Sarcopenia and Mediastinal Adipose Tissue as a Prognostic Marker for Short- and Long-Term Outcomes after Primary Surgical Treatment for Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
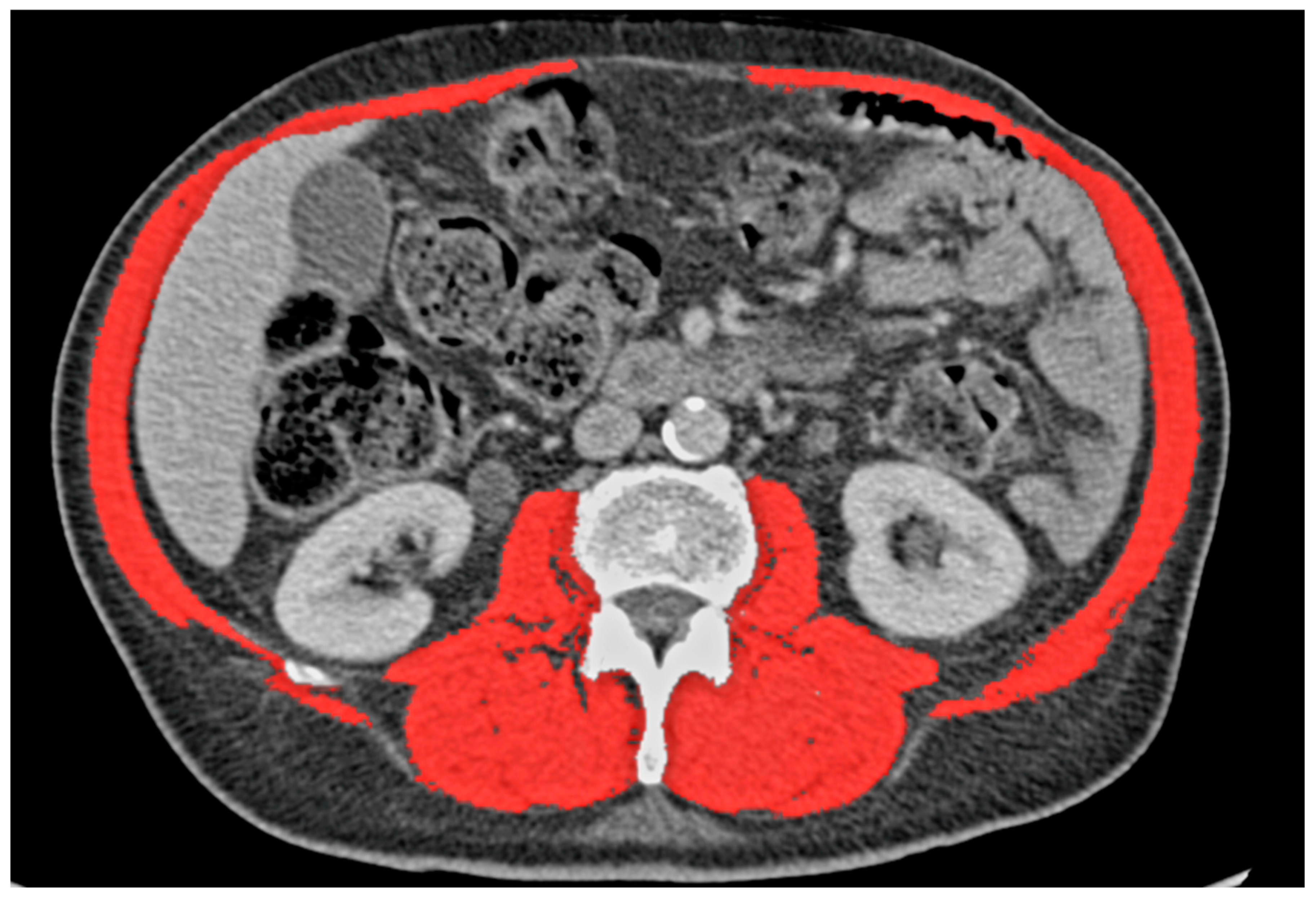
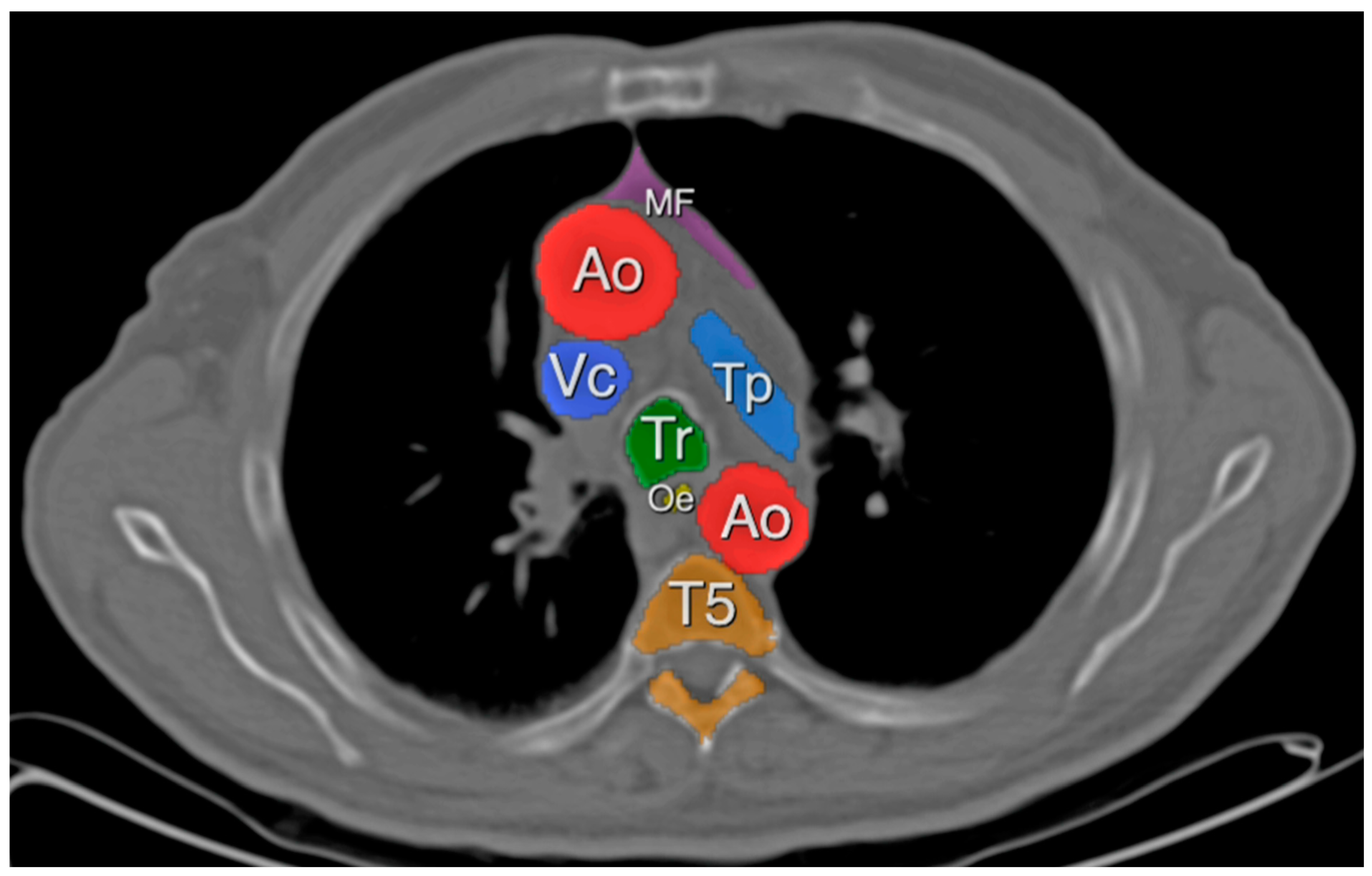
2.3. Definitions
2.4. Statistical Analysis
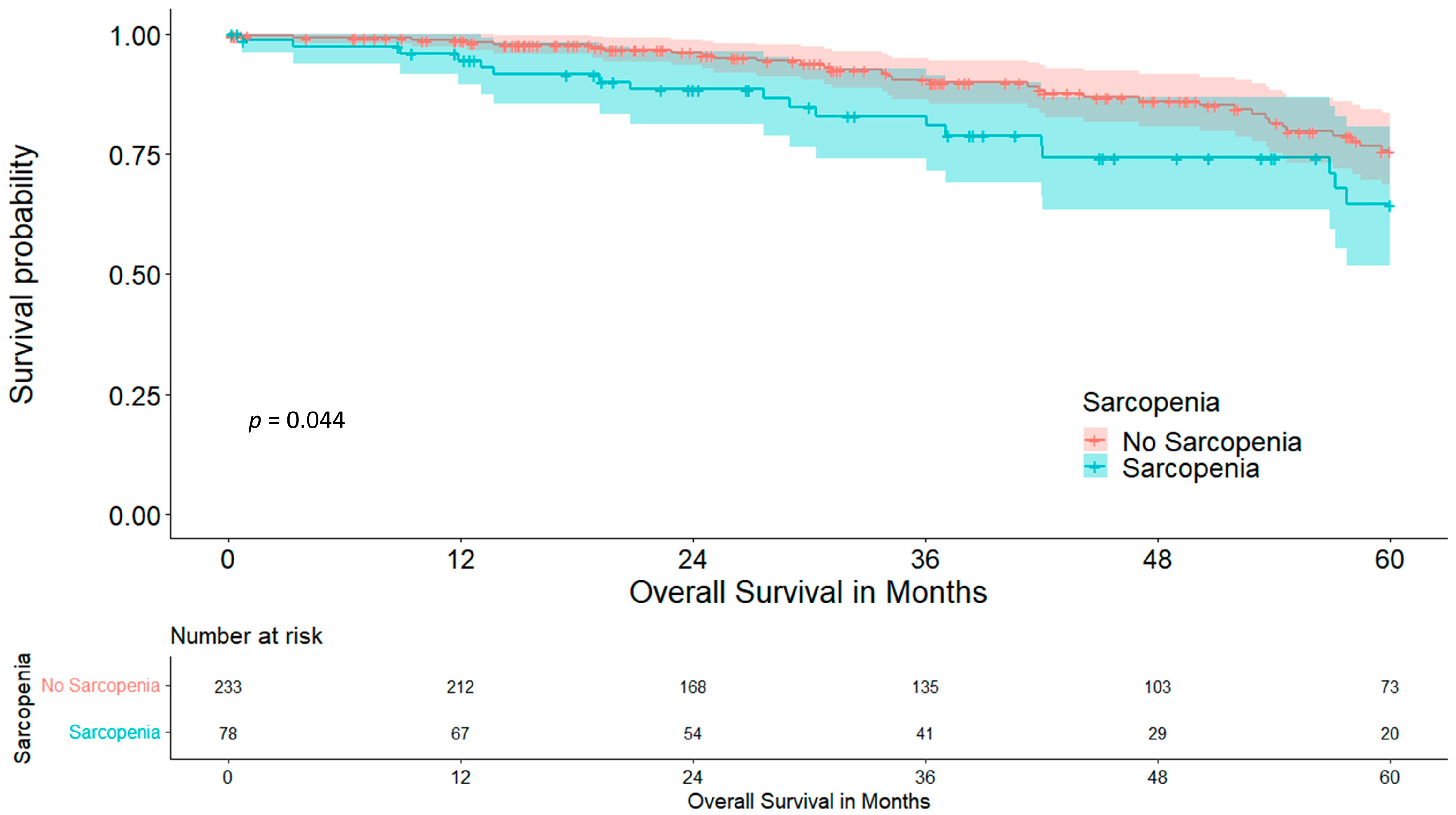
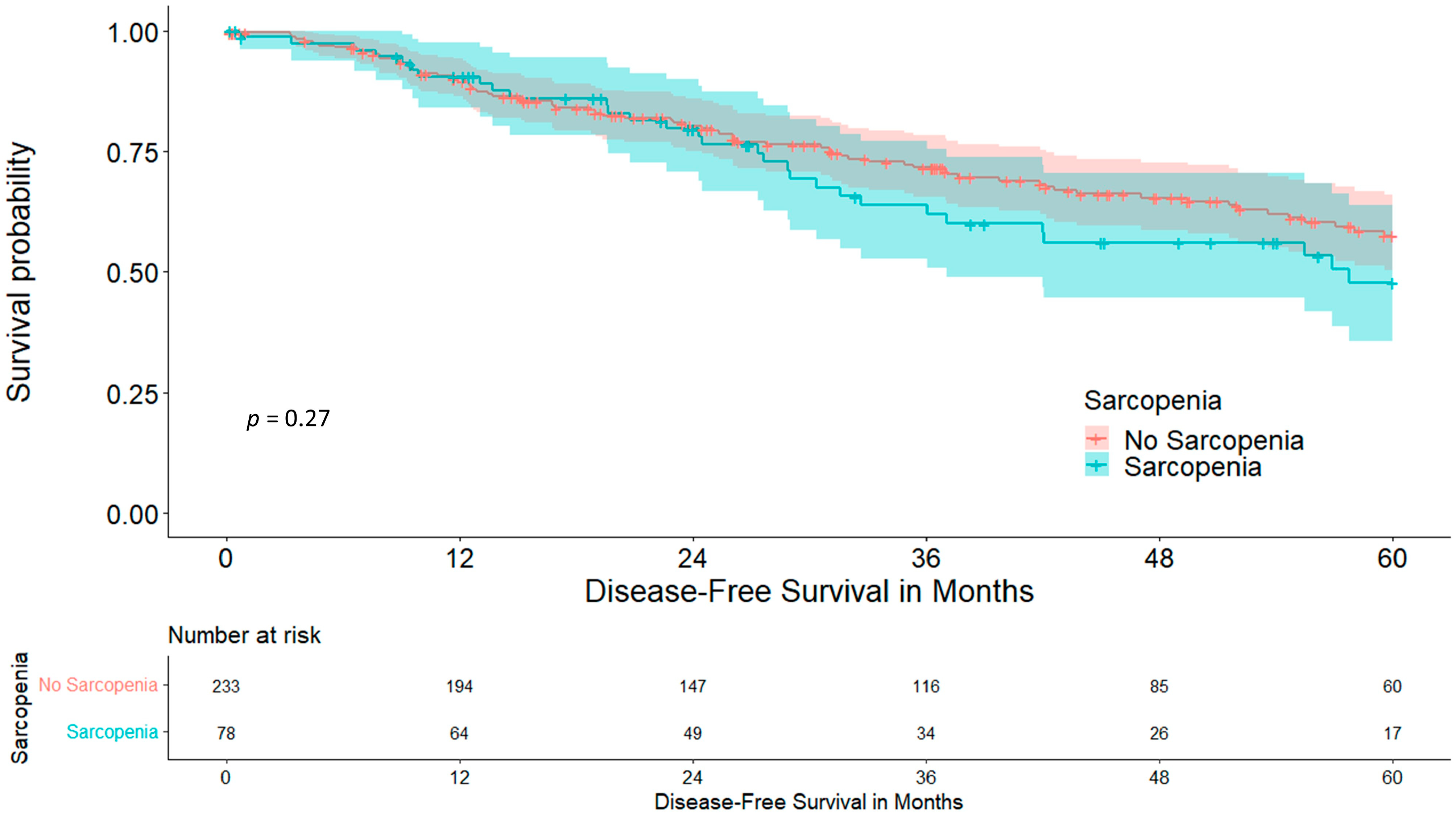
3. Results
3.1. Skeletal Muscle Index
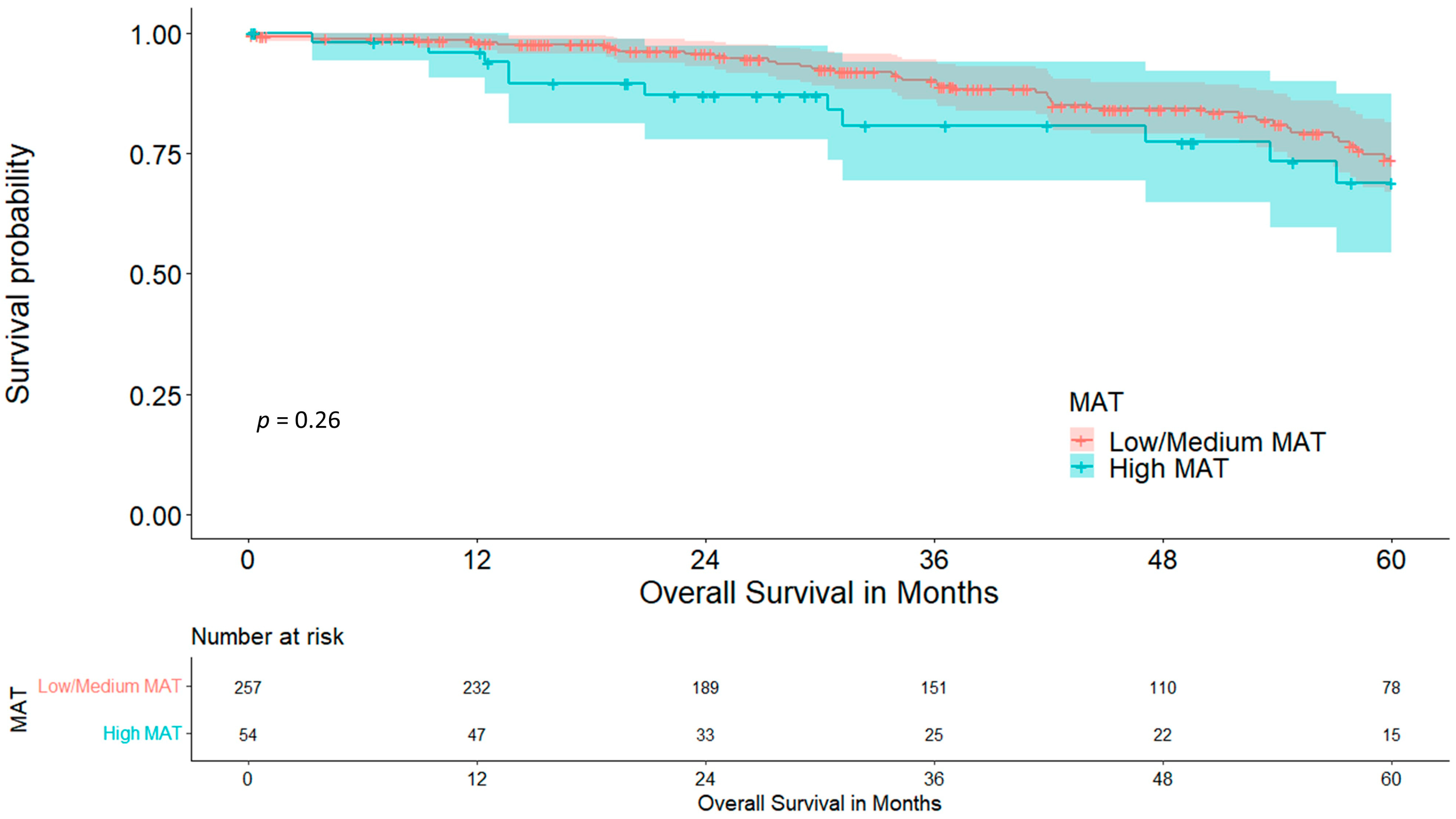
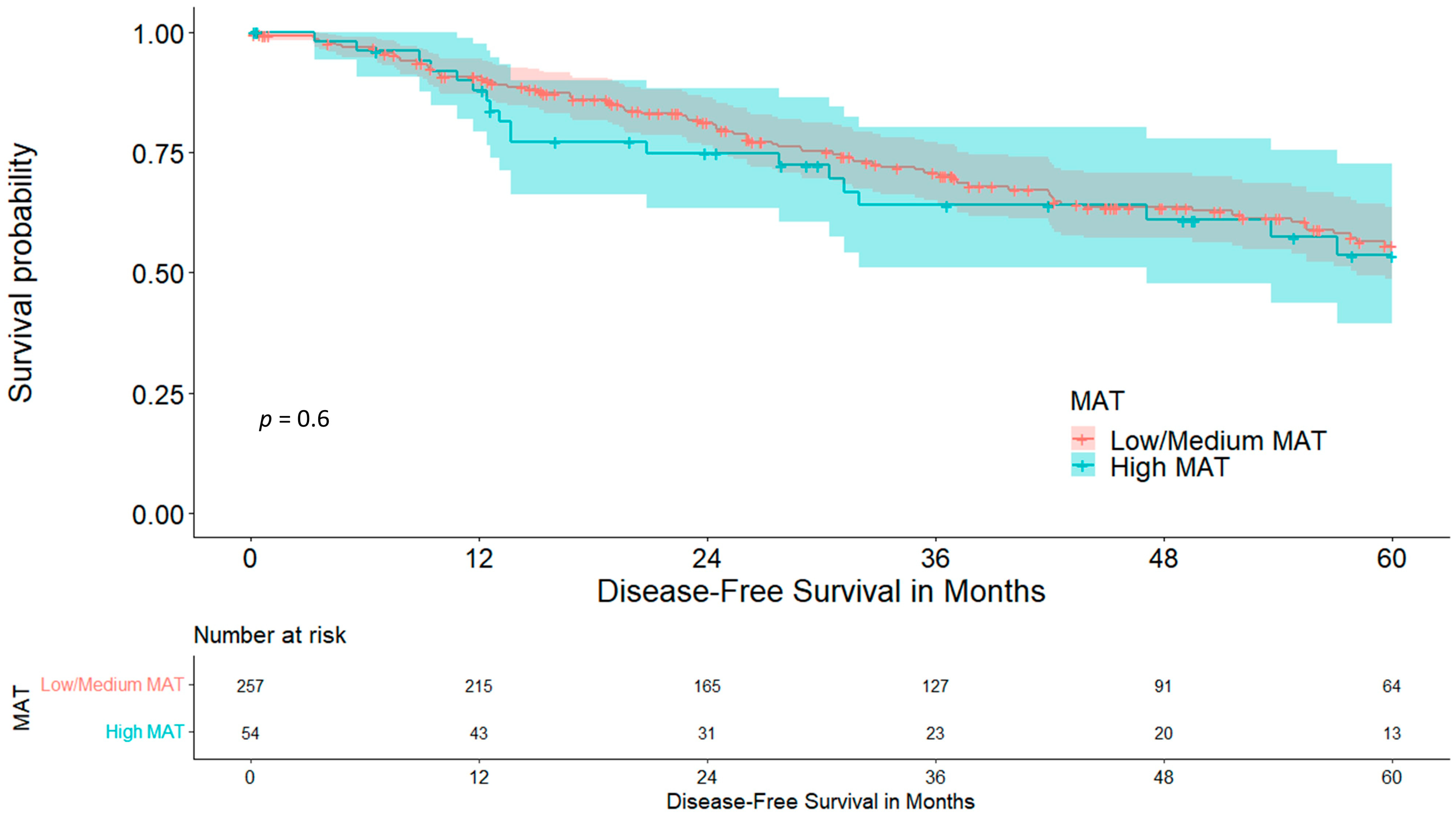
3.2. Mediastinal Adipose Tissue
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Laba, J.M.; Boldt, R.G.; Goodman, C.D.; Palma, D.A.; Senan, S.; VLouie, A. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Ponholzer, F.; Chorazy, K.; Ng, C.; Kocher, F.; Maier, H.; Lucciarini, P.; Öfner, D.; Augustin, F. External validation of risk prediction scores in patients undergoing anatomic video-assisted thoracoscopic resection. Surg. Endosc. 2022, 37, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [PubMed]
