Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Model
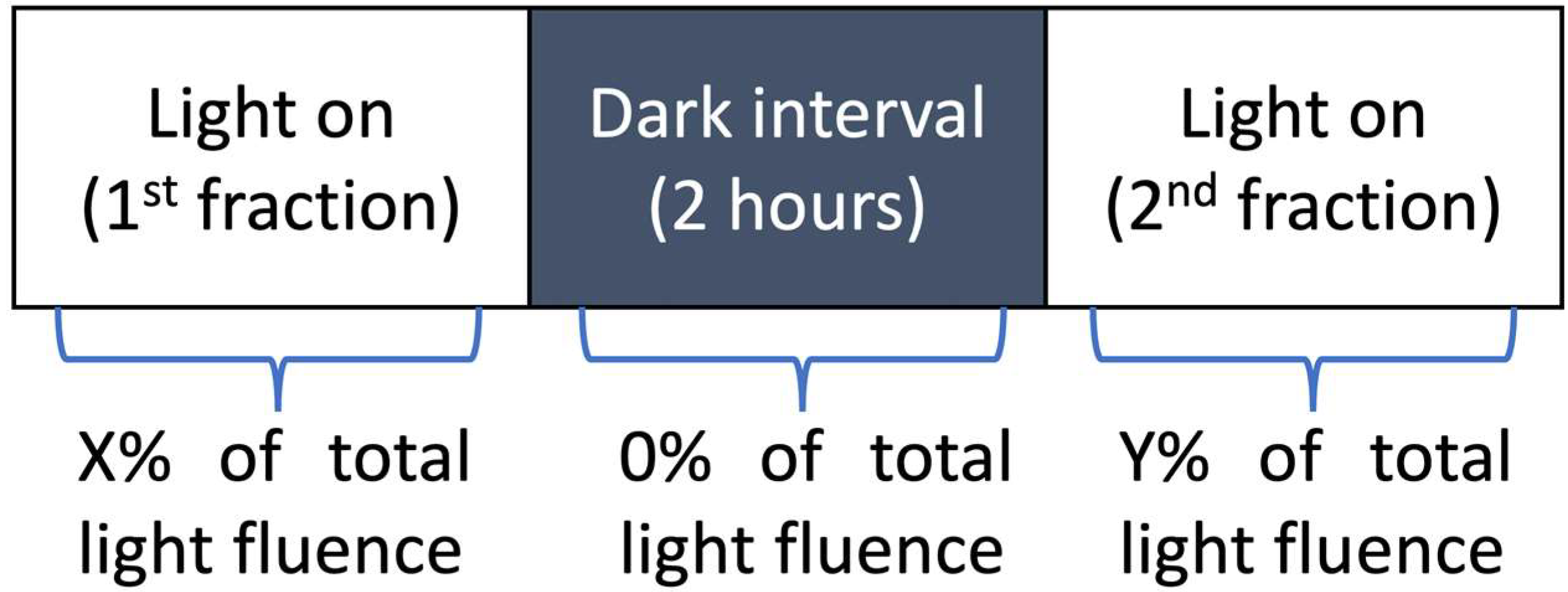
2.2. Photodynamic Therapy Protocol
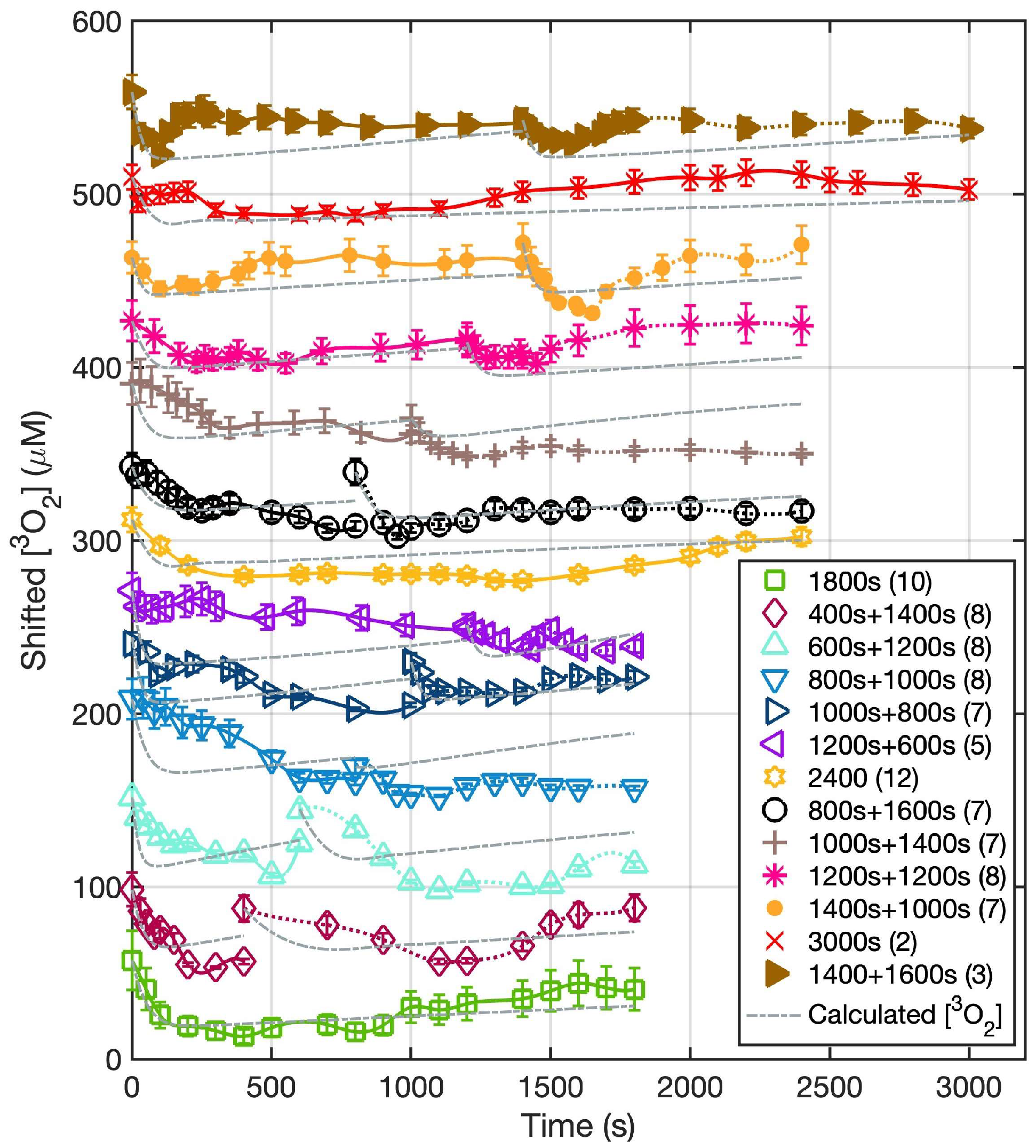
2.3. Measurement of the Oxygen Concentration in Tumors
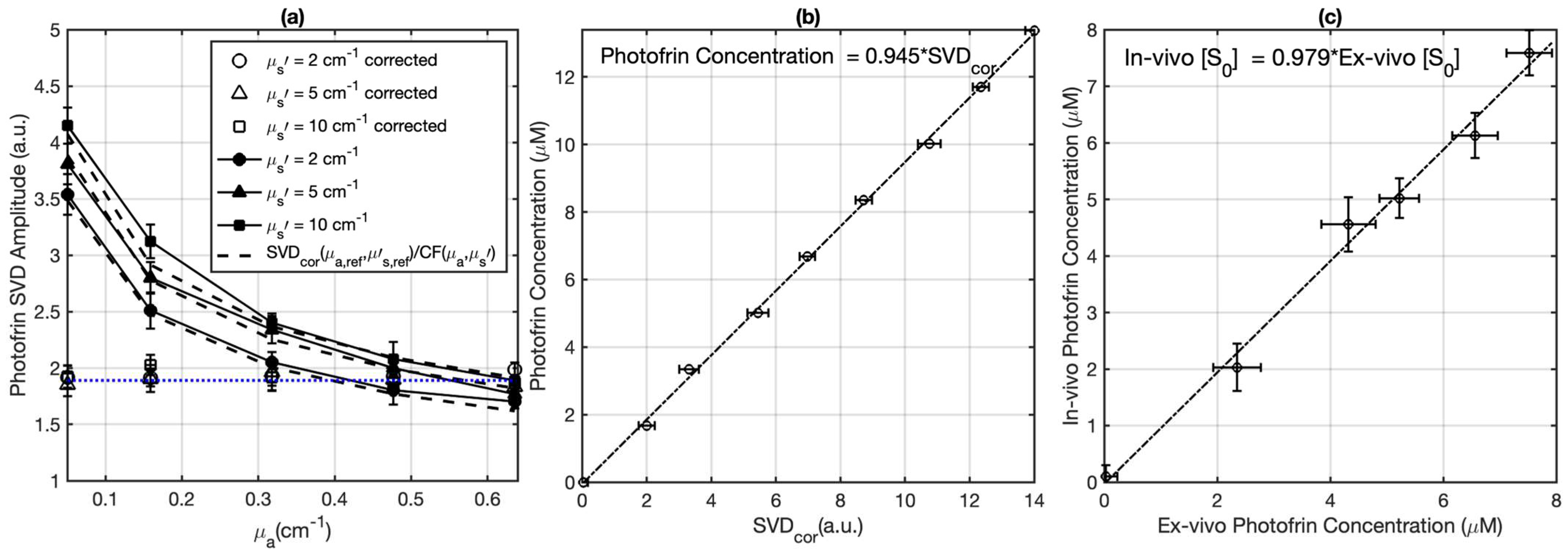
2.4. Validation and Measurement of the Photofrin Concentration in Tumors
2.5. Macroscopic Singlet Oxygen Model for PDT
2.6. Photodynamic Therapy Efficiency Assessment
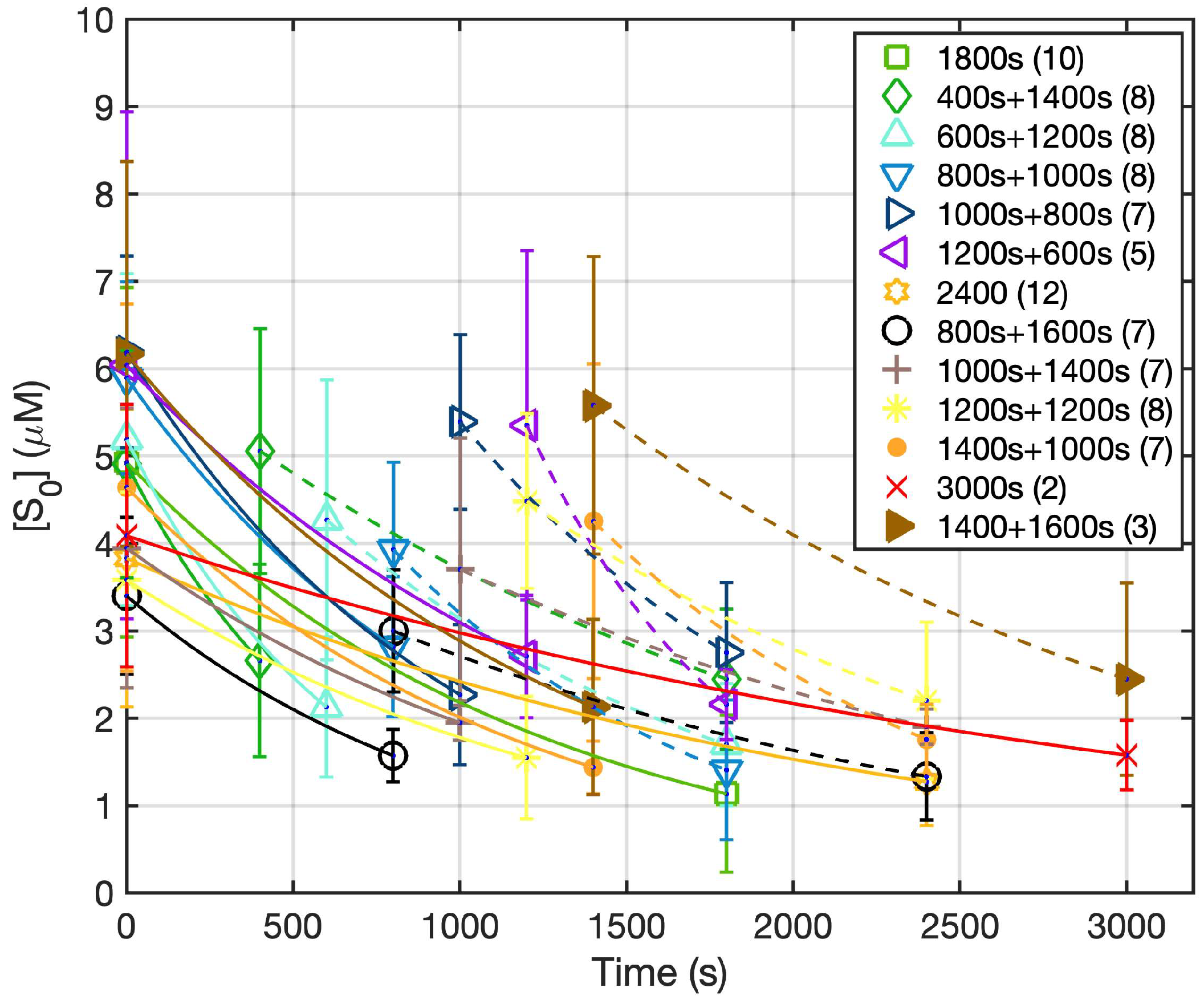
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.A.; Wolfsen, H.C. The role of photodynamic therapy in the esophagus. Gastrointest. Endosc. Clin. N. Am. 2010, 20, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Prazmo, E.J.; Kwasny, M.; Lapinski, M.; Mielczarek, A. Photodynamic Therapy As a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic therapy to control microbial biofilms. Photodiagnosis Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Wang, L.W.; Huang, Z.; Lin, H.; Li, Z.S.; Hetzel, F.; Liu Md, B. Effect of Photofrin-mediated photocytotoxicity on a panel of human pancreatic cancer cells. Photodiagnosis Photodyn. Ther. 2013, 10, 244–251. [Google Scholar] [CrossRef]
- Hajri, A.; Wack, S.; Meyer, C.; Smith, M.K.; Leberquier, C.; Kedinger, M.; Aprahamian, M. In vitro and in vivo efficacy of photofrin and pheophorbide a, a bacteriochlorin, in photodynamic therapy of colonic cancer cells. Photochem. Photobiol. 2002, 75, 140–148. [Google Scholar] [CrossRef]
- Chapman, J.D.; Stobbe, C.C.; Arnfield, M.R.; Santus, R.; Lee, J.; McPhee, M.S. Oxygen dependency of tumor cell killing in vitro by light-activated Photofrin II. Radiat. Res. 1991, 126, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Rainbow, A.J.; Wilson, B.C.; Singh, G. In vivo resistance to photofrin-mediated photodynamic therapy in radiation-induced fibrosarcoma cells resistant to in vitro Photofrin-mediated photodynamic therapy. J. Photochem. Photobiol. B 1999, 49, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sheng, T.; Ong, Y.; Busch, T.M.; Zhu, T.C. Reactive oxygen species explicit dosimetry to predict local tumor growth for Photofrin-mediated photodynamic therapy. Biomed. Opt. Express 2020, 11, 4586–4601. