Pleural Mesothelioma: Treatable Traits of a Heterogeneous Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Pathogenetic Basis
3.1. The Role of Asbestos
3.2. Genetic Basis of the Disease
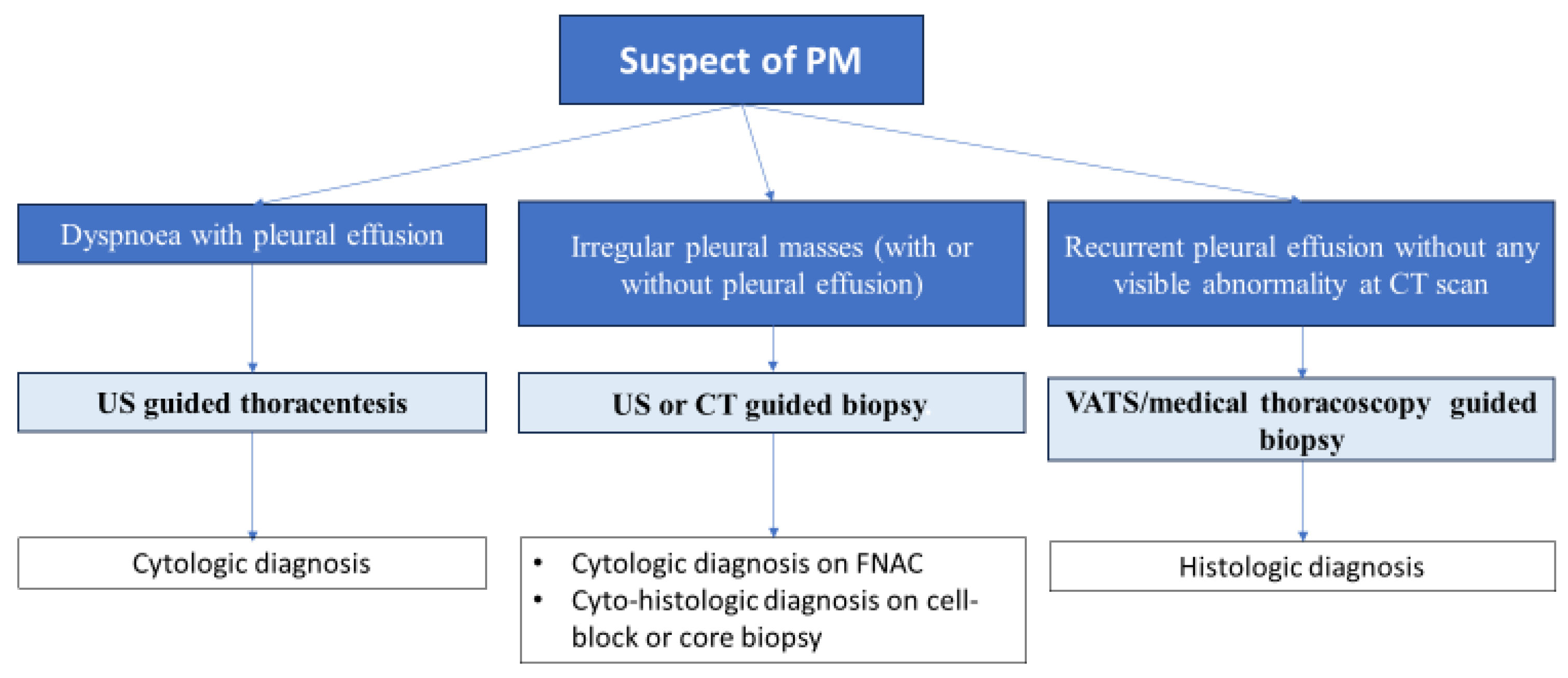
4. Diagnostic Work-Up
4.1. Clinical Presentation
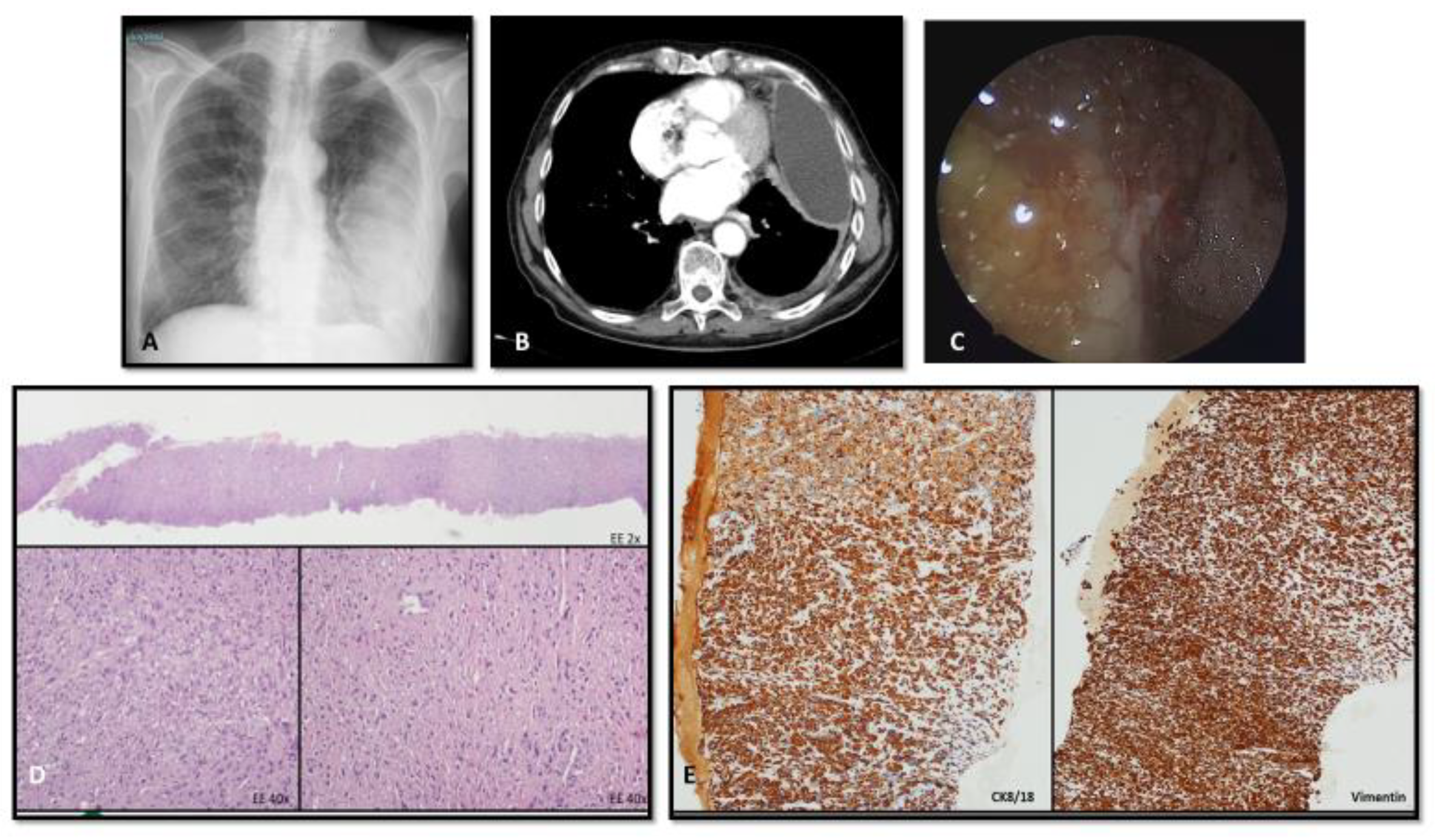
4.2. Imaging
4.3. Bioptic Procedures
4.4. Pathologic Classification and Staging
5. Treatment
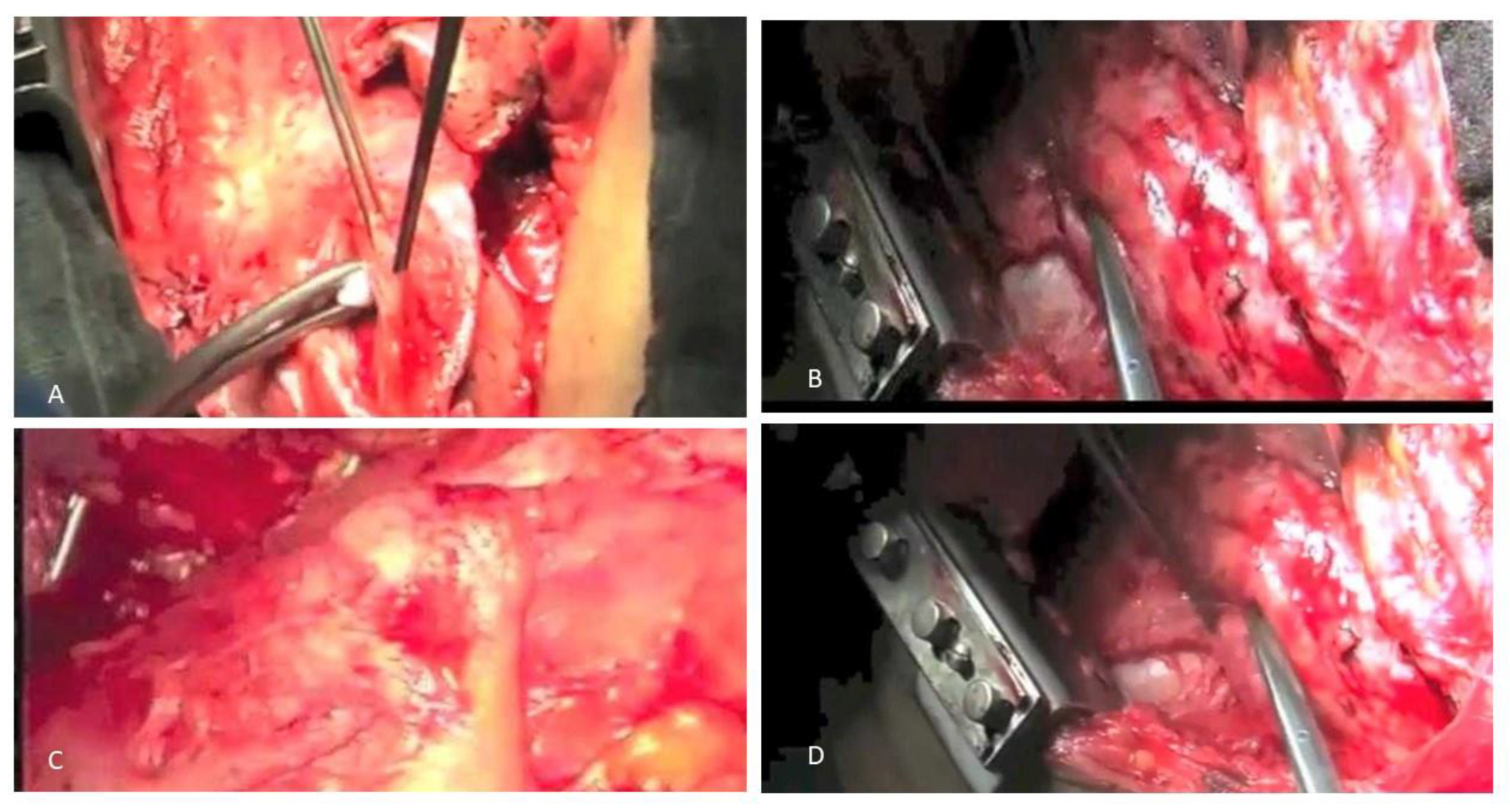
5.1. The Role of Surgery and Radiotherapy: An Update
5.2. Targeting PM Immune Landscape: Current Strategies
5.3. Cell Therapy Approaches
5.3.1. CAR T-Cell Therapy
5.3.2. STING
6. Novel Insights
6.1. Targeting PM Proteomic Profile
6.2. Exploiting PM-OMIC Profile
6.3. Genomic Basis of Novel Trials
6.4. Interfering with Cell Invasive Properties
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaudino, G.; Xue, J.; Yang, H. How Asbestos and Other Fibers Cause Mesothelioma. Transl. Lung Cancer Res. 2020, 9, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.M.; Bortolotto, C.; Benvenuti, S.; Lancia, A.; Filippi, A.R.; Stella, G.M. Malignant Pleural Mesothelioma: Genetic and Microenviromental Heterogeneity as an Unexpected Reading Frame and Therapeutic Challenge. Cancers 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.; Nelson, D.I.; Steenland, K.; Leigh, J.; Concha-Barrientos, M.; Fingerhut, M.; Prüss-Ustün, A. The Global Burden of Disease Due to Occupational Carcinogens. Am. J. Ind. Med. 2005, 48, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Delgermaa, V.; Takahashi, K.; Park, E.K.; Le, G.V.; Hara, T.; Sorahan, T. Les Décés Mondiaux Par Mésothéliome Rapportés á l’Organisation Mondiale de La Santé Entre 1994 et 2008. Bull. World Health Organ. 2011, 89, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Baas, P.; Faivre-Finn, C.; Girard, N.; Nicholson, A.G.; Nowak, A.K.; Opitz, I.; Scherpereel, A.; Reck, M. Malignant Pleural Mesothelioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2022, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Alpert, N.; van Gerwen, M.; Taioli, E. Epidemiology of Mesothelioma in the 21st Century in Europe and the United States, 40 Years after Restricted/Banned Asbestos Use. Transl. Lung Cancer Res. 2020, 9, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, T.; Chen, J.; Ying, S.; Gao, Z.; He, X.; Miao, C.; Yu, M.; Feng, L.; Xia, H.; et al. Hand-Spinning Chrysotile Exposure and Risk of Malignant Mesothelioma: A Case–Control Study in Southeastern China. Int. J. Cancer 2018, 142, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Schumann, S.O.; Kocher, G.; Minervini, F. Epidemiology, Diagnosis and Treatment of the Malignant Pleural Mesothelioma, a Narrative Review of Literature. J. Thorac. Dis. 2021, 13, 2510–2523. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Dakheel-Ali, M.; Marx, M.S.; Thein, K.; Regier, N.G.; Waage, P. HHS Public Access. Physiol. Behav. 2017, 176, 139–148. [Google Scholar]
- Baumann, F.; Ambrosi, J.-P.; Carbone, M. Asbestos Is Not Just Asbestos: An Unrecognised Health Hazard. Lancet. Oncol. 2013, 14, 576–578. [Google Scholar] [CrossRef]
- Carbone, M.; Emri, S.; Dogan, A.U.; Steele, I.; Tuncer, M.; Pass, H.I.; Baris, Y.I. A Mesothelioma Epidemic in Cappadocia: Scientific Developments and Unexpected Social Outcomes. Nat. Rev. Cancer 2007, 7, 147–154. [Google Scholar] [CrossRef]
- Stanton, M.F.; Laynard, M.; Tegeris, A.; Miller, E.; May, M.; Kent, E. Carcinogenicity of Fibrous Glass: Pleural Response in the Rat in Relation to Fiber Dimension. J. Natl. Cancer Inst. 1977, 58, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.L.; Jaurand, M.-C.; Kamp, D.W.; Whysner, J.; Hei, T.K. Role of Mutagenicity in Asbestos Fiber-Induced Carcinogenicity and Other Diseases. J. Toxicol. Environ. Health. B Crit. Rev. 2011, 14, 179–245. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T. In Vitro Studies on the Biologic Effects of Fibers: Correlation with in Vivo Bioassays. Environ. Health Perspect. 1990, 88, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.A.; Grespin, M.; Best, E.A. Asbestos Fiber Length and Its Relation to Disease Risk. Inhal. Toxicol. 2017, 29, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Yang, H. Molecular Pathways: Targeting Mechanisms of Asbestos and Erionite Carcinogenesis in Mesothelioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Donaldson, K.; Decker, U.; Gaering, S.; Kunzendorf, P.; Chevalier, J.; Holm, S.E. A Biopersistence Study Following Exposure to Chrysotile Asbestos Alone or in Combination with Fine Particles. Inhal. Toxicol. 2008, 20, 1009–1028. [Google Scholar] [CrossRef][Green Version]
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; Demay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous Exposure to Chrysotile Asbestos Can Cause Transformation of Human Mesothelial Cells via HMGB1 and TNF-α Signaling. Am. J. Pathol. 2013, 183, 1654–1666. [Google Scholar] [CrossRef]
