Shifting Epidemiology Trends in Tongue Cancer: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Variables
- Eligibility criteria
- Patients ≥18 years old;
- Patients with primary tongue cancer who were diagnosed with biopsy.
- Exclusion criteria
- Patients with recurrent or secondary tongue cancer;
- Patients diagnosed with tongue cancer before 2006 and after 2021.
2.2. Statistical Analysis
3. Results
3.1. Patents’ Characteristics
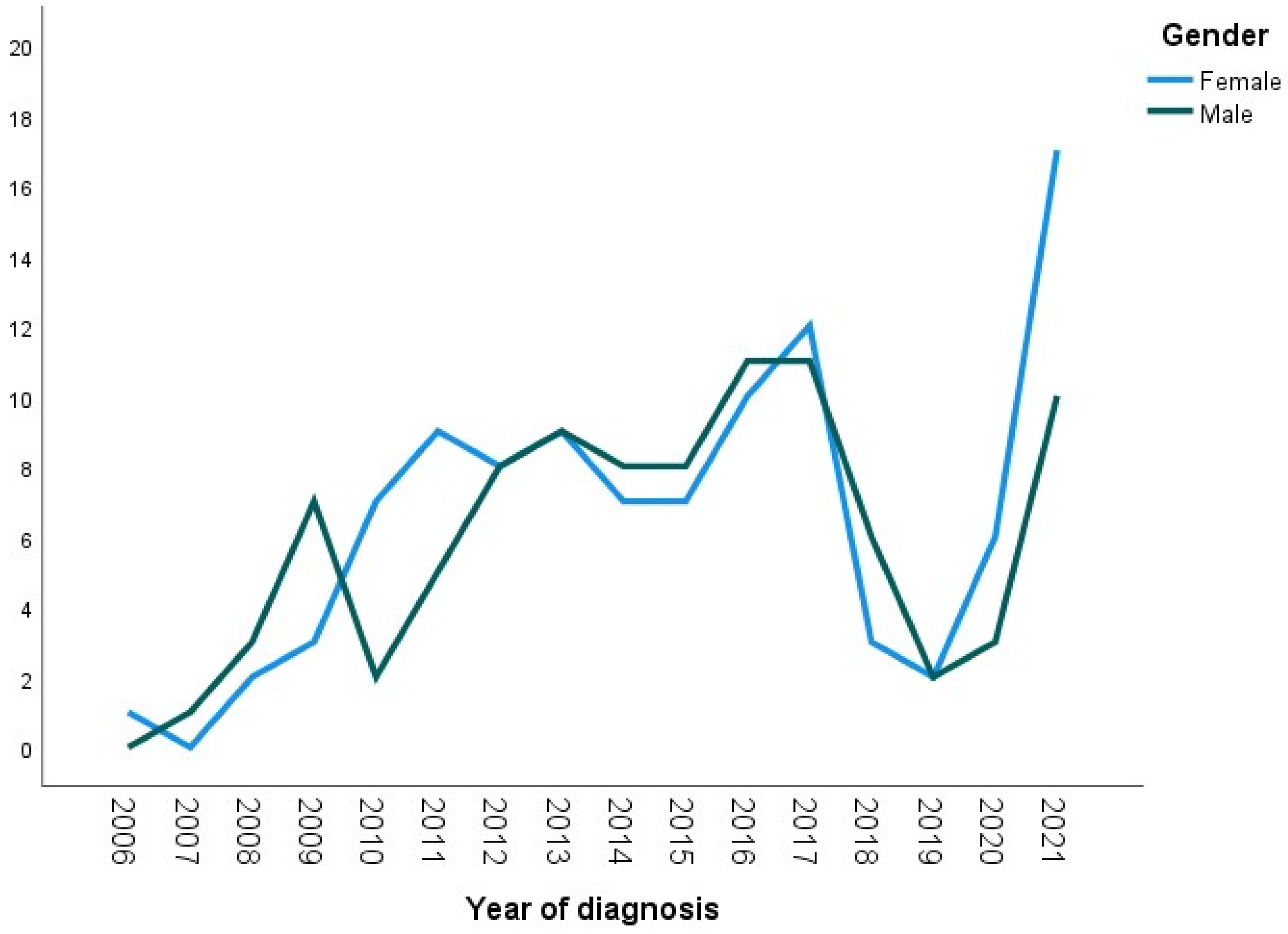
- Gender
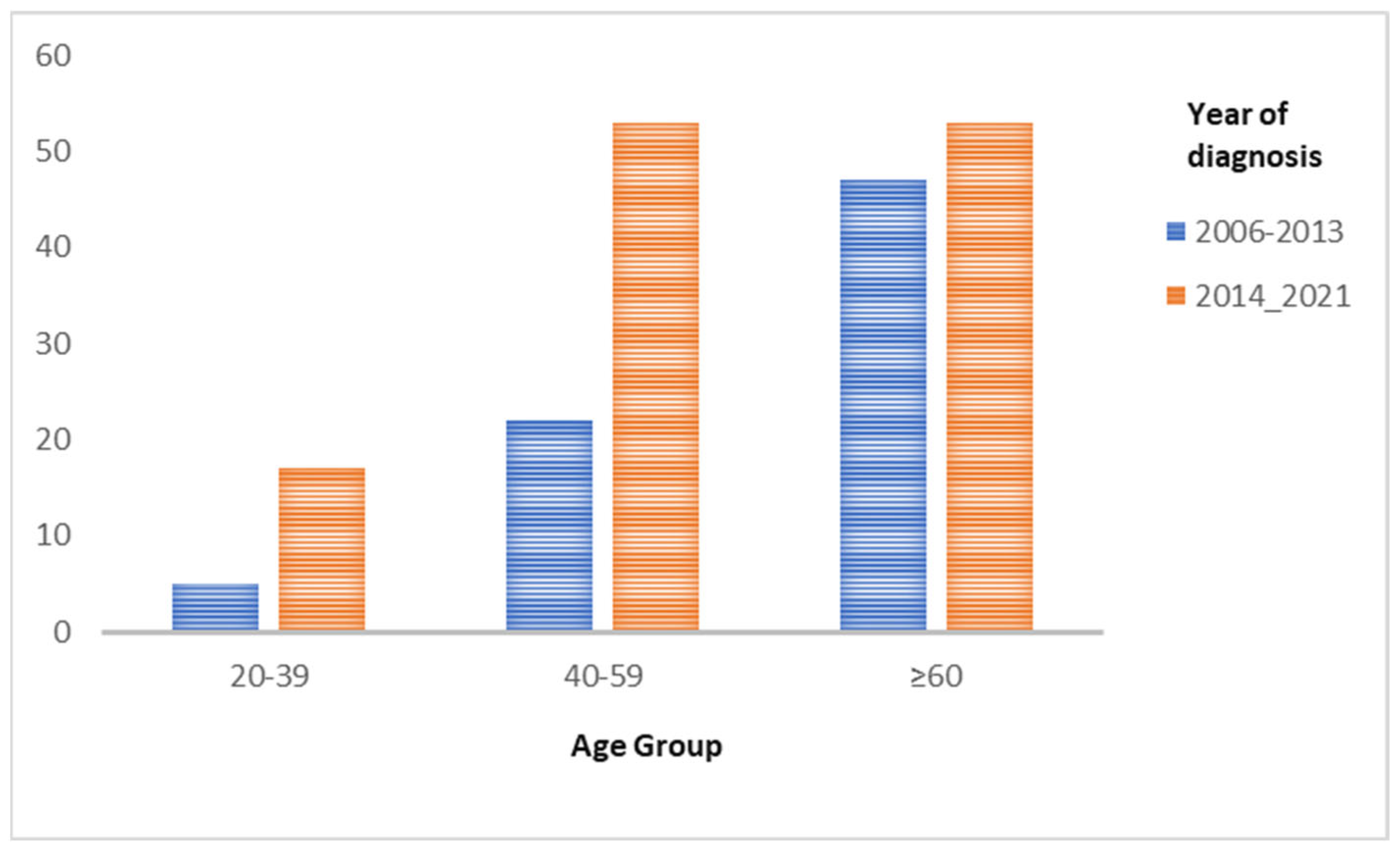
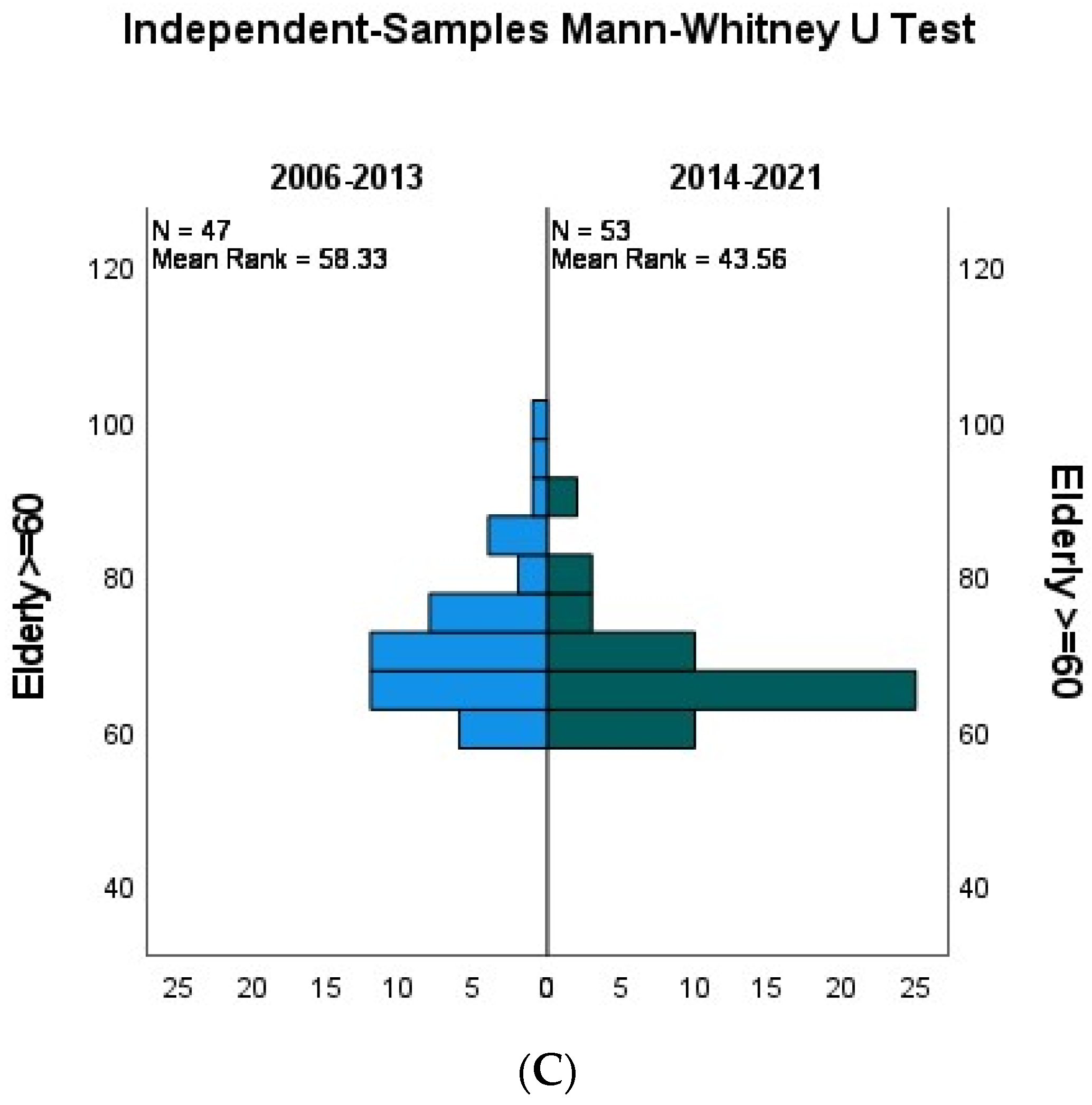
- Age at diagnosis (Figure 2)
- Age from 20 to 39 years
- Age from 40 to 59 years
- Age≥60 years

3.2. Smoking Status
3.3. Family History
3.3.1. Negative Family History
3.3.2. Positive Family History
3.4. Tumor Characteristics
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Tavani, A.; Franceschi, S.; Levi, F.; Corrao, G.; Negri, E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997, 33, 302–312. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 1–334. [Google Scholar]
- Fouad, H.; Commar, A.; Hamadeh, R.R.; El Awa, F.; Shen, Z.; Fraser, C.P. Smoking prevalence in the Eastern Mediterranean Region. East. Mediterr. Health J. 2020, 26, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.-M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Harari, P.M. Oral tongue cancer in young adults less than 40 years of age: Rationale for aggressive therapy. Head Neck 1994, 16, 107–111. [Google Scholar] [CrossRef]
- Patel, S.C.; Carpenter, W.R.; Tyree, S.; Couch, M.E.; Weissler, M.; Hackman, T.; Hayes, D.N.; Shores, C.; Chera, B.S. Increasing Incidence of Oral Tongue Squamous Cell Carcinoma in Young White Women, Age 18 to 44 Years. J. Clin. Oncol. 2011, 29, 1488–1494. [Google Scholar] [CrossRef]
