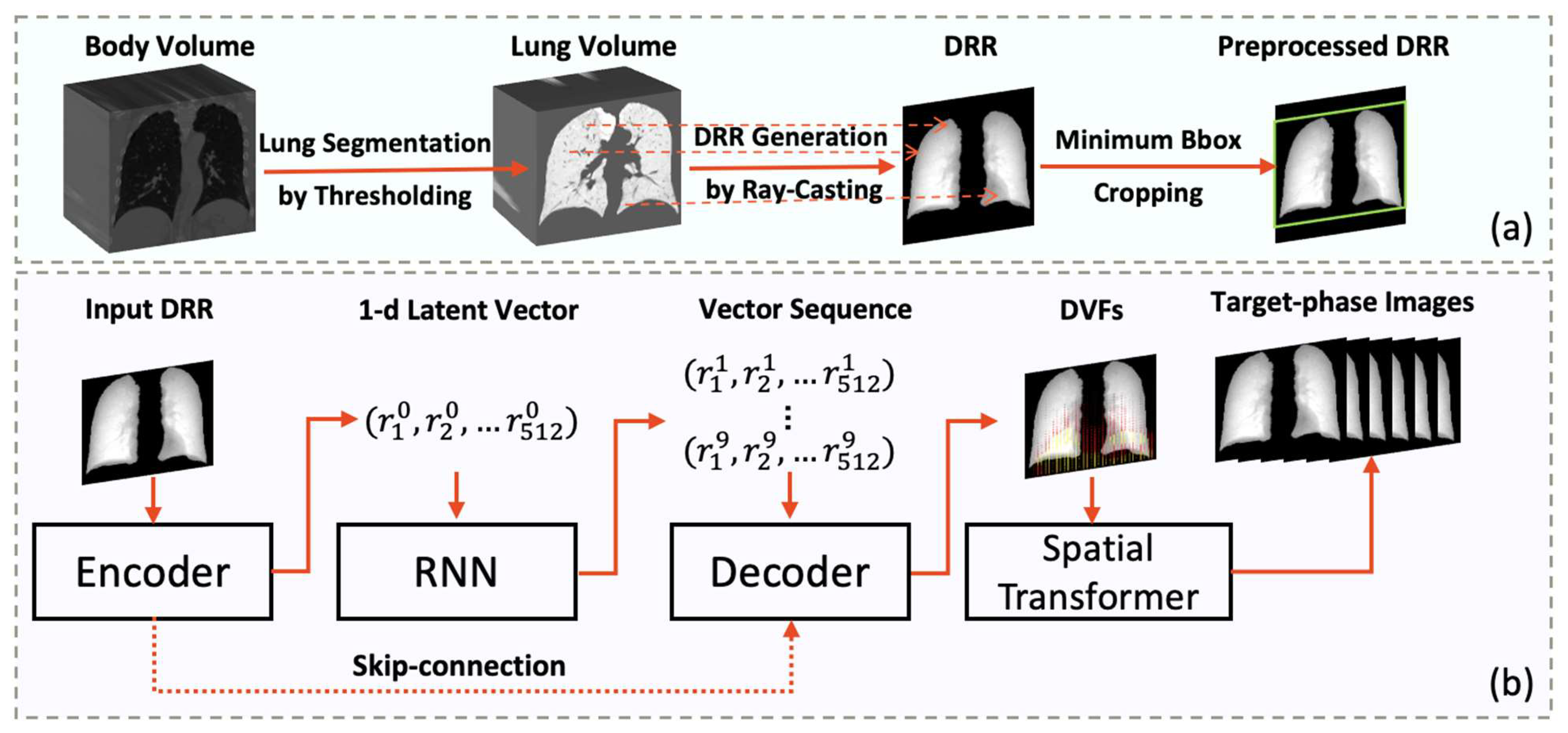
2.2. Network Design
2.2.1. Medical Imaging Generation Model
2.2.2. Loss Function Design
2.2.3. Evaluation Metrics
2.2.4. Experiment Setup
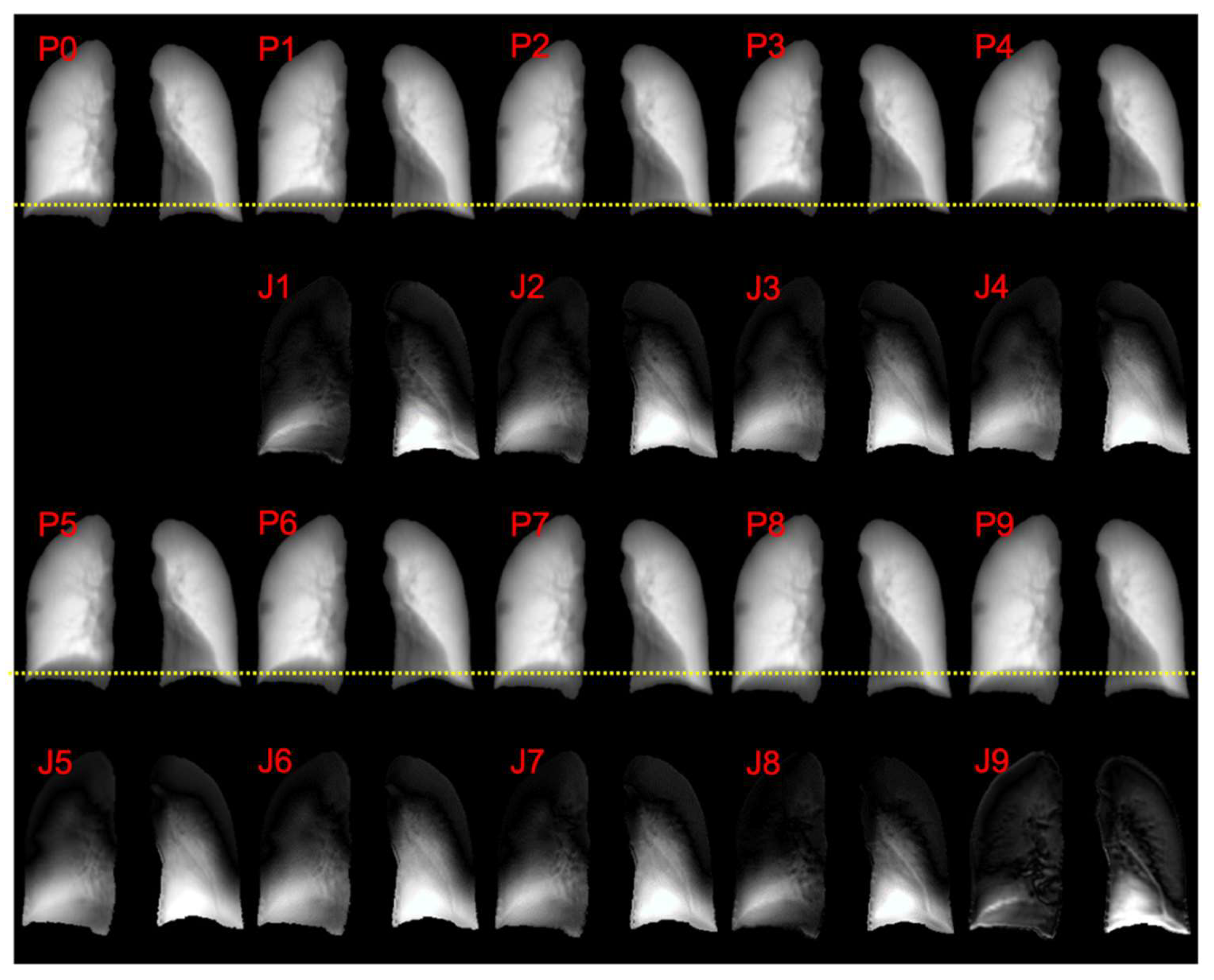
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wielputz, M.O.; Heussel, C.P.; Herth, F.J.; Kauczor, H.U. Radiological diagnosis in lung disease: Factoring treatment options into the choice of diagnostic modality. Dtsch. Arztebl. Int. 2014, 111, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sanada, S.; Okazaki, N.; Kobayashi, T.; Suzuki, M.; Matsui, T.; Matsui, O. Detectability of regional lung ventilation with flat-panel detector-based dynamic radiography. J. Digit. Imaging 2008, 21, 109–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamura, M.; Matsumoto, I.; Saito, D.; Yoshida, S.; Takata, M.; Tanaka, R.; Takemura, H. Dynamic chest radiography: Novel and less-invasive imaging approach for preoperative assessments of pleural invasion and adhesion. Radiol. Case Rep. 2020, 15, 702–704. [Google Scholar] [CrossRef]
- Johnston, D.A.; Brennan, P.C. Reference dose levels for patients undergoing common diagnostic X-ray examinations in Irish hospitals. Br. J. Radiol. 2000, 73, 396–402. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, B.; Hogeweg, L.; Prokop, M. Computer-aided diagnosis in chest radiography: Beyond nodules. Eur. J. Radiol. 2009, 72, 226–230. [Google Scholar] [CrossRef]
- Fraser, R.S.; Müller, N.L.; Colman, N.; Pare, P. Fraser and Paré’s Diagnosis of Diseases of the Chest; Wb Saunders: Philadelphia, PA, USA, 1999; Volumes 1–4. [Google Scholar]