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Kim, M.M.; Penjweini, R.; Zhu, T.C. Macroscopic singlet oxygen modeling for dosimetry of Photofrin-mediated photodynamic therapy: An in-vivo study. J. Biomed. Opt. 2016, 21, 88002. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Curnow, A.; MacRobert, A.J.; Bown, S.G. Comparing and combining light dose fractionation and iron chelation to enhance experimental photodynamic therapy with aminolevulinic acid. Lasers Surg. Med. 2006, 38, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; de Bruijn, H.S.; Star, W.M.; Sterenborg, H.J. Dose and timing of the first light fraction in two-fold illumination schemes for topical ALA-mediated photodynamic therapy of hairless mouse skin. Photochem. Photobiol. 2003, 77, 319–323. [Google Scholar] [CrossRef]
- Tetard, M.C.; Vermandel, M.; Leroy, H.A.; Leroux, B.; Maurage, C.A.; Lejeune, J.P.; Mordon, S.; Reyns, N. Interstitial 5-ALA photodynamic therapy and glioblastoma: Preclinical model development and preliminary results. Photodiagnosis Photodyn. Ther. 2016, 13, 218–224. [Google Scholar] [CrossRef]
- Pinto, M.A.F.; Ferreira, C.B.R.; de Lima, B.E.S.; Molon, A.C.; Ibarra, A.M.C.; Cecatto, R.B.; Dos Santos Franco, A.L.; Rodrigues, M. Effects of 5-ALA mediated photodynamic therapy in oral cancer stem cells. J. Photochem. Photobiol. B 2022, 235, 112552. [Google Scholar] [CrossRef]
- de Vijlder, H.C.; Sterenborg, H.J.; Neumann, H.A.; Robinson, D.J.; de Haas, E.R. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: Five-year follow-up of a randomized, prospective trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; Casas, A.G.; Di Venosa, G.; Gandara, L.; Sterenborg, H.J.; Batlle, A.; Robinson, D.J. Light fractionated ALA-PDT enhances therapeutic efficacy in vitro; the influence of PpIX concentration and illumination parameters. Photochem. Photobiol. Sci. 2013, 12, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J. Clin. Laser Med. Surg. 1996, 14, 289–304. [Google Scholar] [CrossRef]
- Di Venosa, G.; Hermida, L.; Fukuda, H.; Defain, M.V.; Rodriguez, L.; Mamone, L.; MacRobert, A.; Casas, A.; Batlle, A. Comparation of liposomal formulations of ALA Undecanoyl ester for its use in photodynamic therapy. J. Photochem. Photobiol. B 2009, 96, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Hsiao, Y.C.; Chiang, Y.F.; Chang, C.J. Topical application of Photofrin((R)) for photodynamic diagnosis of malignant cutaneous neoplasms. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Schaffer, M.; Schaffer, P.M.; Jori, G.; Corti, L.; Sotti, G.; Hofstetter, A.; Duhmke, E. Radiation therapy combined with photofrin or 5-ALA: Effect on Lewis sarcoma tumor lines implanted in mice. Preliminary results. Tumori 2002, 88, 407–410. [Google Scholar] [CrossRef]
- McCaughan, J.S., Jr. Photodynamic therapy: A review. Drugs Aging 1999, 15, 49–68. [Google Scholar] [CrossRef]
- Schweitzer, V.G.; Somers, M.L. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage (Tis-T2N0M0) SqCCa of oral cavity and oropharynx. Lasers Surg. Med. 2010, 42, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Figueiro Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Cengel, K.A. Photodynamic therapy for lung cancer and malignant pleural mesothelioma. Semin. Oncol. 2014, 41, 820–830. [Google Scholar] [CrossRef]
- Kohoutova, D.; Haidry, R.; Banks, M.; Butt, M.A.; Dunn, J.; Thorpe, S.; Lovat, L. Long-term outcomes of the randomized controlled trial comparing 5-aminolaevulinic acid and Photofrin photodynamic therapy for Barrett’s oesophagus related neoplasia. Scand. J. Gastroenterol. 2018, 53, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.V.; Belchetz, P.E.; Saxena, A.; Igbaseimokumo, U.; Thomson, S.; Nelson, M.; Stringer, M.R.; Holroyd, J.A.; Brown, S.B. Effect of photodynamic therapy on recurrent pituitary adenomas: Clinical phase I/II trial—An early report. Br. J. Neurosurg. 2000, 14, 317–325. [Google Scholar] [CrossRef]