- Larson, D.; Powers, A.; Ambrosi, J.-P.; Tanji, M.; Napolitano, A.; Flores, E.G.; Baumann, F.; Pellegrini, L.; Jennings, C.J.; Buck, B.J.; et al. Investigating Palygorskite’s Role in the Development of Mesothelioma in Southern Nevada: Insights into Fiber-Induced Carcinogenicity. J. Toxicol. Environ. Health. B Crit. Rev. 2016, 19, 213–230. [Google Scholar] [CrossRef]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R.J.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific Clues for Prevention, Diagnosis, and Therapy. CA. Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef]
- Nagai, H.; Ishihara, T.; Lee, W.-H.; Ohara, H.; Okazaki, Y.; Okawa, K.; Toyokuni, S. Asbestos Surface Provides a Niche for Oxidative Modification. Cancer Sci. 2011, 102, 2118–2125. [Google Scholar] [CrossRef]
- Xu, A.; Wu, L.J.; Santella, R.M.; Hei, T.K. Role of Oxyradicals in Mutagenicity and DNA Damage Induced by Crocidolite Asbestos in Mammalian Cells. Cancer Res. 1999, 59, 5922–5926. [Google Scholar]
- Toyokuni, S. Iron Addiction with Ferroptosis-Resistance in Asbestos-Induced Mesothelial Carcinogenesis: Toward the Era of Mesothelioma Prevention. Free Radic. Biol. Med. 2019, 133, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Nino, M.E.; Blumen, S.R.; Sabo-Attwood, T.; Pass, H.; Carbone, M.; Testa, J.R.; Altomare, D.A.; Mossman, B.T. HGF Mediates Cell Proliferation of Human Mesothelioma Cells through a PI3K/MEK5/Fra-1 Pathway. Am. J. Respir. Cell Mol. Biol. 2008, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, V.C.; Yang, L.; Scavo, L.M.; Ernst, J.D.; Boylan, A.M. Asbestos Induces Apoptosis of Human and Rabbit Pleural Mesothelial Cells via Reactive Oxygen Species. J. Clin. Investig. 1996, 98, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bocchetta, M.; Kroczynska, B.; Elmishad, A.G.; Chen, Y.; Liu, Z.; Bubici, C.; Mossman, B.T.; Pass, H.I.; Testa, J.R.; et al. TNF-Alpha Inhibits Asbestos-Induced Cytotoxicity via a NF-KappaB-Dependent Pathway, a Possible Mechanism for Asbestos-Induced Oncogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 10397–10402. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Yang, H. Mesothelioma: Recent Highlights. Ann. Transl. Med. 2017, 5, 238. [Google Scholar] [CrossRef]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed Necrosis Induced by Asbestos in Human Mesothelial Cells Causes High-Mobility Group Box 1 Protein Release and Resultant Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, Y.; Menges, C.W.; Talarchek, J.; Cai, K.Q.; Klein-Szanto, A.J.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Cheung, M.; Mossman, B.T.; Shukla, A.; et al. Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev. Res. 2016, 9, 406–414. [Google Scholar] [CrossRef]
- Thompson, J.K.; Shukla, A.; Leggett, A.L.; Munson, P.B.; Miller, J.M.; MacPherson, M.B.; Beuschel, S.L.; Pass, H.I.; Shukla, A. Extracellular Signal Regulated Kinase 5 and Inflammasome in Progression of Mesothelioma. Oncotarget 2018, 9, 293–305. [Google Scholar] [CrossRef]
- Pellegrini, L.; Xue, J.; Larson, D.; Pastorino, S.; Jube, S.; Forest, K.H.; Saad-Jube, Z.S.; Napolitano, A.; Pagano, I.; Negi, V.S.; et al. HMGB1 Targeting by Ethyl Pyruvate Suppresses Malignant Phenotype of Human Mesothelioma. Oncotarget 2017, 8, 22649–22661. [Google Scholar] [CrossRef] [PubMed]
- Jube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer Cell Secretion of the DAMP Protein HMGB1 Supports Progression in Malignant Mesothelioma. Cancer Res. 2012, 72, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Affar, E.B.; Carbone, M. BAP1 Regulates Different Mechanisms of Cell Death. Cell Death Dis. 2018, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Amelio, I.; Affar, E.B.; Brugarolas, J.; Cannon-Albright, L.A.; Cantley, L.C.; Cavenee, W.K.; Chen, Z.; Croce, C.M.; Andrea, A.D.; et al. Consensus Report of the 8 and 9th Weinman Symposia on Gene x Environment Interaction in Carcinogenesis: Novel Opportunities for Precision Medicine. Cell Death Differ. 2018, 25, 1885–1904. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.; Cleveland, D.W. Rebuilding Chromosomes After Catastrophe: Emerging Mechanisms of Chromothripsis. Trends Cell Biol. 2017, 27, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Emi, M.; Hashimoto-Tamaoki, T.; Ohmuraya, M.; Sato, A.; Tsujimura, T.; Hasegawa, S.; Nakano, T.; Nasu, M.; Pastorino, S.; et al. High-Density Array-CGH with Targeted NGS Unmask Multiple Noncontiguous Minute Deletions on Chromosome 3p21 in Mesothelioma. Proc. Natl. Acad. Sci. USA 2016, 113, 13432–13437. [Google Scholar] [CrossRef]