- van Monsjou, H.S.; Wreesmann, V.B.; Brekel, M.W.v.D.; Balm, A.J. Head and neck squamous cell carcinoma in young patients. Oral Oncol. 2013, 49, 1097–1102. [Google Scholar] [CrossRef]
- Harris, S.L.; Kimple, R.J.; Hayes, D.N.; Couch, M.E.; Rosenman, J.G. Never-smokers, never-drinkers: Unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck 2009, 32, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Auluck, A.; Hislop, G.; Bajdik, C.; Poh, C.; Zhang, L.; Rosin, M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population. Cancer 2010, 116, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Poling, J.S.; Ma, X.-J.; Bui, S.; Luo, Y.; Li, R.; Koch, W.M.; Westra, W.H. Human papillomavirus (HPV) status of non-tobacco related squamous cell carcinomas of the lateral tongue. Oral Oncol. 2014, 50, 306–310. [Google Scholar] [CrossRef]
- Beena, V.; Chauhan, I.; Heera, R.; Rajeev, R. Oral cancer in young non-habituè females: A report of four cases and review of the literature. Aust. Dent. J. 2011, 56, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Attar, E.; Dey, S.; Hablas, A.; Seifeldin, I.A.; Ramadan, M.; Rozek, L.S.; Soliman, A.S. Head and neck cancer in a developing country: A population-based perspective across 8 years. Oral Oncol. 2010, 46, 591–596. [Google Scholar] [CrossRef]
- Tota, J.E.; Anderson, W.F.; Coffey, C.; Califano, J.; Cozen, W.; Ferris, R.L.; John, M.S.; Cohen, E.E.; Chaturvedi, A.K. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017, 67, 146–152. [Google Scholar] [CrossRef]
- Ng, J.H.; Iyer, N.G.; Tan, M.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef]
- Ghantous, Y.; Yaffi, V.; Abu-Elnaaj, I. Oral cavity cancer: Epidemiology and early diagnosis. Refuat Hapeh Vehashinayim (1993) 2015, 32, 55–63, 71. Available online: http://europepmc.org/abstract/MED/26548152 (accessed on 4 November 2023).