- Busch, T.M.; Xing, X.; Yu, G.; Yodh, A.; Wileyto, E.P.; Wang, H.W.; Durduran, T.; Zhu, T.C.; Wang, K.K. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem. Photobiol. Sci. 2009, 8, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.H.; Kim, M.M.; Finlay, J.C.; Dimofte, A.; Singhal, S.; Glatstein, E.; Cengel, K.A.; Zhu, T.C. PDT dose dosimetry for Photofrin-mediated pleural photodynamic therapy (pPDT). Phys. Med. Biol. 2017, 63, 015031. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Urano, M.; Humm, J.L.; Seshan, V.E.; Li, G.C.; Ling, C.C. Comparison of Helzel and OxyLite systems in the measurements of tumor partial oxygen pressure (pO2). Radiat. Res. 2008, 169, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Penjweini, R.; Kim, M.M.; Ong, Y.H.; Zhu, T.C. (1)O(2) determined from the measured PDT dose and (3)O(2) predicts long-term response to Photofrin-mediated PDT. Phys. Med. Biol. 2020, 65, 03LT01. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.H.; Murant, R.S.; Bryant, R.G.; Knox, R.S.; Gibson, S.L.; Hilf, R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat. Res. 1991, 126, 296–303. [Google Scholar] [CrossRef]
- Hu, X.H.; Feng, Y.; Lu, J.Q.; Allison, R.R.; Cuenca, R.E.; Downie, G.H.; Sibata, C.H. Modeling of a type II photofrin-mediated photodynamic therapy process in a heterogeneous tissue phantom. Photochem. Photobiol. 2005, 81, 1460–1468. [Google Scholar] [CrossRef]
- Wang, K.K.; Finlay, J.C.; Busch, T.M.; Hahn, S.M.; Zhu, T.C. Explicit dosimetry for photodynamic therapy: Macroscopic singlet oxygen modeling. J. Biophotonics 2010, 3, 304–318. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Nichols, M.G.; Foster, T.H. The mechanism of Photofrin photobleaching and its consequences for photodynamic dosimetry. Photochem. Photobiol. 1997, 65, 135–144. [Google Scholar] [CrossRef]