- Oey, H.; Daniels, M.; Relan, V.; Chee, T.M.; Davidson, M.R.; Yang, I.A.; Ellis, J.J.; Fong, K.M.; Krause, L.; Bowman, R. V Whole-Genome Sequencing of Human Malignant Mesothelioma Tumours and Cell Lines. Carcinogenesis 2019, 40, 724–734. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Peikert, T.; Smadbeck, J.B.; Udell, J.B.M.; Garcia-Rivera, E.; Elsbernd, L.; Erskine, C.L.; Van Keulen, V.P.; Kosari, F.; Murphy, S.J.; et al. Neoantigenic Potential of Complex Chromosomal Rearrangements in Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 276–287. [Google Scholar] [CrossRef]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef]
- Jongsma, J.; van Montfort, E.; Vooijs, M.; Zevenhoven, J.; Krimpenfort, P.; van der Valk, M.; van de Vijver, M.; Berns, A. A Conditional Mouse Model for Malignant Mesothelioma. Cancer Cell 2008, 13, 261–271. [Google Scholar] [CrossRef]
- Altomare, D.A.; Menges, C.W.; Pei, J.; Zhang, L.; Skele-Stump, K.L.; Carbone, M.; Kane, A.B.; Testa, J.R. Activated TNF-Alpha/NF-KappaB Signaling via down-Regulation of Fas-Associated Factor 1 in Asbestos-Induced Mesotheliomas from Arf Knockout Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sekido, Y. NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma. Int. J. Mol. Sci. 2018, 19, 988. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Vaslet, C.A.; Skele, K.L.; De Rienzo, A.; Devarajan, K.; Jhanwar, S.C.; McClatchey, A.I.; Kane, A.B.; Testa, J.R. A Mouse Model Recapitulating Molecular Features of Human Mesothelioma. Cancer Res. 2005, 65, 8090–8095. [Google Scholar] [CrossRef] [PubMed]
- Rehrauer, H.; Wu, L.; Blum, W.; Pecze, L.; Henzi, T.; Serre-Beinier, V.; Aquino, C.; Vrugt, B.; de Perrot, M.; Schwaller, B.; et al. How Asbestos Drives the Tissue towards Tumors: YAP Activation, Macrophage and Mesothelial Precursor Recruitment, RNA Editing, and Somatic Mutations. Oncogene 2018, 37, 2645–2659. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, T.; Obenauf, A.C.; Murali, R.; Fried, I.; Griewank, K.G.; Ulz, P.; Windpassinger, C.; Wackernagel, W.; Loy, S.; Wolf, I.; et al. Germline Mutations in BAP1 Predispose to Melanocytic Tumors. Nat. Genet. 2011, 43, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Bononi, A.; Giorgi, C.; Patergnani, S.; Larson, D.; Verbruggen, K.; Tanji, M.; Pellegrini, L.; Signorato, V.; Olivetto, F.; Pastorino, S.; et al. BAP1 Regulates IP3R3-Mediated Ca(2+) Flux to Mitochondria Suppressing Cell Transformation. Nature 2017, 546, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 Mutations Predispose to Malignant Mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef]
- Bononi, A.; Yang, H.; Giorgi, C.; Patergnani, S.; Pellegrini, L.; Su, M.; Xie, G.; Signorato, V.; Pastorino, S.; Morris, P.; et al. Germline BAP1 Mutations Induce a Warburg Effect. Cell Death Differ. 2017, 24, 1694–1704. [Google Scholar] [CrossRef]
- Finn, R.S.; Brims, F.J.H.; Gandhi, A.; Olsen, N.; Musk, A.W.; Maskell, N.A.; Lee, Y.C.G. Postmortem Findings of Malignant Pleural Mesothelioma: A Two-Center Study of 318 Patients. Chest 2012, 142, 1267–1273. [Google Scholar] [CrossRef]
- Cardinale, L.; Ardissone, F.; Gned, D.; Sverzellati, N.; Piacibello, E.; Veltri, A. Diagnostic Imaging and Workup of Malignant Pleural Mesothelioma. Acta Biomed. 2017, 88, 134–142. [Google Scholar] [CrossRef]
- Bianco, A.; Valente, T.; de Rimini, M.L.; Sica, G.; Fiorelli, A. Clinical Diagnosis of Malignant Pleural Mesothelioma. J. Thorac. Dis. 2018, 10, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Patz, E.F.J.; Shaffer, K.; Piwnica-Worms, D.R.; Jochelson, M.; Sarin, M.; Sugarbaker, D.J.; Pugatch, R.D. Malignant Pleural Mesothelioma: Value of CT and MR Imaging in Predicting Resectability. AJR Am. J. Roentgenol. 1992, 159, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Heelan, R.T.; Rusch, V.W.; Begg, C.B.; Panicek, D.M.; Caravelli, J.F.; Eisen, C. Staging of Malignant Pleural Mesothelioma: Comparison of CT and MR Imaging. AJR Am. J. Roentgenol. 1999, 172, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Sandach, P.; Seifert, R.; Fendler, W.P.; Hautzel, H.; Herrmann, K.; Maier, S.; Plönes, T.; Metzenmacher, M.; Ferdinandus, J. A Role for PET/CT in Response Assessment of Malignant Pleural Mesothelioma. Semin. Nucl. Med. 2022, 52, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, H.; Metintas, M.; Entok, E.; Ak, G.; Ak, I.; Dundar, E.; Erginel, S. Clinical Value of Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in Differentiation of Malignant Mesothelioma from Asbestos-Related Benign Pleural Disease: An Observational Pilot Study. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2009, 4, 1480–1484. [Google Scholar] [CrossRef]