- Gonzalez, M.; Riera March, A. Tongue Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: http://europepmc.org/books/NBK562324 (accessed on 4 November 2023).
- Moore, S.; Johnson, N.; Pierce, A.; Wilson, D. The epidemiology of tongue cancer: A review of global incidence. Oral Dis. 2000, 6, 75–84. [Google Scholar] [CrossRef]
- Langdon, J.D.; Ral, A.D. Oral Cancer and Sex Why do Females do better? J. Maxillofac. Surg. 1979, 7, 177–181. [Google Scholar] [CrossRef]
- Saba, N.F.; Goodman, M.; Ward, K.; Flowers, C.; Ramalingam, S.; Owonikoko, T.; Chen, A.; Grist, W.; Wadsworth, T.; Beitler, J.J.; et al. Gender and Ethnic Disparities in Incidence and Survival of Squamous Cell Carcinoma of the Oral Tongue, Base of Tongue, and Tonsils: A Surveillance, Epidemiology and End Results Program-Based Analysis. Oncology 2011, 81, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; D’ecclesiis, O.; De Berardinis, R.; Gaeta, A.; Martinoli, C.; Piana, A.F.; Maffini, F.; Gandini, S.; Ansarin, M.; Chiocca, S. The prognostic role of sex and hemoglobin levels in patients with oral tongue squamous cell carcinoma. Front. Oncol. 2022, 12, 1018886. [Google Scholar] [CrossRef]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An international classification of diseases for the twenty-first century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef]
- Ghandour, L.; Chalak, A.; El-Aily, A.; Yassin, N.; Nakkash, R.; Tauk, M.; El Salibi, N.; Heffron, M.; Afifi, R. Alcohol consumption in the Arab region: What do we know, why does it matter, and what are the policy implications for youth harm reduction? Int. J. Drug Policy 2016, 28, 10–33. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, F.; Khabour, O.; Alzoubi, K.; Al-shatnawi, S. Attitudes and beliefs related to reporting alcohol consumption in research studies: A case from Jordan. Pragmatic Obs. Res. 2018, 9, 55–61. [Google Scholar] [CrossRef]