- Jitwongwai, S.; Lertudomphonwanit, C.; Junhasavasdikul, T.; Fuangfa, P.; Tanpowpong, P.; Gesprasert, G.; Treepongkaruna, S. Low psoas muscle index as an unfavorable factor in children with end-stage liver disease undergoing liver transplantation. Pediatr. Transplant. 2021, 25, e13996. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Shiotsuka, J.; Kawarai Lefor, A.; Sanui, M. The Psoas Muscle Index Is Associated with Prognosis in Elderly Patients Undergoing Cardiovascular Surgery. Anesth. Pain. Med. 2021, 11, e118608. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Kong, M.; Geng, N.; Song, W.; Guo, G.; Duan, Z.; Han, Y.; Chen, Y. Psoas Muscle Index Can Be Used to Predict Long-Term Mortality in Young Male Patients With Acute-on-Chronic Liver Failure. Front. Nutr. 2022, 9, 811826. [Google Scholar] [CrossRef]
- Ozeki, N.; Kawaguchi, K.; Fukui, T.; Nakamura, S.; Hakiri, S.; Mori, S.; Goto, M.; Iwano, S.; Yokoi, K.; Chen-Yoshikawa, T.F. Psoas muscle mass in patients undergoing lung cancer surgery: A prognostic difference between squamous cell carcinoma and adenocarcinoma. Int. J. Clin. Oncol. 2020, 25, 876–884. [Google Scholar] [CrossRef]
- Verhoek, O.G.; Jungblut, L.; Lauk, O.; Blüthgen, C.; Opitz, I.; Frauenfelder, T.; Martini, K. Sarcopenia, Precardial Adipose Tissue and High Tumor Volume as Outcome Predictors in Surgically Treated Pleural Mesothelioma. Diagnostics 2022, 12, 99. [Google Scholar] [CrossRef]
- Cano Megías, M.; Guisado Vasco, P.; Bouarich, H.; Aguilera, I.L.; La Arriba-de Fuente G de Rodríguez-Puyol, D. Tejido graso epicárdico, calcificación arterial coronaria y mortalidad en pacientes con enfermedad renal crónica avanzada y hemodiálisis. Nefrologia (Engl. Ed.) 2021, 41, 174–181. [Google Scholar] [CrossRef]