- Frauenfelder, T.; Kestenholz, P.; Hunziker, R.; Nguyen, T.D.L.; Fries, M.; Veit-Haibach, P.; Husmann, L.; Stahel, R.; Weder, W.; Opitz, I. Use of Computed Tomography and Positron Emission Tomography/Computed Tomography for Staging of Local Extent in Patients with Malignant Pleural Mesothelioma. J. Comput. Assist. Tomogr. 2015, 39, 160–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basu, S.; Saboury, B.; Torigian, D.A.; Alavi, A. Current Evidence Base of FDG-PET/CT Imaging in the Clinical Management of Malignant Pleural Mesothelioma: Emerging Significance of Image Segmentation and Global Disease Assessment. Mol. Imaging Biol. 2011, 13, 801–811. [Google Scholar] [CrossRef]
- Sharif, S.; Zahid, I.; Routledge, T.; Scarci, M. Does Positron Emission Tomography Offer Prognostic Information in Malignant Pleural Mesothelioma? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 806–811. [Google Scholar] [CrossRef]
- Yamamuro, M.; Gerbaudo, V.H.; Gill, R.R.; Jacobson, F.L.; Sugarbaker, D.J.; Hatabu, H. Morphologic and Functional Imaging of Malignant Pleural Mesothelioma. Eur. J. Radiol. 2007, 64, 356–366. [Google Scholar] [CrossRef]
- Qureshi, N.R.; Rahman, N.M.; Gleeson, F.V. Thoracic Ultrasound in the Diagnosis of Malignant Pleural Effusion. Thorax 2009, 64, 139–143. [Google Scholar] [CrossRef]
- Henderson, D.W.; Reid, G.; Kao, S.C.; van Zandwijk, N.; Klebe, S. Challenges and Controversies in the Diagnosis of Mesothelioma: Part 1. Cytology-Only Diagnosis, Biopsies, Immunohistochemistry, Discrimination between Mesothelioma and Reactive Mesothelial Hyperplasia, and Biomarkers. J. Clin. Pathol. 2013, 66, 847–853. [Google Scholar] [CrossRef]
- Baas, P.; Fennell, D.; Kerr, K.M.; Van Schil, P.E.; Haas, R.L.; Peters, S. Malignant Pleural Mesothelioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26 (Suppl. 5), v31–v39. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Chansky, K.; Rice, D.C.; Pass, H.I.; Kindler, H.L.; Shemanski, L.; Billé, A.; Rintoul, R.C.; Batirel, H.F.; Thomas, C.F.; et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 2089–2099. [Google Scholar] [CrossRef]
- Rice, D.; Chansky, K.; Nowak, A.; Pass, H.; Kindler, H.; Shemanski, L.; Opitz, I.; Call, S.; Hasegawa, S.; Kernstine, K.; et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant Pleural Mesothelioma: An Update on Investigation, Diagnosis and Treatment. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2016, 25, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Sauter, J.L.; Dacic, S.; Galateau-Salle, F.; Attanoos, R.L.; Butnor, K.J.; Churg, A.; Husain, A.N.; Kadota, K.; Khoor, A.; Nicholson, A.G.; et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 608–622. [Google Scholar] [CrossRef]
- Mastromarino, M.G.; Lenzini, A.; Aprile, V.; Alì, G.; Bacchin, D.; Korasidis, S.; Ambrogi, M.C.; Lucchi, M. New Insights in Pleural Mesothelioma Classification Update: Diagnostic Traps and Prognostic Implications. Diagnostics 2022, 12, 2905. [Google Scholar] [CrossRef]
- Hysi, I.; Le Pimpec-Barthes, F.; Alifano, M.; Venissac, N.; Mouroux, J.; Regnard, J.-F.; Riquet, M.; Porte, H. Lymph Node Involvement and Metastatic Lymph Node Ratio Influence the Survival of Malignant Pleural Mesothelioma: A French Multicenter Retrospective Study. Oncol. Rep. 2014, 31, 415–421. [Google Scholar] [CrossRef]
- Berzenji, L.; Van Schil, P.E.; Carp, L. The Eighth TNM Classification for Malignant Pleural Mesothelioma. Transl. Lung Cancer Res. 2018, 7, 543–549. [Google Scholar] [CrossRef]
- Lim, E.; Darlison, L.; Edwards, J.; Elliott, D.; Fennell, D.A.; Popat, S.; Rintoul, R.C.; Waller, D.; Ali, C.; Bille, A.; et al. Mesothelioma and Radical Surgery 2 (MARS 2): Protocol for a Multicentre Randomised Trial Comparing (Extended) Pleurectomy Decortication versus No (Extended) Pleurectomy Decortication for Patients with Malignant Pleural Mesothelioma. BMJ Open 2020, 10, e038892. [Google Scholar] [CrossRef]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-Pleural Pneumonectomy versus No Extra-Pleural Pneumonectomy for Patients with Malignant Pleural Mesothelioma: Clinical Outcomes of the Mesothelioma and Radical Surgery (MARS) Randomised Feasibility Study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- Rice, D.; Rusch, V.; Pass, H.; Asamura, H.; Nakano, T.; Edwards, J.; Giroux, D.J.; Hasegawa, S.; Kernstine, K.H.; Waller, D.; et al. Recommendations for Uniform Definitions of Surgical Techniques for Malignant Pleural Mesothelioma: A Consensus Report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interes. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1304–1312. [Google Scholar] [CrossRef]
