Clonality Analysis for the Relationship between the Pulmonary Combined Neuroendocrine Carcinoma and “the So-Called Reported Histologic Transformation”
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Immunohistochemistry for Determining the Expression of p53 and Rb
2.3. Tissue Dissection for C-NEC
2.4. Whole Exome Sequencing for C-NEC
2.5. Data Processing for C-NEC
2.6. Clonal Analysis for C-NEC
2.7. Statistical Analyses
3. Results
3.1. Clinicopathological Characteristics of the Patients
3.2. Expression of p53 and Rb Assessed Using IHC
3.3. Molecular Alterations
3.3.1. Mutations in TP53 and RB1
3.3.2. EGFR Mutation
3.3.3. Molecular Alterations in Other Common Genes
3.4. Clonal Evolution Analysis for C-NEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangum, M.D.; Greco, F.A.; Hainsworth, J.D.; Hande, K.R.; Johnson, D.H. Combined small-cell and non-small-cell lung cancer. J. Clin. Oncol. 1989, 7, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E.; Hasleton, P.S.; Colby, T.V.; Sheppard, M.N.; Falk, R.; Travis, W.D. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Iyoda, A.; Roh, M.S.; Sica, G.; Kuk, D.; Sima, C.S.; Pietanza, M.C.; Park, B.J.; Travis, W.D.; Rusch, V.W. Neoadjuvant and Adjuvant Chemotherapy in Resected Pulmonary Large Cell Neuroendocrine Carcinomas: A Single Institution Experience. Ann. Thorac. Surg. 2011, 92, 1180–1187, discussion 6–7. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, A.E.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Wang, K.; Elkadi, O.R.; Tarasen, A.; Foulke, L.; Sheehan, E.C.; Otto, A.G.; Palmer, G.; Yelensky, R.; Lipson, D.; et al. Next-generation sequencing reveals frequent consistent genomic alterations in small cell undifferentiated lung cancer. J. Clin. Pathol. 2014, 67, 772–776. [Google Scholar] [CrossRef]
- Miyoshi, T.; Umemura, S.; Matsumura, Y.; Mimaki, S.; Tada, S.; Makinoshima, H.; Ishii, G.; Udgawa, H.; Matsumoto, S.; Yoh, K.; et al. Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin. Cancer Res. 2017, 23, 757–765. [Google Scholar] [CrossRef]
- Rekhtman, N.; Pietanza, M.C.; Hellmann, M.D.; Naidoo, J.; Arora, A.; Won, H.; Halpenny, D.F.; Wang, H.; Tian, S.K.; Litvak, A.M.; et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma–like and Non–Small Cell Carcinoma–like Subsets. Clin. Cancer Res. 2016, 22, 3618–3629. [Google Scholar] [CrossRef]
- The Clinical Lung Cancer Genome Project, Network Genomic Medicine NGM. A genomics-based classification of human lung tumors. Sci. Transl. Med. 2013, 5, 209ra153. [Google Scholar]