- Chen, O.; Sharma, A.; Ahmad, I.; Bourji, N.; Nestoiter, K.; Hua, P.; Hua, B.; Ivanov, A.; Yossef, J.; Klem, I.; et al. Correlation between pericardial, mediastinal, and intrathoracic fat volumes with the presence and severity of coronary artery disease, metabolic syndrome, and cardiac risk factors. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cikim, A.S.; Topal, E.; Harputluoglu, M.; Keskin, L.; Zengin, Z.; Cikim, K.; Ozdemir, R.; Aladag, M.; Yologlu, S. Epicardial adipose tissue, hepatic steatosis and obesity. J. Endocrinol. Investig. 2007, 30, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, D.; Orsso, C.E.; Chohan, K.; Orchanian-Cheff, A.; Nourouzpour, S.; Nicholson, J.M.; Elangeswaran, B.; Vagaon, A.; Fidler, L.; Singer, L.G.; et al. Clinical outcomes associated with computed tomography-based body composition measures in lung transplantation: A systematic review. Transpl. Int. 2020, 33, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Awwad, A.; Macdonald, I.A.; Lobo, D.N. A comparison of two different software packages for analysis of body composition using computed tomography images. Nutrition 2019, 57, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Volbers, B.; Staykov, D.; Wagner, I.; Dörfler, A.; Saake, M.; Schwab, S.; Bardutzky, J. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur. J. Neurol. 2011, 18, 1323–1328. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Goulson, R.L.; Ross, B.E.; Wang, N.C.; Sullivan, J.A.; Su, G.L.; Wang, S.C. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J. Nutr. Health Aging 2017, 21, 180–185. [Google Scholar] [CrossRef]
- Marchiori, E.; Hochhegger, B.; Zanetti, G. Anterior mediastinal mass. J. Bras. Pneumol. 2018, 44, 3. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 30 August 2023).