- Qin, C.; Yao, D.; Shi, Y.; Song, Z. Computer-aided detection in chest radiography based on artificial intelligence: A survey. Biomed. Eng. Online 2018, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Roshan, R.K.; Deiveega, S.M. Chest X ray image enhancement using deep contrast diffusion learning. Optik 2023, 279, 170751. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wu, J.-X.; Li, C.-M.; Chen, P.-Y.; Pai, N.-S.; Kuo, Y.-C. Enhancement of Chest X-ray Images to Improve Screening Accuracy Rate Using Iterated Function System and Multilayer Fractional-Order Machine Learning Classifier. IEEE Photonics J. 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, X.; Huang, Z.; Li, W.; Zhang, H. Low-dose chest X-ray image super-resolution using generative adversarial nets with spectral normalization. Biomed. Signal Process. Control 2020, 55, 101600. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ghosh, A. ENResNet: A novel residual neural network for chest X-ray enhancement based COVID-19 detection. Biomed. Signal Process. Control 2022, 72, 103286. [Google Scholar] [CrossRef]
- Rahman, T.; Khandakar, A.; Kadir, M.A.; Islam, K.R.; Islam, K.F.; Mazhar, R.; Hamid, T.; Islam, M.T.; Kashem, S.; Mahbub, Z.B.; et al. Reliable Tuberculosis Detection Using Chest X-ray With Deep Learning, Segmentation and Visualization. IEEE Access 2020, 8, 191586–191601. [Google Scholar] [CrossRef]