- Shiao, T.-H.; Chang, Y.-L.; Yu, C.-J.; Chang, Y.-C.; Hsu, Y.-C.; Chang, S.-H.; Shih, J.-Y.; Yang, P.-C. Epidermal Growth Factor Receptor Mutations in Small Cell Lung Cancer: A Brief Report. J. Thorac. Oncol. 2011, 6, 195–198. [Google Scholar] [CrossRef]
- Ohashi, K.; Maruvka, Y.E.; Michor, F.; Pao, W. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor–Resistant Disease. J. Clin. Oncol. 2013, 31, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Duan, H.; Liu, X.; Zhou, L.; Liang, Z. Genetic alterations and protein expression in combined small cell lung cancers and small cell lung cancers arising from lung adenocarcinomas after therapy with tyrosine kinase inhibitors. Oncotarget 2016, 7, 34240–34249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, J.; Kim, S.; Kim, S.; Youk, J.; Park, S.; An, Y.; Keam, B.; Kim, D.-W.; Heo, D.S.; et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 2017, 35, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Leblay, N.; Thunnissen, E.; van Suylen, R.J.; den Bakker, M.; Groen, H.J.; Smit, E.F.; Damhuis, R.; van den Broek, E.C.; Charbrier, A.; et al. Molecular Subtypes of Pulmonary Large-cell Neuroendocrine Carcinoma Predict Chemotherapy Treatment Outcome. Clin. Cancer Res. 2018, 24, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zakowski, M.F.; Ladanyi, M.; Kris, M.G. Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome Group. EGFR mutations in small-cell lung cancers in patients who have never smoked. N. Engl. J. Med. 2006, 355, 213–215. [Google Scholar] [CrossRef]
- Marcoux, N.; Gettinger, S.N.; O’Kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Fujita, S.; Masago, K.; Katakami, N.; Yatabe, Y. Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J. Thorac. Oncol. 2016, 11, e67–e72. [Google Scholar] [CrossRef]
- Abdallah, N.; Nagasaka, M.; Abdulfatah, E.; Shi, D.; Wozniak, A.J.; Sukari, A. Non-small cell to small cell lung cancer on PD-1 inhibitors: Two cases on potential histologic transformation. Lung Cancer Targets Ther. 2018, 9, 85–90. [Google Scholar] [CrossRef]
- Lee, M.; Patel, D.; Jofre, S.; Fidvi, S.; Suhrland, M.; Cohen, P.; Cheng, H. Large Cell Neuroendocrine Carcinoma Transformation as a Mechanism of Acquired Resistance to Osimertinib in Non-small Cell Lung Cancer: Case Report and Literature Review. Clin. Lung Cancer 2022, 23, e276–e282. [Google Scholar] [CrossRef]