- Nainani, P.; Paliwal, A.; Nagpal, N.; Agrawal, M. Sex hormones in gender-specific risk for head and neck cancer: A review. J. Int. Soc. Prev. Commun. Dent. 2014, 4, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Izzo, G.; Carinci, F.; Campisi, G.; Muzio, L.L.; D’Amato, S.; Mazzotta, M.; Cannavale, R.; Ferrara, D.; Minucci, S. Expression of Sexual Hormones Receptors in Oral Squamous Cell Carcinoma. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 129–132. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef]
- Goldemberg, D.C.; de Araújo, L.H.L.; Antunes, H.S.; de Melo, A.C.; Thuler, L.C.S. Tongue cancer epidemiology in Brazil: Incidence, morbidity and mortality. Head Neck 2018, 40, 1834–1844. [Google Scholar] [CrossRef]
- Paderno, A.; Morello, R.; Piazza, C. Tongue carcinoma in young adults: A review of the literature. Acta Otorhinolaryngol. Ital. 2018, 38, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Knopf, A.; Lempart, J.; Bas, M.; Slotta-Huspenina, J.; Mansour, N.; Fritsche, M.K. Oncogenes and tumor suppressor genes in squamous cell carcinoma of the tongue in young patients. Oncotarget 2015, 6, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Lassig, A.A.D.; Lindgren, B.R.; Fernandes, P.; Cooper, S.; Ardeshipour, F.; Schotzko, C.; Yueh, B. The effect of young age on outcomes in head and neck cancer. Laryngoscope 2013, 123, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Grant, J.R.; Etzel, C.J.; Roberts, D.; Wei, Q.; Sturgis, E.M. Matched Analysis of Survival in Patients with Squamous Cell Carcinoma of the Head and Neck Diagnosed Before and After 40 Years of Age. Arch. Otolaryngol. Neck Surg. 2004, 130, 869–873. [Google Scholar] [CrossRef]