- Souza, J.C.; Diniz, J.O.B.; Ferreira, J.L.; Da Silva, G.L.F.; Silva, A.C.; de Paiva, A.C. An automatic method for lung segmentation and reconstruction in chest X-ray using deep neural networks. Comput. Methods Programs Biomed. 2019, 177, 285–296. [Google Scholar]
- Stirenko, S.; Kochura, Y.; Alienin, O.; Rokovyi, O.; Gordienko, Y.; Gang, P.; Zeng, W. Chest X-ray Analysis of Tuberculosis by Deep Learning with Segmentation and Augmentation. In Proceedings of the 2018 IEEE 38th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 24–26 April 2018; pp. 422–428. [Google Scholar]
- Yang, D.; Ren, G.; Ni, R.; Huang, Y.H.; Lam, N.F.D.; Sun, H.; Wan, S.B.N.; Wong, M.F.E.; Chan, K.K.; Tsang, H.C.H.; et al. Deep learning attention-guided radiomics for COVID-19 chest radiograph classification. Quant. Imaging Med. Surg. 2023, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Perumal, E.; Tiwari, P.; Shorfuzzaman, M.; Gupta, D. Deep learning and evolutionary intelligence with fusion-based feature extraction for detection of COVID-19 from chest X-ray images. Multimed. Syst. 2022, 28, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Jain, J.S.; Bansal, P.; Gupta, S. Feature Extraction and Classification of Chest X-ray Images Using CNN to Detect Pneumonia. In Proceedings of the 2020 10th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 29–31 January 2020; pp. 227–231. [Google Scholar]