- Kassambara, A.; Kosinski, M.; Biecek, P.; Scheipl, F. Drawing Survival Curves Using ‘ggplot2’ 2023. [cited 2023 Oct 2]. Available online: https://cran.r-project.org/web/packages/survminer/survminer.pdf (accessed on 30 August 2023).
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing, 3rd ed.; Elsevier Science & Technology Books: San Diego, CA, USA, 2013; Statistical Modeling and Decision Science; Available online: http://www.sciencedirect.com/science/book/9780123869838 (accessed on 30 August 2023).
- Pagano, R.R. Understanding Statistics in the Behavioral Sciences, 9th ed.; Wadsworth/Cengage Learning: Belmont, CA, USA, 2010. [Google Scholar]
- Rasch, D.; Guiard, V. The robustness of parametric statistical methods. Psychol. Sci. 2004, 46, 175–208. [Google Scholar]
- Box, G.E.; Tidwell, P.W. Transformation of the Independent Variables. Technometrics 1962, 4, 531–550. [Google Scholar] [CrossRef]
- Brunelli, A.; Cicconi, S.; Decaluwe, H.; Szanto, Z.; Falcoz, P.E. Parsimonious Eurolung risk models to predict cardiopulmonary morbidity and mortality following anatomic lung resections: An updated analysis from the European Society of Thoracic Surgeons database. Eur. J. Cardiothorac. Surg. 2020, 57, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ponholzer, F.; Ng, C.; Maier, H.; Lucciarini, P.; Öfner, D.; Augustin, F. Risk factors, complications and costs of prolonged air leak after video-assisted thoracoscopic surgery for primary lung cancer. J. Thorac. Dis. 2023, 15, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical Ventilation-induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Varela, G.; Jiménez, M.F.; Novoa, N.; Aranda, J.L. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur. J. Cardiothorac. Surg. 2005, 27, 329–333. [Google Scholar] [CrossRef]
- Topan, M.-M.; Sporea, I.; Dănilă, M.; Popescu, A.; Ghiuchici, A.-M.; Lupuşoru, R.; Şirli, R. Impact of Sarcopenia on Survival and Clinical Outcomes in Patients With Liver Cirrhosis. Front. Nutr. 2021, 8, 766451. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support. Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef]
- Tsukioka, T.; Izumi, N.; Mizuguchi, S.; Kyukwang, C.; Komatsu, H.; Toda, M.; Hara, K.; Miyamoto, H.; Nishiyama, N. Positive correlation between sarcopenia and elevation of neutrophil/lymphocyte ratio in pathological stage IIIA (N2-positive) non-small cell lung cancer patients. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 716–722. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. Available online: https://pubmed.ncbi.nlm.nih.gov/24875653/ (accessed on 10 September 2023). [CrossRef] [PubMed]
- Lo, J.H.; Kin, P.U.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 2020, 23, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Morano, M.T.; Araújo, A.S.; Nascimento, F.B.; da Silva, G.F.; Mesquita, R.; Pinto, J.S.; de Moraes Filho, M.O.; Pereira, E.D. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: A pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 53–58. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Yang, M.; Su, J.; Liu, J.; Che, G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: A randomized controlled trial. J. Surg. Res. 2017, 209, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ponholzer, F.; Kroepfl, V.; Ng, C.; Maier, H.; Kocher, F.; Lucciarini, P.; Öfner, D.; Augustin, F. Delay to surgical treatment in lung cancer patients and its impact on survival in a video-assisted thoracoscopic lobectomy cohort. Sci. Rep. 2021, 11, 4914. [Google Scholar] [CrossRef] [PubMed]
- Bingül, E.S.; Şentürk, N.M.; Kaynar, A.M. Prehabilitation: A narrative review focused on exercise therapy for the prevention of postoperative pulmonary complications following lung resection. Front. Med. 2023, 10, 1196981. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; Caro A de Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef]