- Flores, R.M.; Pass, H.I.; Seshan, V.E.; Dycoco, J.; Zakowski, M.; Carbone, M.; Bains, M.S.; Rusch, V.W. Extrapleural Pneumonectomy versus Pleurectomy/Decortication in the Surgical Management of Malignant Pleural Mesothelioma: Results in 663 Patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 620–626.e3. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Giroux, D.; Kennedy, C.; Ruffini, E.; Cangir, A.K.; Rice, D.; Pass, H.; Asamura, H.; Waller, D.; Edwards, J.; et al. Initial Analysis of the International Association for the Study of Lung Cancer Mesothelioma Database. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Richards, W.G.; Bueno, R. Extrapleural Pneumonectomy in the Treatment of Epithelioid Malignant Pleural Mesothelioma: Novel Prognostic Implications of Combined N1 and N2 Nodal Involvement Based on Experience in 529 Patients. Ann. Surg. 2014, 260, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., 3rd; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef]
- McMillan, R.R.; Berger, A.; Sima, C.S.; Lou, F.; Dycoco, J.; Rusch, V.; Rizk, N.P.; Jones, D.R.; Huang, J. Thirty-Day Mortality Underestimates the Risk of Early Death after Major Resections for Thoracic Malignancies. Ann. Thorac. Surg. 2014, 98, 1765–1769. [Google Scholar] [CrossRef][Green Version]
- Batirel, H.F.; Metintas, M.; Caglar, H.B.; Ak, G.; Yumuk, P.F.; Yildizeli, B.; Yuksel, M. Adoption of Pleurectomy and Decortication for Malignant Mesothelioma Leads to Similar Survival as Extrapleural Pneumonectomy. J. Thorac. Cardiovasc. Surg. 2016, 151, 478–484. [Google Scholar] [CrossRef]
- Cho, B.C.J.; Feld, R.; Leighl, N.; Opitz, I.; Anraku, M.; Tsao, M.-S.; Hwang, D.M.; Hope, A.; de Perrot, M. A Feasibility Study Evaluating Surgery for Mesothelioma After Radiation Therapy: The “SMART” Approach for Resectable Malignant Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 397–402. [Google Scholar] [CrossRef]
- Falanga, F.; Rinaldi, P.; Primiceri, C.; Bortolotto, C.; Oneta, O.; Agustoni, F.; Morbini, P.; Saracino, L.; Eleftheriou, D.; Sottotetti, F.; et al. Feasibility and Safety of Extended Pleurectomy/Decortication for Malignant Pleural Mesothelioma. A Single Group Experience. Thorac. cancer 2022, 13, 2792–2798. [Google Scholar] [CrossRef]
- de Perrot, M.; Dong, Z.; Bradbury, P.; Patsios, D.; Keshavjee, S.; Leighl, N.B.; Hope, A.; Feld, R.; Cho, J. Impact of Tumour Thickness on Survival after Radical Radiation and Surgery in Malignant Pleural Mesothelioma. Eur. Respir. J. 2017, 49, 1601428. [Google Scholar] [CrossRef]
- van Ruth, S.; Baas, P.; Haas, R.L.M.; Rutgers, E.J.T.; Verwaal, V.J.; Zoetmulder, F.A.N. Cytoreductive Surgery Combined with Intraoperative Hyperthermic Intrathoracic Chemotherapy for Stage I Malignant Pleural Mesothelioma. Ann. Surg. Oncol. 2003, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; DeLaney, T.F.; Tochner, Z.; Smith, P.E.; Temeck, B.K.; Pogrebniak, H.W.; Kranda, K.C.; Russo, A.; Friauf, W.S.; Cole, J.W. Intrapleural Photodynamic Therapy: Results of a Phase I Trial. Ann. Surg. Oncol. 1994, 1, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Murrer, L.; Zoetmulder, F.A.; Stewart, F.A.; Ris, H.B.; van Zandwijk, N.; Peterse, J.L.; Rutgers, E.J. Photodynamic Therapy as Adjuvant Therapy in Surgically Treated Pleural Malignancies. Br. J. Cancer 1997, 76, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for Newly Diagnosed Pleural Mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Tsao, A.S.; Lindwasser, O.W.; Adjei, A.A.; Adusumilli, P.S.; Beyers, M.L.; Blumenthal, G.M.; Bueno, R.; Burt, B.M.; Carbone, M.; Dahlberg, S.E.; et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1655–1667. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive Genomic Analysis of Malignant Pleural Mesothelioma Identifies Recurrent Mutations, Gene Fusions and Splicing Alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Mutti, L.; Peikert, T.; Robinson, B.W.S.; Scherpereel, A.; Tsao, A.S.; de Perrot, M.; Woodard, G.A.; Jablons, D.M.; Wiens, J.; Hirsch, F.R.; et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1269–1283. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical Safety and Activity of Pembrolizumab in Patients with Malignant Pleural Mesothelioma (KEYNOTE-028): Preliminary Results from a Non-Randomised, Open-Label, Phase 1b Trial. Lancet. Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Metaxas, Y.; Rivalland, G.; Mauti, L.A.; Klingbiel, D.; Kao, S.; Schmid, S.; Nowak, A.K.; Gautschi, O.; Bartnick, T.; Hughes, B.G.; et al. Pembrolizumab as Palliative Immunotherapy in Malignant Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.-S.; Brown, C.; Hughes, B.G.; Karikios, D.J.; John, T.; Kao, S.C.-H.; Leslie, C.; Cook, A.M.; et al. Durvalumab with First-Line Chemotherapy in Previously Untreated Malignant Pleural Mesothelioma (DREAM): A Multicentre, Single-Arm, Phase 2 Trial with a Safety Run-In. Lancet. Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-Line Nivolumab plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus Placebo in Patients with Relapsed Malignant Mesothelioma (CONFIRM): A Multicentre, Double-Blind, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.; Zheng, H.; Adusumilli, P.S. Immunotherapy for Malignant Pleural Mesothelioma: Current Status and Future Directions. Transl. Lung Cancer Res. 2017, 6, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.S.; Forde, P.M.; Hughes, B.; Sun, Z.; Brown, C.; Ramalingam, S.; Cook, A.; Lesterhuis, W.J.; Yip, S.; O’Byrne, K.; et al. Protocol of DREAM3R: DuRvalumab with ChEmotherapy as First-Line TreAtment in Advanced Pleural Mesothelioma-a Phase 3 Randomised Trial. BMJ Open 2022, 12, e057663. [Google Scholar] [CrossRef]