- Blanchard, P.; Belkhir, F.; Temam, S.; El Khoury, C.; De Felice, F.; Casiraghi, O.; Patrikidou, A.; Mirghani, H.; Levy, A.; Even, C.; et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: A single-institution case-matched analysis. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Garavello, W.; Spreafico, R.; Gaini, R.M. Oral tongue cancer in young patients: A matched analysis. Oral Oncol. 2007, 43, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Hilly, O.; Shkedy, Y.; Hod, R.; Soudry, E.; Mizrachi, A.; Hamzany, Y.; Bachar, G.; Shpitzer, T. Carcinoma of the oral tongue in patients younger than 30 years: Comparison with patients older than 60 years. Oral Oncol. 2013, 49, 987–990. [Google Scholar] [CrossRef]
- Park, J.-O.; Sun, D.-I.; Cho, K.-J.; Joo, Y.-H.; Yoo, H.-J.; Kim, M.-S. Clinical Outcome of Squamous Cell Carcinoma of the Tongue in Young Patients: A Stage-Matched Comparative Analysis. Clin. Exp. Otorhinolaryngol. 2010, 3, 161–165. [Google Scholar] [CrossRef]
- Tagliabue, M.; Belloni, P.; De Berardinis, R.; Gandini, S.; Chu, F.; Zorzi, S.; Fumagalli, C.; Santoro, L.; Chiocca, S.; Ansarin, M. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. 2021, 10, 2566–2578. [Google Scholar] [CrossRef]
- Llewellyn, C.D.; Linklater, K.; Bell, J.; Johnson, N.W.; Warnakulasuriya, S. An analysis of risk factors for oral cancer in young people: A case-control study. Oral Oncol. 2004, 40, 304–313. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for the Eastern Mediterranean. The Tobacco Industry’s Tactics and Plans to Undermine Control Efforts in Egypt and North Africa; World Health Organization, Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2003. [Google Scholar]
- Yurekli, A.; Nassar, H. The Economics of Tobacco in Egypt: A New Analysis of Demand. 2003. Available online: https://escholarship.org/uc/item/5pv1f39j (accessed on 4 November 2023).