- Sanders, K.J.C.; Ash, S.Y.; Washko, G.R.; Mottaghy, F.M.; Schols, A.M.W.J. Imaging approaches to understand disease complexity: Chronic obstructive pulmonary disease as a clinical model. J. Appl. Physiol. 2018, 124, 512–520. [Google Scholar] [CrossRef]
| Factor | Total (n = 311) | Non-Sarcopenic (n = 233) | Sarcopenic (n = 78) | p-Value |
|---|---|---|---|---|
| Mean Age in years (range) | 64.66 (15–83) | 64.09 (15–83) | 66.38 (36–83) | 0.099 |
| Sex (%) | 0.009 | |||
| Female | 149 (47.9) | 122 (52.4) | 27 (34.6) | |
| Male | 162 (52.1) | 111 (47.6) | 51 (65.4) | |
| Mean BMI (range) | 25.5 (14.1–42.3) | 26.30 (15.42–42.29) | 23.14 (14.13–38.53) | <0.001 |
| Mean Height in cm (range) | 170 (145–196) | 168 (145–196) | 174 (152–192) | <0.001 |
| Mean Weight in kg (range) | 73.63 (38.0–118.0) | 74.68 (43–117) | 70.50 (38–118) | 0.032 |
| Mean aCCI (range) | 3.13 (0–8) | 3.04 (0–8) | 3.38 (0–8) | 0.102 |
| Mean SMCA in cm2 (range) | 131.49 (72.93–199.26) | 137.68 (83.06–199.26) | 112.98 (72.93–167.22) | <0.001 |
| Mean SMI in cm2/m2 (range) | 45.52 (26.43–70.22) | 48.37 (34.51–70.22) | 37.01 (26.43–45.38) | <0.001 |
| Mean FEV1% (range) | 82.52 (34.0–154.8) | 82.45 (34.0–154.8) | 82.75 (48.0–135.7) | 0.889 |
| Mean ppoFEV1% (range) | 65.83 (32.21–130.36) | 65.17 (32.21–130.36) | 66.02 (37.89–107.13) | 0.641 |
| Postoperative Complications (%) | 0.023 | |||
| No Complication | 161 (51.8) | 129 (55.4) | 32 (41.0) | |
| Minor Complication | 111 (35.7) | 81 (34.8) | 30 (38.5) | |
| Major Complication | 39 (12.5) | 23 (9.9) | 16 (20.5) | |
| MAT group (%) | 0.117 | |||
| Low MAT | 56 (18.0) | 37 (15.9) | 19 (24.4) | |
| Medium MAT | 201 (64.6) | 151 (64.8) | 50 (64.1) | |
| High MAT | 54 (17.4) | 45 (19.3) | 9 (11.5) | |
| Coronary Artery Disease (%) | 14 (4.5) | 11 (4.7) | 3 (3.8) | 1.000 |
| Cerebrovascular Disease (%) | 29 (9.3) | 22 (9.4) | 7 (9.0) | 1.000 |
| Arterial Hypertension (%) | 140 (45.0) | 112 (48.1) | 28 (35.9) | 0.067 |
| Liver Disease (%) | 23 (7.4) | 17 (7.3) | 6 (7.7) | 1.000 |
| COPD (%) | 103 (33.1) | 75 (32.2) | 28 (35.9) | 0.579 |
| Emphysema (%) | 97 (31.2) | 71 (30.5) | 26 (33.3) | 0.673 |
| Diabetes Mellitus (%) | 40 (12.9) | 32 (13.7) | 8 (10.3) | 0.558 |
| Location of Tumour (%) | 0.268 | |||
| Central | 68 (21.9) | 55 (23.6) | 13 (16.7 | |
| Peripheral | 243 (78.1) | 178 (76.4) | 65 (83.3) | |
| Tumour Diameter in mm (range) | 21.50 (5.0–62.0) | 22.35 (5.0–62.0) | 18.95 (8.0–53.0) | 0.005 |
| UICC Stage (%) | 0.313 | |||
| IA | 254 (81.7) | 187 (80.3) | 67 (85.9) | |
| IB | 57 (18.3) | 46 (19.7) | 11 (14.1) |
| Variables in Equation | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | ||
| Lower | Upper | |||||||
| Sarcopenia | 0.912 | 0.382 | 5.699 | 1 | 0.017 | 2.489 | 1.177 | 5.261 |
| Age | 0.016 | 0.020 | 0.653 | 1 | 0.419 | 1.016 | 0.978 | 1.056 |
| Sex | −0.394 | 0.388 | 1.031 | 1 | 0.310 | 0.674 | 0.315 | 1.443 |
| ppoFEV1% | −0.043 | 0.015 | 8.031 | 1 | 0.005 | 0.958 | 0.931 | 0.987 |
| Conversion to Thoracotomy | 0.758 | 0.957 | 0.627 | 1 | 0.429 | 2.134 | 0.327 | 13.931 |
| Extended Resection | 0.882 | 0.721 | 1.497 | 1 | 0.221 | 2.415 | 0.588 | 9.912 |
| Coronary Artery Disease | −1.013 | 1.082 | 0.875 | 1 | 0.349 | 0.363 | 0.044 | 3.030 |
| Cerebrovascular Disease | −0.179 | 0.655 | 0.075 | 1 | 0.785 | 0.836 | 0.231 | 3.021 |
| Chronic Kidney Disease | −0.503 | 0.845 | 0.354 | 1 | 0.552 | 0.605 | 0.115 | 3.170 |
| Constant | −0.462 | 1.538 | 0.090 | 1 | 0.764 | 0.630 | ||
| Variables in Equation | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | ||
| Lower | Upper | |||||||
| Sarcopenia | 0.624 | 0.329 | 3.606 | 1 | 0.058 | 1.867 | 0.980 | 3.555 |
| Age | 0.026 | 0.015 | 2.827 | 1 | 0.093 | 1.026 | 0.996 | 1.058 |
| Sex | 0.265 | 0.302 | 0.772 | 1 | 0.380 | 1.304 | 0.721 | 2.356 |
| ppoFEV1% | −0.012 | 0.011 | 1.329 | 1 | 0.249 | 0.988 | 0.967 | 1.009 |
| Conversion to Thoracotomy | −12.376 | 397.674 | 0.001 | 1 | 0.975 | 0.000 | 0.000 | . |
| Extended Resection | 0.941 | 0.482 | 3.803 | 1 | 0.051 | 2.562 | 0.995 | 6.595 |