- Shen, L.; Zhao, W.; Xing, L. Patient-specific reconstruction of volumetric computed tomography images from a single projection view via deep learning. Nat. Biomed. Eng. 2019, 3, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.R.; Athavale, A.M.; Sahni, A.; Sukhal, S.; Saini, A.; Itteera, M.; Zhukovsky, S.; Vernik, J.; Abraham, M.; Joshi, A.; et al. Deep learning model to predict the need for mechanical ventilation using chest X-ray images in hospitalised patients with COVID-19. BMJ Innov. 2021, 7, 261–270. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Qin, W.; Liang, X.; Xu, J.; Xiong, J.; Xie, Y. Magnetic resonance image (MRI) synthesis from brain computed tomography (CT) images based on deep learning methods for magnetic resonance (MR)-guided radiotherapy. Quant. Imaging Med. Surg. 2020, 10, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.; Nunes, J.C.; Chourak, H.; Largent, A.; Tahri, S.; Acosta, O.; De Crevoisier, R.; Lafond, C.; Barateau, A. Deep learning methods to generate synthetic CT from MRI in radiotherapy: A literature review. Phys. Med. 2021, 89, 265–281. [Google Scholar] [CrossRef]
- Castillo, R.; Castillo, E.; Guerra, R.; Johnson, V.E.; McPhail, T.; Garg, A.K.; Guerrero, T. A framework for evaluation of deformable image registration spatial accuracy using large landmark point sets. Phys. Med. Biol. 2009, 54, 1849–1870. [Google Scholar] [CrossRef] [PubMed]
- Kipritidis, J.; Tahir, B.A.; Cazoulat, G.; Hofman, M.S.; Siva, S.; Callahan, J.; Hardcastle, N.; Yamamoto, T.; Christensen, G.E.; Reinhardt, J.M.; et al. The VAMPIRE challenge: A multi-institutional validation study of CT ventilation imaging. Med. Phys. 2019, 46, 1198–1217. [Google Scholar] [CrossRef]