- Izumi, H.; Yamasaki, A.; Ueda, Y.; Sumikawa, T.; Maeta, H.; Nakamoto, S.; Shimizu, E. Squamous Cell Carcinoma Transformation from EGFR-mutated Lung Adenocarcinoma: A Case Report and Literature Review. Clin. Lung Cancer 2018, 19, e63–e66. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Chen, H.; Jia, J.; Wang, L.; Feng, H.; Wang, H.; Song, Z.; Yang, N.; Zhang, Y. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer 2021, 155, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Y.; Ying, J.; Cai, W.; Li, J.; Lee, K.Y.; Ricciuti, B.; Pacheco, J.; Xing, P. Whole exome sequencing (WES) analysis of transformed small cell lung cancer (SCLC) from lung adenocarcinoma (LUAD). Transl. Lung Cancer Res. 2020, 9, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, Y.; Wang, H.; Jia, T.; Wang, E.; Luo, Y.; Wei, Y.; Qin, Z.; Ma, X. Small cell lung cancer transformation: From pathogenesis to treatment. Semin. Cancer Biol. 2022, 86, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, A.J. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Cerbone, M.; De Luca, C.; Russo, D.; Di Maio, A.; De Marco, M.; Turco, M.C.; Insabato, L.; Zullo, F. Diagnostic accuracy of p53 immunohistochemistry as surrogate of TP53 sequencing in endometrial cancer. Pathol. Res. Pract. 2020, 216, 153025. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385.E18. [Google Scholar] [CrossRef]
- Martínez-Jiménez, F.; Muiños, F.; Sentís, I.; Deu-Pons, J.; Reyes-Salazar, I.; Arnedo-Pac, C.; Mularoni, L.; Pich, O.; Bonet, J.; Kranas, H.; et al. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 2020, 20, 273. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Levacq, D.; D’Haene, N.; de Wind, R.; Remmelink, M.; Berghmans, T. Histological transformation of ALK rearranged adenocarcinoma into small cell lung cancer: A new mechanism of resistance to ALK inhibitors. Lung Cancer 2016, 102, 38–41. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-W.; Su, K.-Y.; Su, T.-J.; Chang, C.-C.; Lin, J.-W.; Lee, Y.-H.; Yu, S.-L.; Chen, J.-S.; Hsieh, M.-S. Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer. Lung Cancer 2018, 125, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Tsuta, K.; Furuta, K.; Watanabe, S.-I.; Asamura, H.; Ohe, Y.; Maeshima, A.M.; Shibata, T.; Masuda, N.; Matsuno, Y. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci. 2007, 98, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Yokobori, T.; Mogi, A.; Shimizu, K.; Yajima, T.; Kosaka, T.; Ohtaki, Y.; Obayashi, K.; Nakazawa, S.; Gombodorj, N.; et al. Genetic and Immunohistochemical Studies Investigating the Histogenesis of Neuroendocrine and Carcinomatous Components of Combined Neuroendocrine Carcinoma. Ann. Surg. Oncol. 2019, 26, 1744–1750. [Google Scholar] [CrossRef]