- Nichols, M.G.; Foster, T.H. Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids. Phys. Med. Biol. 1994, 39, 2161–2181. [Google Scholar] [CrossRef] [PubMed]
- Dysart, J.S.; Singh, G.; Patterson, M.S. Calculation of singlet oxygen dose from photosensitizer fluorescence and photobleaching during mTHPC photodynamic therapy of MLL cells. Photochem. Photobiol. 2005, 81, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, J.P.; Gavaghan, D.J.; Hahn, C.E. Mathematical modelling of oxygen transport to tissue. J. Math. Biol. 2002, 44, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Head. Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Q.; Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Yu, Z.; Wang, L.; Zhong, F.; Zhou, H. Real-time imaging mitochondrial viscosity dynamic during mitophagy mediated by photodynamic therapy. Anal. Chim. Acta 2021, 1178, 338847. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammacher, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, e2206121. [Google Scholar] [CrossRef]
- Ong, Y.H.; Zhu, T.C. Analytic function for predicting light fluence rate of circular fields on a semi-infinite turbid medium. Opt. Express 2016, 24, 26261–26281. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.M.; Baumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef]
- Chen, B.; Pogue, B.W.; Goodwin, I.A.; O’Hara, J.A.; Wilmot, C.M.; Hutchins, J.E.; Hoopes, P.J.; Hasan, T. Blood flow dynamics after photodynamic therapy with verteporfin in the RIF-1 tumor. Radiat. Res. 2003, 160, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Takagi, S.; Hoshino, Y.; Okumura, M.; Fujinaga, T. Antitumor effects and blood flow dynamics after photodynamic therapy using benzoporphyrin derivative monoacid ring A in KLN205 and LM8 mouse tumor models. Cancer