- Vandemeulebroucke, J.; Rit, S.; Kybic, J.; Clarysse, P.; Sarrut, D. Spatiotemporal motion estimation for respiratory-correlated imaging of the lungs. Med. Phys. 2011, 38, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015, Munich, Germany, 5–9 October 2015; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Jaderberg, M.; Simonyan, K.; Zisserman, A.; Kavukcuoglu, K. Spatial transformer networks. Adv. Neural Inf. Process. Syst. 2015, 2015, 2017–2025. [Google Scholar]
- Shiraishi, J.; Katsuragawa, S.; Ikezoe, J.; Matsumoto, T.; Kobayashi, T.; Komatsu, K.-I.; Matsui, M.; Fujita, H.; Kodera, Y.; Doi, K. Development of a Digital Image Database for Chest Radiographs With and Without a Lung Nodule. Am. J. Roentgenol. 2000, 174, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kalra, M.K.; Nitiwarangkul, C.; Patti, J.A.; Homayounieh, F.; Padole, A.; Rao, P.; Putha, P.; Muse, V.V.; Sharma, A.; et al. Deep learning in chest radiography: Detection of findings and presence of change. PLoS ONE 2018, 13, e0204155. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Ebrahimian, S.; McDermott, S.; Lee, S.; Naccarato, L.; Di Capua, J.F.; Wu, M.Y.; Zhang, E.W.; Muse, V.; Miller, B.; et al. Association of Artificial Intelligence–Aided Chest Radiograph Interpretation with Reader Performance and Efficiency. JAMA Netw. Open 2022, 5, e2229289. [Google Scholar] [CrossRef]


| Dataset Resource | Number of Patients |
|---|---|
| DIR Lab 4D-CT dataset [22] | 10 |
| POPI dataset [24] | 5 |
| VAMPIRE challenge 4D CT dataset [23] | 12 |