- Belderbos, R.A.; Baas, P.; Berardi, R.; Cornelissen, R.; Fennell, D.A.; Van Meerbeeck, J.P.; Scherpereel, A.; Vroman, H.; Aerts, J.G.J.V. A Multicenter, Randomized, Phase II/III Study of Dendritic Cells Loaded with Allogeneic Tumor Cell Lysate (MesoPher) in Subjects with Mesothelioma as Maintenance Therapy after Chemotherapy: DENdritic Cell Immunotherapy for Mesothelioma (DENIM) Trial. Transl. Lung Cancer Res. 2019, 8, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, D.W.; Cornelissen, R.; Bezemer, K.; Baart, S.J.; Aerts, J.G.J.V. Long-Term Follow-up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase i/Ii Trials. Vaccines 2021, 9, 525. [Google Scholar] [CrossRef]
- Field, G.C.; Pavlyk, I.; Szlosarek, P.W. Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules 2023, 28, 2150. [Google Scholar] [CrossRef]
- Chintala, N.K.; Restle, D.; Quach, H.; Saini, J.; Bellis, R.; Offin, M.; Beattie, J.; Adusumilli, P.S. CAR T-Cell Therapy for Pleural Mesothelioma: Rationale, Preclinical Development, and Clinical Trials. Lung Cancer 2021, 157, 48–59. [Google Scholar] [CrossRef]
- Romero, D. Uncovering Adagrasib Resistance CAR T Cells Show Promise in Mesothelioma. Nat. Rev. Clin. Oncol. 2021, 18, 541. [Google Scholar] [CrossRef]
- Klampatsa, A.; Dimou, V.; Albelda, S.M. Mesothelin-Targeted CAR-T Cell Therapy for Solid Tumors. Expert Opin. Biol. Ther. 2021, 21, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II Clinical Trial of Amatuximab, a Chimeric Antimesothelin Antibody with Pemetrexed and Cisplatin in Advanced Unresectable Pleural Mesothelioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab Ravtansine: A Novel Mesothelin-Targeting Antibody-Drug Conjugate Cures Tumors with Heterogeneous Target Expression Favored by Bystander Effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef]
- Hassan, R.; Blumenschein, G.R.J.; Moore, K.N.; Santin, A.D.; Kindler, H.L.; Nemunaitis, J.J.; Seward, S.M.; Thomas, A.; Kim, S.K.; Rajagopalan, P.; et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Y.; Subramanyam, B.; Sarapa, N.; Golfier, S.; Dinter, H. Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors. Clin. Cancer Drugs 2016, 3, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hollevoet, K.; Mason-Osann, E.; Liu, X.; Imhof-Jung, S.; Niederfellner, G.; Pastan, I. In Vitro and in Vivo Activity of the Low-Immunogenic Antimesothelin Immunotoxin RG7787 in Pancreatic Cancer. Mol. Cancer Ther. 2014, 13, 2040–2049. [Google Scholar] [CrossRef]
- Sivick, K.E.; Desbien, A.L.; Glickman, L.H.; Reiner, G.L.; Corrales, L.; Surh, N.H.; Hudson, T.E.; Vu, U.T.; Francica, B.J.; Banda, T.; et al. Magnitude of Therapeutic STING Activation Determines CD8(+) T Cell-Mediated Anti-Tumor Immunity. Cell Rep. 2018, 25, 3074–3085.e5. [Google Scholar] [CrossRef]
- Marcus, A.; Mao, A.J.; Lensink-Vasan, M.; Wang, L.; Vance, R.E.; Raulet, D.H. Tumor-Derived CGAMP Triggers a STING-Mediated Interferon Response in Non-Tumor Cells to Activate the NK Cell Response. Immunity 2018, 49, 754–763.e4. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-Cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D.; et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018, 8, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Voabil, P.; de Bruijn, M.; Roelofsen, L.M.; Hendriks, S.H.; Brokamp, S.; van den Braber, M.; Broeks, A.; Sanders, J.; Herzig, P.; Zippelius, A.; et al. An Ex Vivo Tumor Fragment Platform to Dissect Response to PD-1 Blockade in Cancer. Nat. Med. 2021, 27, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Saha, S.; Bettke, J.; Nagar, R.; Parrales, A.; Iwakuma, T.; van der Velden, A.W.M.; Martinez, L.A. Mutant P53 Suppresses Innate Immune Signaling to Promote Tumorigenesis. Cancer Cell 2021, 39, 494–508.e5. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.; Gray, E.E.; Brunette, R.L.; Stetson, D.B. DNA Tumor Virus Oncogenes Antagonize the CGAS-STING DNA-Sensing Pathway. Science 2015, 350, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Knelson, E.H.; Ivanova, E.V.; Tarannum, M.; Campisi, M.; Lizotte, P.H.; Booker, M.A.; Ozgenc, I.; Noureddine, M.; Meisenheimer, B.; Chen, M.; et al. Activation of Tumor-Cell STING Primes NK-Cell Therapy. Cancer Immunol. Res. 2022, 10, 947–961. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Palmer, D.C.; Robeson, A.C.; Shou, P.; Bommiasamy, H.; Laurie, S.J.; Willis, C.; Dotti, G.; Vincent, B.G.; Restifo, N.P.; et al. STING Agonist Promotes CAR T Cell Trafficking and Persistence in Breast Cancer. J. Exp. Med. 2021, 218, e20200844. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zou, J.; Lai, C.-T.; Zeng, T.; Peng, J.; Zou, W.; Cao, B.; Liu, D.; Zhu, L.-Y.; et al. Comprehensive Analysis of the Glutathione S-Transferase Mu (GSTM) Gene Family in Ovarian Cancer Identifies Prognostic and Expression Significance. Front. Oncol. 2022, 12, 968547. [Google Scholar] [CrossRef]