Lett. 2007, 248, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Choi, B.; Ramirez-San-Juan, J.C.; Nelson, J.S.; Osann, K.; Kelly, K.M. Microvascular blood flow dynamics associated with photodynamic therapy, pulsed dye laser irradiation and combined regimens. Lasers Surg. Med. 2006, 38, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.C.; Mitra, S.; Patterson, M.S.; Foster, T.H. Photobleaching kinetics of Photofrin in vivo and in multicell tumour spheroids indicate two simultaneous bleaching mechanisms. Phys. Med. Biol. 2004, 49, 4837–4860. [Google Scholar] [CrossRef]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; van der Ploeg-van den Heuvel, A.; Sterenborg, H.J.; Robinson, D.J. Fractionated illumination after topical application of 5-aminolevulinic acid on normal skin of hairless mice: The influence of the dark interval. J. Photochem. Photobiol. B 2006, 85, 184–190. [Google Scholar] [CrossRef]
- Penjweini, R.; Kim, M.M.; Liu, B.; Zhu, T.C. Evaluation of the 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide (HPPH) mediated photodynamic therapy by macroscopic singlet oxygen modeling. J. Biophotonics 2016, 9, 1344–1354. [Google Scholar] [CrossRef]
| Parameter | Definition | Value |
|---|---|---|
| ξ (cm2s−1mW−1) | Specific oxygen consumption rate | 3.7 × 10−3 |
| σ (μM−1) | Specific photobleaching ratio | 7.6 × 10−5 |
| δ (μM) | Low-concentration correction | 33 |
| β (μM) | Oxygen quenching threshold concentration | 11.9 |
| g (μM/s) | Macroscopic oxygen maximum perfusion rate | 0.76 |
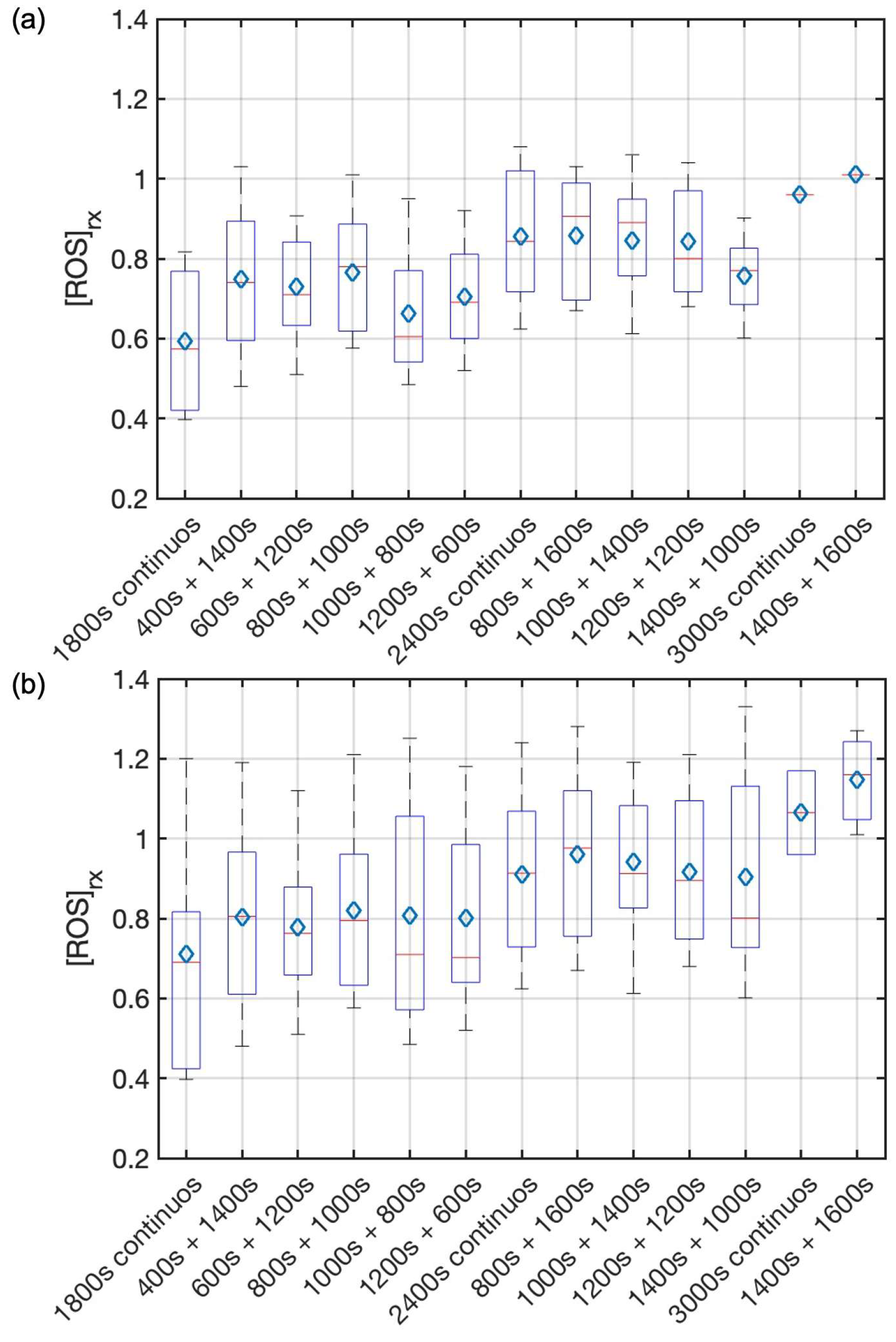
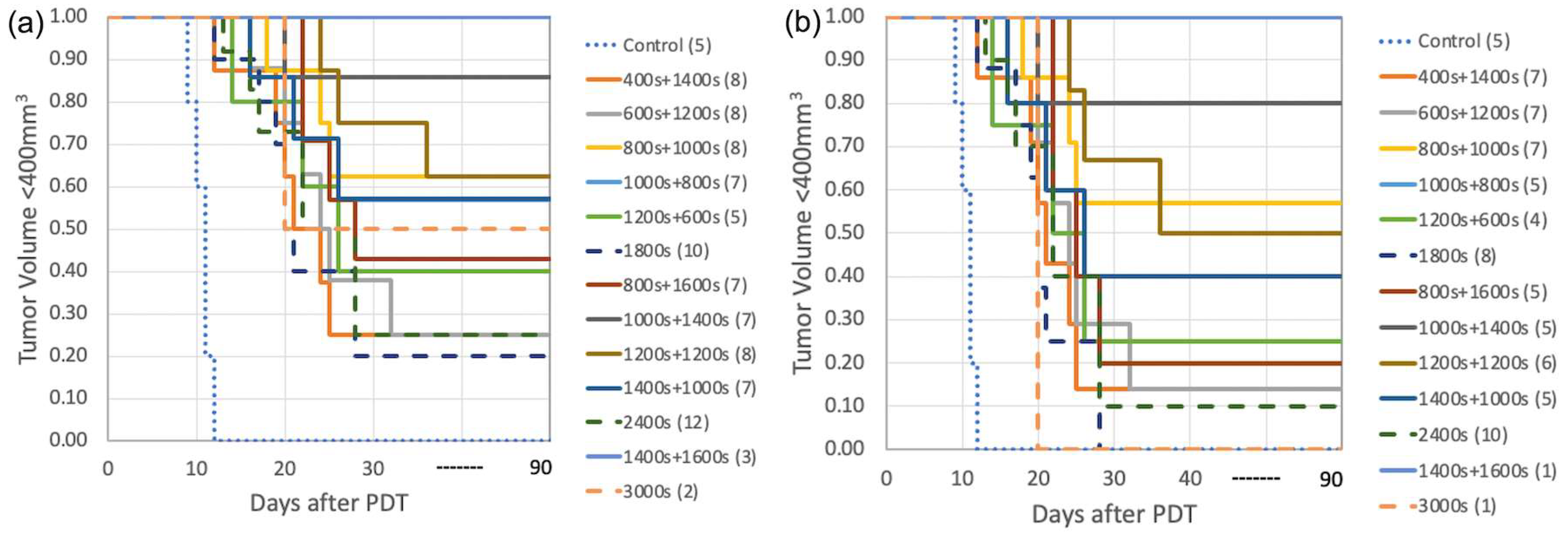
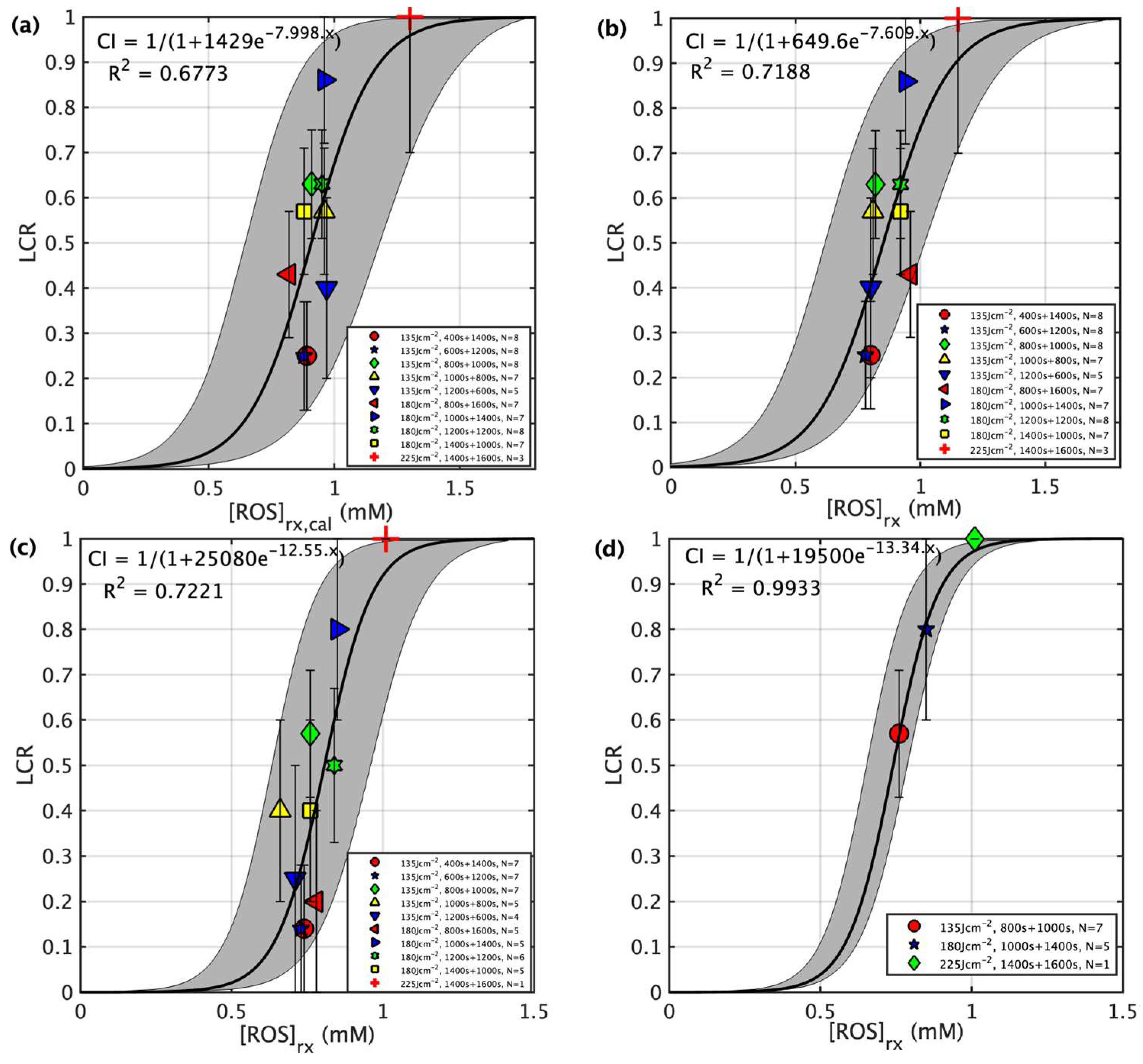
| Groups | # of Mice | Treatment Schemes | In-Air Fluence Rate ‡ (mW/cm2) | [3O2]0 # (μM) | [S0]0 # (μM) | [S0]end ## (μM) | PDT Dose * (μMJ/cm2) | Mean [ROS]rx,cal (mM) | Mean [ROS]rx (mM) | LCR ** |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | |
| 1 | 8 | 400 s + 1400 s | 74.4 ± 2.9 | 48.4 ± 9.1 | 4.9 ± 1.3 | 2.5 ± 1.0 | 401.6 ± 103.2 | 0.89 ± 0.19 | 0.80 ± 0.24 | 0.25 ± 0.12 |
| 2 | 8 | 600 s + 1200 s | 73.5 ± 3.2 | 30.8 ± 5.6 | 5.2 ± 1.9 | 1.7 ± 0.7 | 329.9 ± 83.2 | 0.68 ± 0.15 | 0.78 ± 0.19 | 0.25 ± 0.12 |
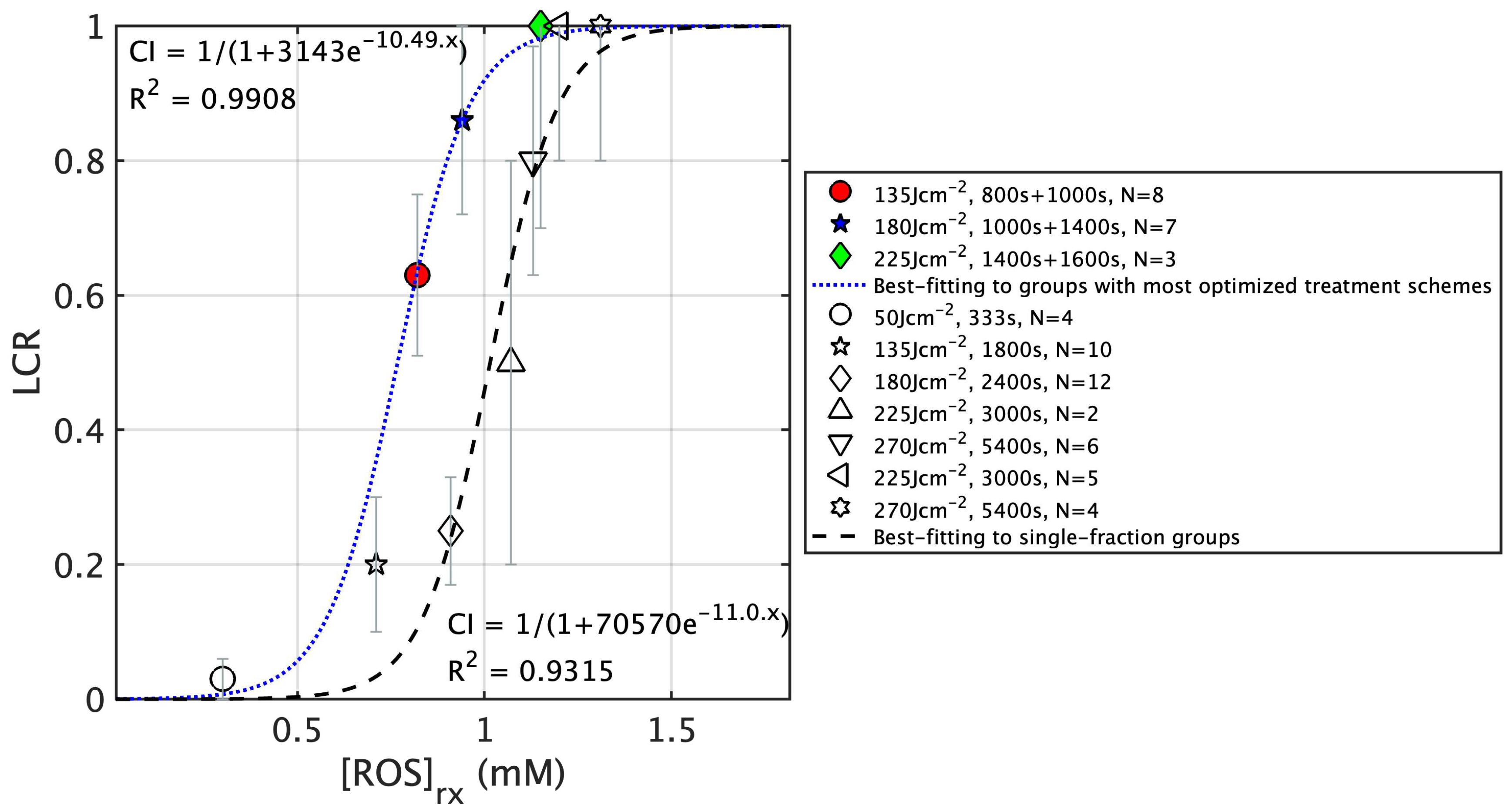
| 3 | 8 | 800 s + 1000 s | 75.5 ± 2.8 | 58.4 ± 12.4 | 4.9 ± 1.1 | 1.4 ± 0.8 | 328.2 ± 117.3 | 0.81 ± 0.18 | 0.82 ± 0.22 | 0.63 ± 0.12 |
| 4 | 7 | 1000 s + 800 s | 76.0 ± 3.0 | 47.3 ± 7.5 | 6.2 ± 2.3 | 2.8 ± 1.1 | 467.1 ± 73.5 | 0.96 ± 0.26 | 0.81 ± 0.29 | 0.57 ± 0.14 |
| 5 | 5 | 1200 s + 600 s | 75.8 ± 1.7 | 51.0 ± 10.4 | 7.1 ± 2.9 | 2.2 ± 0.4 | 475.1 ± 92.6 | 0.97 ± 0.25 | 0.80 ± 0.26 | 0.40 ± 0.20 |
| 6 | 10 | 1800 s | 74.2 ± 2.6 | 57.4 ± 15.2 | 4.9 ± 2.0 | 1.1 ± 0.3 | 317.5 ± 56.2 | 0.79 ± 0.24 | 0.71 ± 0.30 | 0.20 ± 0.10 |
| 7 | 7 | 800 s + 1600 s | 77.1 ± 2.8 | 52.7 ± 9.8 | 2.4 ± 0.9 | 1.3 ± 0.5 | 315.6 ± 93.4 | 0.82 ± 0.18 | 0.96 ± 0.22 | 0.43 ± 0.14 |
| 8 | 7 | 1000 s + 1400 s | 72.6 ± 3.1 | 50.6 ± 12.4 | 4.0 ± 1.6 | 0.9 ± 0.2 | 339.0 ± 101.3 | 0.95 ± 0.14 | 0.94 ± 0.21 | 0.86 ± 0.14 |
| 9 | 8 | 1200 s + 1200 s | 73.8 ± 3.3 | 46.8 ± 10.2 | 3.6 ± 1.0 | 2.2 ± 0.9 | 403.1 ± 73.1 | 0.86 ± 0.17 | 0.92 ± 0.19 | 0.63 ± 0.12 |
| 10 | 7 | 1400 s + 1000 s | 74.1 ± 1.5 | 33.4 ± 8.9 | 4.6 ± 2.1 | 1.8 ± 0.4 | 409.4 ± 83.6 | 0.88 ± 0.21 | 0.92 ± 0.27 | 0.57 ± 0.14 |
| 11 | 12 | 2400 s | 75.3 ± 2.0 | 42.0 ± 7.3 | 3.8 ± 1.7 | 1.3 ± 0.5 | 348.7 ± 38.5 | 0.83 ± 0.11 | 0.91 ± 0.20 | 0.25 ± 0.08 |
| 12 | 3 | 1400 s + 1600 s | 74.6 ± 1.1 | 49.0 ± 5.3 | 6.2 ± 2.2 | 2.5 ± 1.1 | 705.9 ± 228.3 | 1.50 ± 0.10 | 1.15 ± 0.13 | 1.0 ± 0.3 *** |
| 13 | 2 | 3000 s | 75.3 ± 0.9 | 39.9 ± 3.6 | 4.1 ± 1.5 | 1.6 ± 0.4 | 508.0 ± 198.8 | 1.15 ± 0.13 | 1.07 ± 0.15 | 0.50 ± 0.50 |
| Groups | # of Mice | Time (s) | [S0]0 (μM) | [S0]end (μM) | PDT Dose (μMJ/cm2) | Mean [ROS]rx (mM) | LCR |
|---|---|---|---|---|---|---|---|