| Coronary Artery Disease | 0.898 | 0.621 | 2.091 | 1 | 0.148 | 2.454 | 0.727 | 8.288 |
| Cerebrovascular Disease | 0.399 | 0.484 | 0.680 | 1 | 0.409 | 1.491 | 0.577 | 3.849 |
| BMI | −0.019 | 0.036 | 0.276 | 1 | 0.599 | 0.981 | 0.914 | 1.053 |
| Factor | Total (n = 311) | Low/Medium MAT (n = 257) | High MAT (n = 54) | p-Value |
|---|---|---|---|---|
| Mean Age in years (range) | 64.66 (15–83) | 63.83 (15–83) | 68.61 (51–80) | <0.001 |
| Sex (%) | 0.881 | |||
| Female | 149 (47.9) | 124 (48.2) | 25 (46.3) | |
| Male | 162 (52.1) | 133 (51.8) | 29 (53.7) | |
| Mean BMI (range) | 25.5 (14.1–42.3) | 24.42 (14.1–42.3) | 30.68 (23.7–39.8) | <0.001 |
| Mean Height in cm (range) | 170 (145–196) | 169.57 (147–190) | 170.17 (145–196) | 0.663 |
| Mean Weight in kg (range) | 73.63 (38.0–118.0) | 70.42 (38–102) | 88.88 (65–118) | <0.001 |
| Mean aCCI (range) | 3.13 (0–8) | 3.05 (0–8) | 3.46 (1–6) | 0.092 |
| Mean SMCA in cm2 (range) | 131.49 (72.93–199.26) | 129.27 (72.93–199.26) | 142.02 (87.05–194.24) | 0.005 |
| Mean SMI in cm2/m2 (range) | 45.52 (26.43–70.22) | 44.80 (26.43–70.22) | 48.94 (32.11–68.82) | 0.002 |
| Mean FEV1% (range) | 82.52 (34.0–154.8) | 82.34 (34.0–154.8) | 83.38 (48.0–118.0) | 0.677 |
| Mean ppoFEV1% (range) | 65.83 (32.21–130.36) | 65.22 (32.21–130.36) | 66.16 (37.89–89.83) | 0.656 |
| Postoperative Complications (%) | 0.225 | |||
| No Complication | 161 (51.8) | 132 (51.4) | 29 (53.7) | |
| Minor Complication | 111 (35.7) | 89 (34.6) | 22 (40.7) | |
| Major Complication | 39 (12.5) | 36 (14.0) | 3 (5.6) | |
| Sacropenia group (%) | 0.124 | |||
| No Sarcopenia | 233 (74.9) | 188 (73.2) | 45 (83.3) | |
| Sarcopenia | 78 (25.1) | 69 (26.8) | 9 (16.7) | |
| Coronary Artery Disease (%) | 14 (4.5) | 11 (4.3) | 3 (5.6) | 0.717 |
| Cerebrovascular Disease (%) | 29 (9.3) | 25 (9.7) | 4 (7.4) | 0.798 |
| Arterial Hypertension (%) | 140 (45.0) | 109 (42.4) | 31 (57.4) | 0.051 |
| Liver Disease (%) | 23 (7.4) | 16 (6.2) | 7 (13.0) | 0.092 |
| COPD (%) | 103 (33.1) | 96 (37.4) | 7 (13.0) | <0.001 |
| Emphysema (%) | 97 (31.2) | 84 (32.7) | 13 (24.1) | 0.259 |
| Diabetes Mellitus (%) | 40 (12.9) | 26 (10.1) | 14 (25.9) | 0.003 |
| Location of Tumour (%) | 0.469 | |||
| Central | 68 (21.9) | 54 (21.0) | 14 (25.9) | |
| Peripheral | 243 (78.1) | 203 (79.0) | 40 (74.1) | |
| Tumour Diameter in mm (range) | 21.50 (5.0–62.0) | 21.35 (5.0–62.0) | 22.17 (8.0–47.0) | 0.594 |
| UICC Stage (%) | 0.564 | |||
| IA | 254 (81.7) | 208 (80.9) | 46 (85.2) | |
| IB | 57 (18.3) | 49 (19.1) | 8 (14.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponholzer, F.; Groemer, G.; Ng, C.; Maier, H.; Lucciarini, P.; Kocher, F.; Öfner, D.; Gassner, E.; Schneeberger, S.; Augustin, F. Sarcopenia and Mediastinal Adipose Tissue as a Prognostic Marker for Short- and Long-Term Outcomes after Primary Surgical Treatment for Lung Cancer. Cancers 2023, 15, 5666. https://doi.org/10.3390/cancers15235666
Ponholzer F, Groemer G, Ng C, Maier H, Lucciarini P, Kocher F, Öfner D, Gassner E, Schneeberger S, Augustin F. Sarcopenia and Mediastinal Adipose Tissue as a Prognostic Marker for Short- and Long-Term Outcomes after Primary Surgical Treatment for Lung Cancer. Cancers. 2023; 15(23):5666. https://doi.org/10.3390/cancers15235666
Chicago/Turabian StylePonholzer, Florian, Georg Groemer, Caecilia Ng, Herbert Maier, Paolo Lucciarini, Florian Kocher, Dietmar Öfner, Eva Gassner, Stefan Schneeberger, and Florian Augustin. 2023. "Sarcopenia and Mediastinal Adipose Tissue as a Prognostic Marker for Short- and Long-Term Outcomes after Primary Surgical Treatment for Lung Cancer" Cancers 15, no. 23: 5666. https://doi.org/10.3390/cancers15235666
APA StylePonholzer, F., Groemer, G., Ng, C., Maier, H., Lucciarini, P., Kocher, F., Öfner, D., Gassner, E., Schneeberger, S., & Augustin, F. (2023). Sarcopenia and Mediastinal Adipose Tissue as a Prognostic Marker for Short- and Long-Term Outcomes after Primary Surgical Treatment for Lung Cancer. Cancers, 15(23), 5666. https://doi.org/10.3390/cancers15235666