- Cerne, D.; Melkic, E.; Trost, Z.; Sok, M.; Marc, J. Lipoprotein Lipase Activity and Gene Expression in Lung Cancer and in Adjacent Noncancer Lung Tissue. Exp. Lung Res. 2007, 33, 217–225. [Google Scholar] [CrossRef]
- Trempolec, N.; Degavre, C.; Doix, B.; Brusa, D.; Corbet, C.; Feron, O. Acidosis-Induced TGF-Β2 Production Promotes Lipid Droplet Formation in Dendritic Cells and Alters Their Potential to Support Anti-Mesothelioma T Cell Response. Cancers 2020, 12, 1284. [Google Scholar] [CrossRef]
- Englinger, B.; Laemmerer, A.; Moser, P.; Kallus, S.; Röhrl, C.; Pirker, C.; Baier, D.; Mohr, T.; Niederstaetter, L.; Meier-Menches, S.M.; et al. Lipid Droplet-Mediated Scavenging as Novel Intrinsic and Adaptive Resistance Factor against the Multikinase Inhibitor Ponatinib. Int. J. Cancer 2020, 147, 1680–1693. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.; Pickup, M.; Pang, Y.; Gorska, A.E.; Li, Z.; Chytil, A.; Geng, Y.; Gray, J.W.; Moses, H.L.; Yang, L. Gr-1+CD11b+ Myeloid Cells Tip the Balance of Immune Protection to Tumor Promotion in the Premetastatic Lung. Cancer Res. 2010, 70, 6139–6149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Zhang, L.; Chen, Y.; Ha, M. LINC01140 Inhibits Nonsmall Cell Lung Cancer Progression and Cisplatin Resistance through the MiR-4742-5p/TACC1 Axis. J. Biochem. Mol. Toxicol. 2022, 36, e23048. [Google Scholar] [CrossRef]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Chew, S.H.; Toyokuni, S. Malignant Mesothelioma as an Oxidative Stress-Induced Cancer: An Update. Free Radic. Biol. Med. 2015, 86, 166–178. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.-W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular Matrix Stiffness and Composition Jointly Regulate the Induction of Malignant Phenotypes in Mammary Epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-Associated Extracellular Matrix Genes Link Cancer-Associated Fibroblasts to Immune Evasion and Immunotherapy Failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Ollila, H.; Mäyränpää, M.I.; Paavolainen, L.; Paajanen, J.; Välimäki, K.; Sutinen, E.; Wolff, H.; Räsänen, J.; Kallioniemi, O.; Myllärniemi, M.; et al. Prognostic Role of Tumor Immune Microenvironment in Pleural Epithelioid Mesothelioma. Front. Oncol. 2022, 12, 870352. [Google Scholar] [CrossRef] [PubMed]
- Creaney, J.; Patch, A.-M.; Addala, V.; Sneddon, S.A.; Nones, K.; Dick, I.M.; Lee, Y.C.G.; Newell, F.; Rouse, E.J.; Naeini, M.M.; et al. Comprehensive Genomic and Tumour Immune Profiling Reveals Potential Therapeutic Targets in Malignant Pleural Mesothelioma. Genome Med. 2022, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Lievense, L.A.; Cornelissen, R.; Bezemer, K.; Kaijen-Lambers, M.E.H.; Hegmans, J.P.J.J.; Aerts, J.G.J. V Pleural Effusion of Patients with Malignant Mesothelioma Induces Macrophage-Mediated T Cell Suppression. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.F.; Vaitsi, P.C.; Pappas, A.G.; Iliopoulou, M.; Kosti, C.N.; Psarra, K.; Kalomenidis, I.T. CSF1/CSF1R Axis Blockade Limits Mesothelioma and Enhances Efficiency of Anti-PDL1 Immunotherapy. Cancers 2021, 13, 2546. [Google Scholar] [CrossRef]
- Torricelli, F.; Donati, B.; Reggiani, F.; Manicardi, V.; Piana, S.; Valli, R.; Lococo, F.; Ciarrocchi, A. Spatially Resolved, High-Dimensional Transcriptomics Sorts out the Evolution of Biphasic Malignant Pleural Mesothelioma: New Paradigms for Immunotherapy. Mol. Cancer 2023, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Panou, V.; Gadiraju, M.; Wolin, A.; Weipert, C.M.; Skarda, E.; Husain, A.N.; Patel, J.D.; Rose, B.; Zhang, S.R.; Weatherly, M.; et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Chmielecki, J.; Goparaju, C.; Heguy, A.; Dolgalev, I.; Carbone, M.; Seepo, S.; Meyerson, M.; Pass, H.I. Whole-Exome Sequencing Reveals Frequent Genetic Alterations in BAP1, NF2, CDKN2A, and CUL1 in Malignant Pleural Mesothelioma. Cancer Res. 2015, 75, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Schelch, K.; Emminger, D.; Zitta, B.; Johnson, T.G.; Kopatz, V.; Eder, S.; Ries, A.; Stefanelli, A.; Heffeter, P.; Hoda, M.A.; et al. Targeting YB-1 via Entinostat Enhances Cisplatin Sensitivity of Pleural Mesothelioma in Vitro and in Vivo. Cancer Lett. 2023, 574, 216395. [Google Scholar] [CrossRef]