| Control | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 7 | 400 s + 1400 s | 4.3 ± 1.1 | 1.9 ± 1.0 | 318.4 ± 111.3 | 0.74 ± 0.20 | 0.14 ± 0.14 |
| 2 | 7 | 600 s + 1200 s | 5.0 ± 1.6 | 1.7 ± 0.8 | 320.5 ± 72.4 | 0.73 ± 0.14 | 0.14 ± 0.14 |
| 3 | 7 | 800 s + 1000 s | 4.3 ± 1.1 | 1.8 ± 0.9 | 297.5 ± 113.9 | 0.76 ± 0.16 | 0.57 ± 0.14 |
| 4 | 5 | 1000 s + 800 s | 5.9 ± 2.0 | 2.4 ± 1.0 | 459.4 ± 82.5 | 0.66 ± 0.18 | 0.40 ± 0.20 |
| 5 | 4 | 1200 s + 600 s | 6.3 ± 1.9 | 1.9 ± 0.6 | 394.4 ± 79.9 | 0.71 ± 0.17 | 0.25 ± 0.25 |
| 6 | 8 | 1800 s | 4.5 ± 2.1 | 1.4 ± 0.6 | 286.5 ± 62.6 | 0.59 ± 0.18 | 0.00 ± 0.12 |
| 7 | 5 | 800 s + 1600 s | 2.2 ± 0.7 | 0.9 ± 0.5 | 222.8 ± 101.0 | 0.78 ± 0.16 | 0.20 ± 0.20 |
| 8 | 5 | 1000 s + 1400 s | 3.7 ± 1.5 | 1.1 ± 0.4 | 308.0 ± 103.1 | 0.85 ± 0.16 | 0.80 ± 0.20 |
| 9 | 6 | 1200 s + 1200 s | 3.5 ± 0.9 | 2.0 ± 1.0 | 381.7 ± 68.4 | 0.84 ± 0.15 | 0.50 ± 0.17 |
| 10 | 5 | 1400 s + 1000 s | 3.9 ± 2.4 | 1.7 ± 0.5 | 380.2 ± 89.2 | 0.76 ± 0.11 | 0.40 ± 0.20 |
| 11 | 10 | 2400 s | 3.8 ± 1.9 | 1.2 ± 0.7 | 340.1 ± 47.1 | 0.86 ± 0.17 | 0.10 ± 0.10 |
| 12 | 1 | 1400 s + 1600 s | 4.1 | 2.4 | 606.5 ± 210.9 | 1.01 | 1.0 |
| 13 | 1 | 3000 s | 3.7 | 1.2 | 427.4 ± 202.7 | 0.96 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Yang, W.; Ong, Y.; Busch, T.M.; Zhu, T.C. Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival. Cancers 2023, 15, 5682. https://doi.org/10.3390/cancers15235682
Sun H, Yang W, Ong Y, Busch TM, Zhu TC. Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival. Cancers. 2023; 15(23):5682. https://doi.org/10.3390/cancers15235682
Chicago/Turabian StyleSun, Hongjing, Weibing Yang, Yihong Ong, Theresa M. Busch, and Timothy C. Zhu. 2023. "Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival" Cancers 15, no. 23: 5682. https://doi.org/10.3390/cancers15235682
APA StyleSun, H., Yang, W., Ong, Y., Busch, T. M., & Zhu, T. C. (2023). Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival. Cancers, 15(23), 5682. https://doi.org/10.3390/cancers15235682