- Fouda, S.; Kelany, M.; Moustafa, N.; Abushouk, A.I.; Hassane, A.; Sleem, A.; Mokhtar, O.; Negida, A.; Bassiony, M. Tobacco smoking in Egypt: A scoping literature review of its epidemiology and control measures. East. Mediterr. Health J. 2018, 24, 198–215. [Google Scholar] [CrossRef] [PubMed]
| 2006–2013 | 2014–2021 | p-Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | 0.927 | ||||
| Females | 39 | 52.7% | 64 | 52% | |
| Males | 35 | 47.3% | 59 | 48% | |
| Age | 0.018 | ||||
| 20–39 | 5 | 6.8% | 17 | 13.8% | 0.039 |
| 40–49 | 22 | 29.7% | 53 | 43.1% | 0.912 |
| ≥60 | 47 | 63.5% | 53 | 43.1% | 0.011 |
| Age | 0.127 | ||||
| <40 | 5 | 6.8% | 17 | 13.8% | |
| ≥40 | 69 | 93.2% | 106 | 86.2% | |
| Smoking status | 0.973 | ||||
| Smoker | 17 | 23% | 28 | 22.8% | |
| Non-Smoker | 57 | 77% | 95 | 77.2% | |
| Family history of cancer | 0.002 | ||||
| Positive | 1 | 1.5% | 19 | 16.8% | |
| Negative | 64 | 98.5% | 94 | 83.2% | |
| Type of cancer reported in the family History | |||||
| Head and neck | 0 | 7 | |||
| Gynecological | 1 | 8 | |||
| GIT | 0 | 7 | |||
| Others | 0 | 3 | |||
| 2006–2013 | 2014–2021 | p-Value | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Site | Lateral | 57 | 77% | 103 | 83.7% | 0.253 |
| Dorsum | 11 | 14.6% | 17 | 13.8% | ||
| Middle/Posterior | 5 | 6.8% | 3 | 2.4% | ||
| Side | Right | 36 | 59% | 61 | 52.6% | 0.414 |
| Left | 25 | 41% | 55 | 47.4% | ||
| Nodal status | Positive | 46 | 62.2% | 41 | 33.3% | <0.001 |
| Negative | 26 | 35.1% | 55 | 44.7% | ||
| Clinical stage | 1 | 10 | 14.3% | 22 | 26.5% | 0.148 |
| 2 | 13 | 18.6% | 16 | 19.3% | ||
| 3 | 5 | 7.1% | 9 | 10.8% | ||
| 4 | 42 | 60% | 36 | 43.4% | ||
| Grade | 1 | 36 | 49.3% | 37 | 45.1% | 0.621 |
| 2 | 30 | 41.1% | 33 | 40.2% | ||
| 3 | 7 | 9.6% | 12 | 14.6% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, Y.; Hamdy, O.; Eldeghedi, M.; Abdelaziz, R.; Med Sidi El Moctar, E.; Alharazin, M.; Awny, S. Shifting Epidemiology Trends in Tongue Cancer: A Retrospective Cohort Study. Cancers 2023, 15, 5680. https://doi.org/10.3390/cancers15235680
Sakr Y, Hamdy O, Eldeghedi M, Abdelaziz R, Med Sidi El Moctar E, Alharazin M, Awny S. Shifting Epidemiology Trends in Tongue Cancer: A Retrospective Cohort Study. Cancers. 2023; 15(23):5680. https://doi.org/10.3390/cancers15235680
Chicago/Turabian StyleSakr, Yara, Omar Hamdy, Maher Eldeghedi, Rabab Abdelaziz, Echreiva Med Sidi El Moctar, Mohammed Alharazin, and Shadi Awny. 2023. "Shifting Epidemiology Trends in Tongue Cancer: A Retrospective Cohort Study" Cancers 15, no. 23: 5680. https://doi.org/10.3390/cancers15235680
APA StyleSakr, Y., Hamdy, O., Eldeghedi, M., Abdelaziz, R., Med Sidi El Moctar, E., Alharazin, M., & Awny, S. (2023). Shifting Epidemiology Trends in Tongue Cancer: A Retrospective Cohort Study. Cancers, 15(23), 5680. https://doi.org/10.3390/cancers15235680