- Bott, M.; Brevet, M.; Taylor, B.S.; Shimizu, S.; Ito, T.; Wang, L.; Creaney, J.; Lake, R.A.; Zakowski, M.F.; Reva, B.; et al. The Nuclear Deubiquitinase BAP1 Is Commonly Inactivated by Somatic Mutations and 3p21.1 Losses in Malignant Pleural Mesothelioma. Nat. Genet. 2011, 43, 668–672. [Google Scholar] [CrossRef]
- Srinivasan, G.; Sidhu, G.S.; Williamson, E.A.; Jaiswal, A.S.; Najmunnisa, N.; Wilcoxen, K.; Jones, D.; George, T.J.J.; Hromas, R. Synthetic Lethality in Malignant Pleural Mesothelioma with PARP1 Inhibition. Cancer Chemother. Pharmacol. 2017, 80, 861–867. [Google Scholar] [CrossRef]
- Passiglia, F.; Bironzo, P.; Righi, L.; Listì, A.; Arizio, F.; Novello, S.; Volante, M.; Scagliotti, G.V. A Prospective Phase II Single-Arm Study of Niraparib Plus Dostarlimab in Patients With Advanced Non-Small-Cell Lung Cancer and/or Malignant Pleural Mesothelioma, Positive for PD-L1 Expression and Germline or Somatic Mutations in the DNA Repair Genes. Rat. Clin. Lung Cancer 2021, 22, e63–e66. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; King, A.; Mohammed, S.; Branson, A.; Brookes, C.; Darlison, L.; Dawson, A.G.; Gaba, A.; Hutka, M.; Morgan, B.; et al. Rucaparib in Patients with BAP1-Deficient or BRCA1-Deficient Mesothelioma (MiST1): An Open-Label, Single-Arm, Phase 2a Clinical Trial. Lancet. Respir. Med. 2021, 9, 593–600. [Google Scholar] [CrossRef]
- Parisi, A.; Rossi, F.; De Filippis, C.; Paoloni, F.; Felicetti, C.; Mammarella, A.; Pecci, F.; Lupi, A.; Berardi, R. Current Evidence and Future Perspectives about the Role of PARP Inhibitors in the Treatment of Thoracic Cancers. Onco. Targets. Ther. 2023, 16, 585–613. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet. Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Witta, S.E.; Jotte, R.M.; Konduri, K.; Neubauer, M.A.; Spira, A.I.; Ruxer, R.L.; Varella-Garcia, M.; Bunn, P.A.J.; Hirsch, F.R. Randomized Phase II Trial of Erlotinib with and without Entinostat in Patients with Advanced Non-Small-Cell Lung Cancer Who Progressed on Prior Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Juergens, R.A.; Wrangle, J.; Vendetti, F.P.; Murphy, S.C.; Zhao, M.; Coleman, B.; Sebree, R.; Rodgers, K.; Hooker, C.M.; Franco, N.; et al. Combination Epigenetic Therapy Has Efficacy in Patients with Refractory Advanced Non-Small Cell Lung Cancer. Cancer Discov. 2011, 1, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Qi, Q.; Peng, X.; Yan, L.; Takabe, K.; Hait, N.C. Class I Histone Deacetylase Inhibitor Suppresses Vasculogenic Mimicry by Enhancing the Expression of Tumor Suppressor and Anti-Angiogenesis Genes in Aggressive Human TNBC Cells. Int. J. Oncol. 2019, 55, 116–130. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Somasekharan, S.P.; Wang, Y.; Cheng, H.; Negri, G.L.; Pan, M.; Wang, X.Q.; Delaidelli, A.; Rafn, B.; Cran, J.; et al. Class I HDAC Inhibitors Enhance YB-1 Acetylation and Oxidative Stress to Block Sarcoma Metastasis. EMBO Rep. 2019, 20, e48375. [Google Scholar] [CrossRef]
- Sidiropoulos, D.N.; Rafie, C.I.; Jang, J.K.; Castanon, S.; Baugh, A.G.; Gonzalez, E.; Christmas, B.J.; Narumi, V.H.; Davis-Marcisak, E.F.; Sharma, G.; et al. Entinostat Decreases Immune Suppression to Promote Antitumor Responses in a HER2+ Breast Tumor Microenvironment. Cancer Immunol. Res. 2022, 10, 656–669. [Google Scholar] [CrossRef]
- Truong, A.S.; Zhou, M.; Krishnan, B.; Utsumi, T.; Manocha, U.; Stewart, K.G.; Beck, W.; Rose, T.L.; Milowsky, M.I.; He, X.; et al. Entinostat Induces Antitumor Immune Responses through Immune Editing of Tumor Neoantigens. J. Clin. Investig. 2021, 131, e138560. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccio, F.R.; Agustoni, F.; Galli, G.; Bortolotto, C.; Saddi, J.; Baietto, G.; Baio, N.; Montini, S.; Putignano, P.; D’Ambrosio, G.; et al. Pleural Mesothelioma: Treatable Traits of a Heterogeneous Disease. Cancers 2023, 15, 5731. https://doi.org/10.3390/cancers15245731
Bertuccio FR, Agustoni F, Galli G, Bortolotto C, Saddi J, Baietto G, Baio N, Montini S, Putignano P, D’Ambrosio G, et al. Pleural Mesothelioma: Treatable Traits of a Heterogeneous Disease. Cancers. 2023; 15(24):5731. https://doi.org/10.3390/cancers15245731
Chicago/Turabian StyleBertuccio, Francesco Rocco, Francesco Agustoni, Giulia Galli, Chandra Bortolotto, Jessica Saddi, Guido Baietto, Nicola Baio, Simone Montini, Paola Putignano, Gioacchino D’Ambrosio, and et al. 2023. "Pleural Mesothelioma: Treatable Traits of a Heterogeneous Disease" Cancers 15, no. 24: 5731. https://doi.org/10.3390/cancers15245731
APA StyleBertuccio, F. R., Agustoni, F., Galli, G., Bortolotto, C., Saddi, J., Baietto, G., Baio, N., Montini, S., Putignano, P., D’Ambrosio, G., Corsico, A. G., Pedrazzoli, P., & Stella, G. M. (2023). Pleural Mesothelioma: Treatable Traits of a Heterogeneous Disease. Cancers, 15(24), 5731. https://doi.org/10.3390/cancers15245731