| Henan Cancer Hospital | 33 |
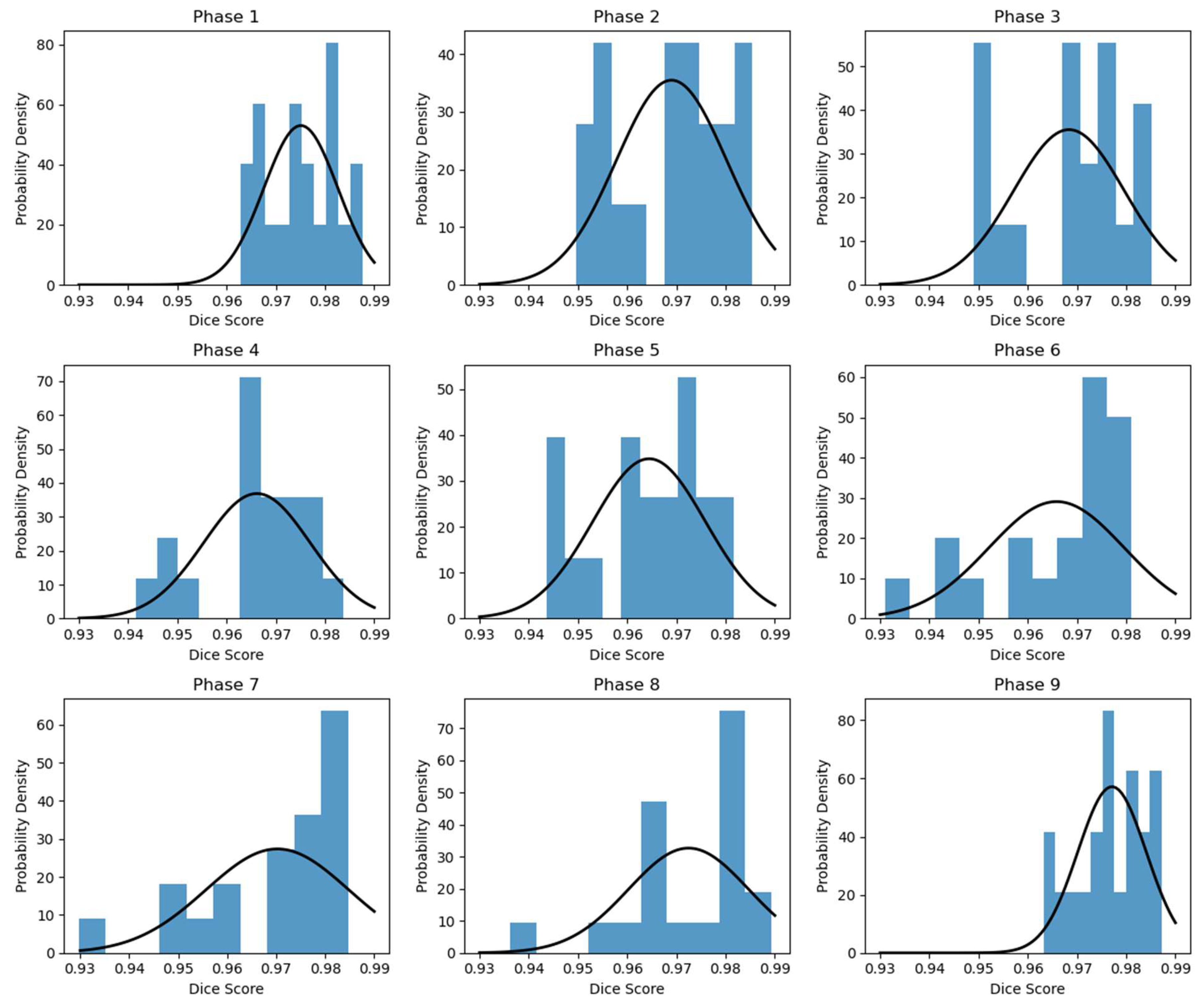
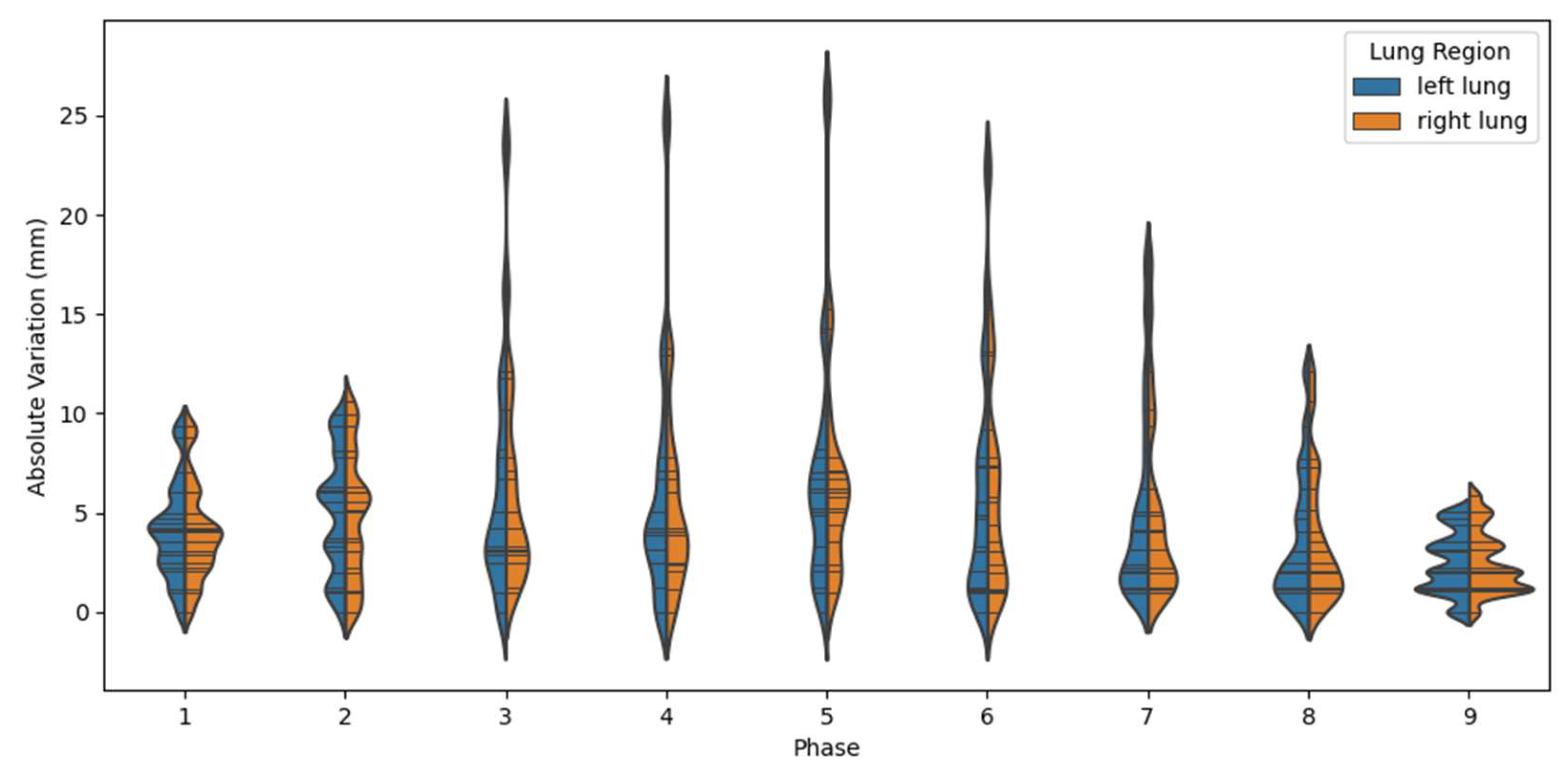
| Phase # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Dice: | 0.975 (0.0075) | 0.969 (0.0112) | 0.968 (0.0112) | 0.966 (0.0108) | 0.964 (0.0114) | 0.966 (0.0137) | 0.970 (0.0146) | 0.972 (0.0122) | 0.977 (0.0070) |
| LLE (mm): | 4.00 (2.60) | 4.73 () | 5.80 () | 4.77 () | 4.36 () | 3.09 () | 1.30 () | 0.28 () | 0.36 () |
| RLE (mm): | 3.93 () | 4.62 () | 5.75 () | 4.76 () | 4.29 () | 3.03 () | 1.35 () | 0.18 () | 0.31 () |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Huang, Y.; Li, B.; Cai, J.; Ren, G. Dynamic Chest Radiograph Simulation Technique with Deep Convolutional Neural Networks: A Proof-of-Concept Study. Cancers 2023, 15, 5768. https://doi.org/10.3390/cancers15245768
Yang D, Huang Y, Li B, Cai J, Ren G. Dynamic Chest Radiograph Simulation Technique with Deep Convolutional Neural Networks: A Proof-of-Concept Study. Cancers. 2023; 15(24):5768. https://doi.org/10.3390/cancers15245768
Chicago/Turabian StyleYang, Dongrong, Yuhua Huang, Bing Li, Jing Cai, and Ge Ren. 2023. "Dynamic Chest Radiograph Simulation Technique with Deep Convolutional Neural Networks: A Proof-of-Concept Study" Cancers 15, no. 24: 5768. https://doi.org/10.3390/cancers15245768
APA StyleYang, D., Huang, Y., Li, B., Cai, J., & Ren, G. (2023). Dynamic Chest Radiograph Simulation Technique with Deep Convolutional Neural Networks: A Proof-of-Concept Study. Cancers, 15(24), 5768. https://doi.org/10.3390/cancers15245768