- Tatematsu, A.; Shimizu, J.; Murakami, Y.; Horio, Y.; Nakamura, S.; Hida, T.; Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor mutations in small cell lung cancer. Clin. Cancer Res. 2008, 14, 6092–6096. [Google Scholar] [CrossRef]
- van Riel, S.; Thunnissen, E.; Heideman, D.; Smit, E.F.; Biesma, B. A patient with simultaneously appearing adenocarcinoma and small-cell lung carcinoma harbouring an identical EGFR exon 19 mutation. Ann. Oncol. 2012, 23, 3188–3189. [Google Scholar] [CrossRef]
- Watanabe, S.; Sone, T.; Matsui, T.; Yamamura, K.; Tani, M.; Okazaki, A.; Kurokawa, K.; Tambo, Y.; Takato, H.; Ohkura, N.; et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer 2013, 82, 370–372. [Google Scholar] [CrossRef]
- Morinaga, R.; Okamoto, I.; Furuta, K.; Kawano, Y.; Sekijima, M.; Dote, K.; Satou, T.; Nishio, K.; Fukuoka, M.; Nakagawa, K. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer 2007, 58, 411–413. [Google Scholar] [CrossRef]
- Popat, S.; Wotherspoon, A.; Nutting, C.; Gonzalez, D.; Nicholson, A.; O’brien, M. Transformation to “high grade” neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer 2013, 80, 1–4. [Google Scholar] [CrossRef]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.S.; Micinski, A.M.; Kastner, D.W.; Guo, B.; Wait, S.J.; Spainhower, K.B.; Conley, C.C.; Chen, O.S.; Guthrie, M.R.; Soltero, D.; et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Res. 2020, 80, PO-120. [Google Scholar] [CrossRef]
- Tolomeo, D.; Traversa, D.; Venuto, S.; Ebbesen, K.K.; Rodríguez, J.L.G.; Tamma, G.; Ranieri, M.; Simonetti, G.; Ghetti, M.; Paganelli, M.; et al. circPVT1 and show a role in cell proliferation, apoptosis, and tumor subtype-definition in small cell lung cancer. Gene Chromosome Cancer 2023, 62, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, S.; Wang, H.; Chi, K.; Ren, W.; Huang, X.; Zhuo, M.; Lin, D. Molecular subtype expression and genomic profiling differ between surgically resected pure and combined small cell lung carcinoma. Hum. Pathol. 2023, 141, 118–129. [Google Scholar] [CrossRef] [PubMed]
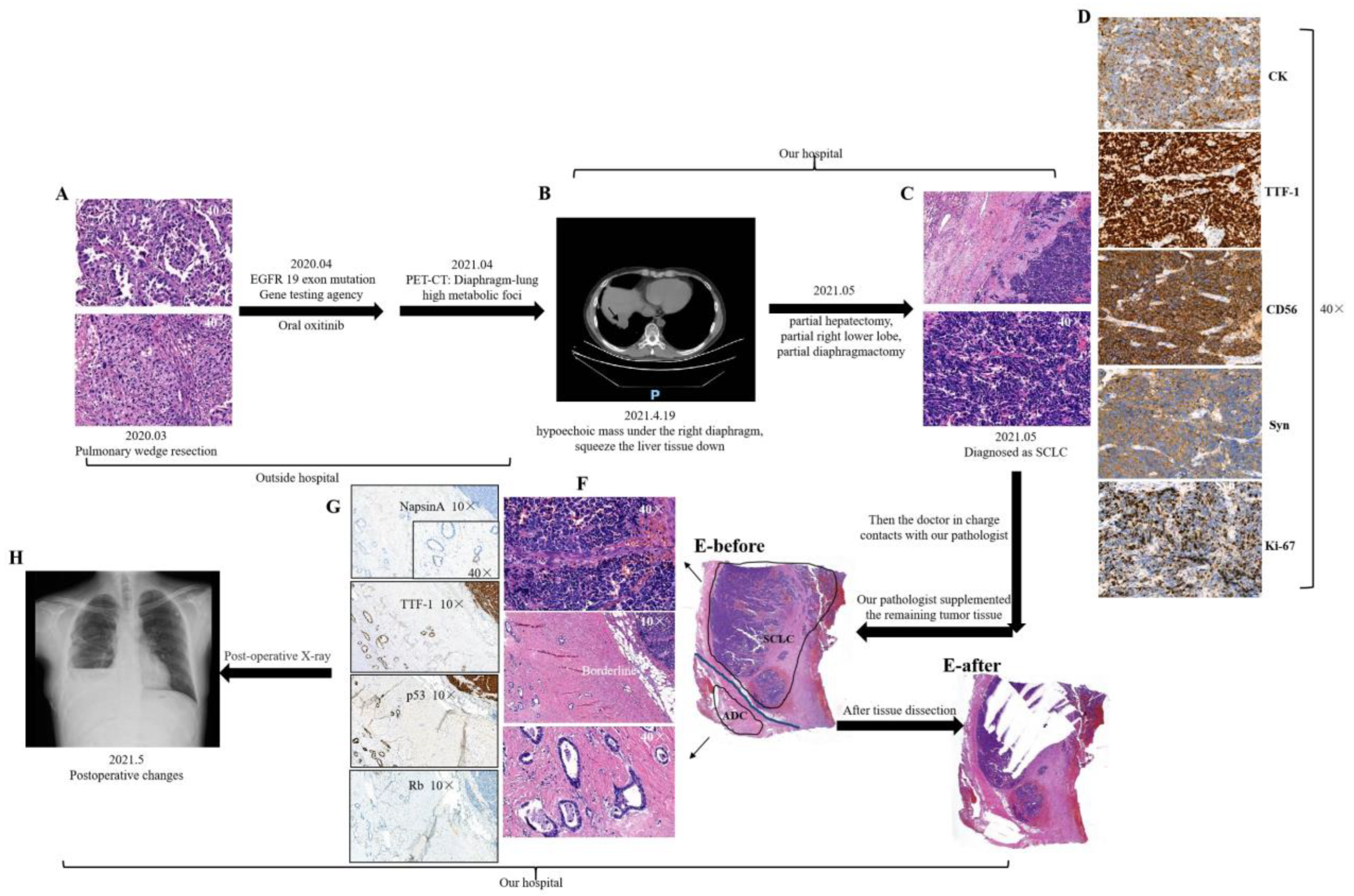
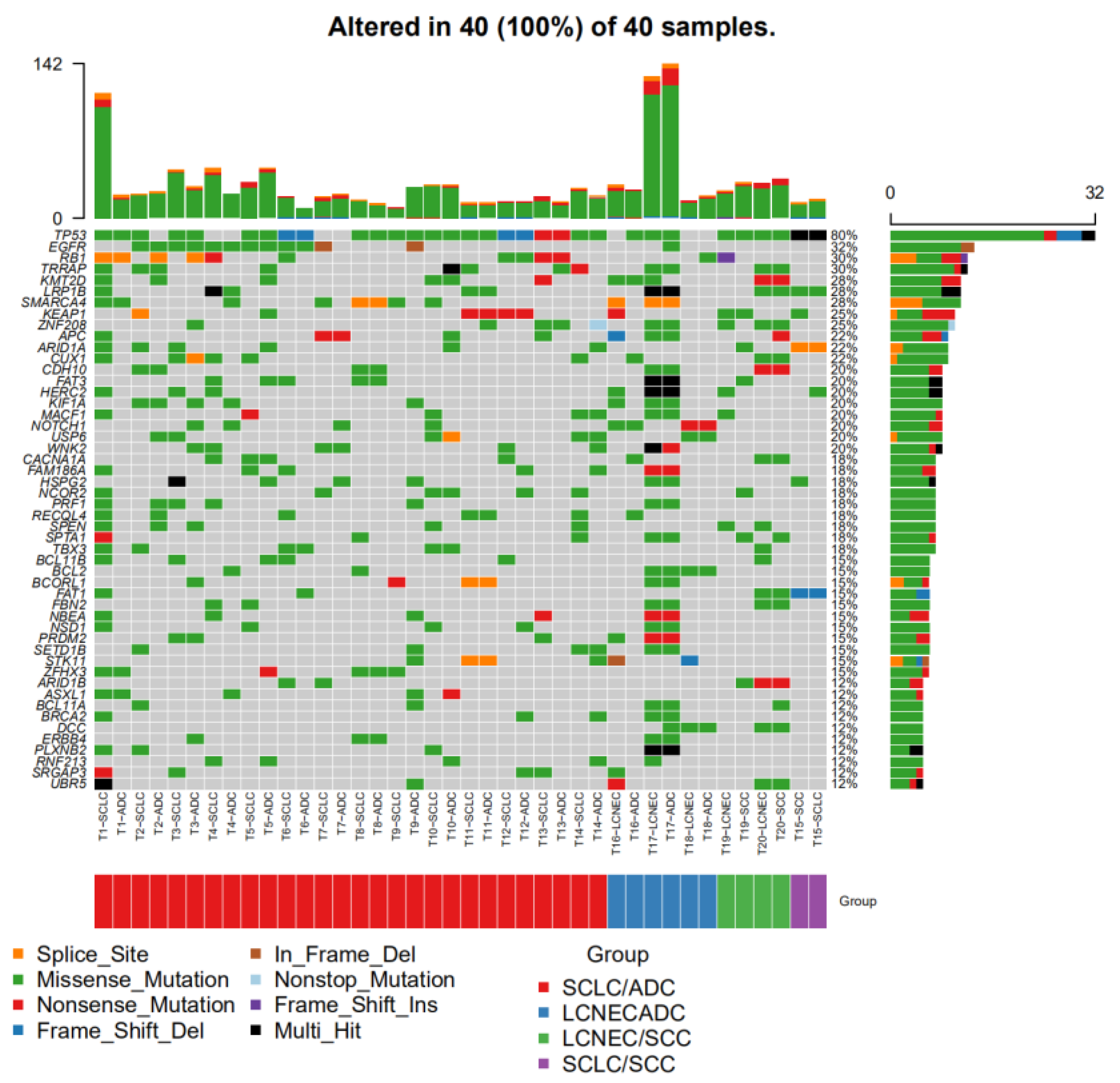
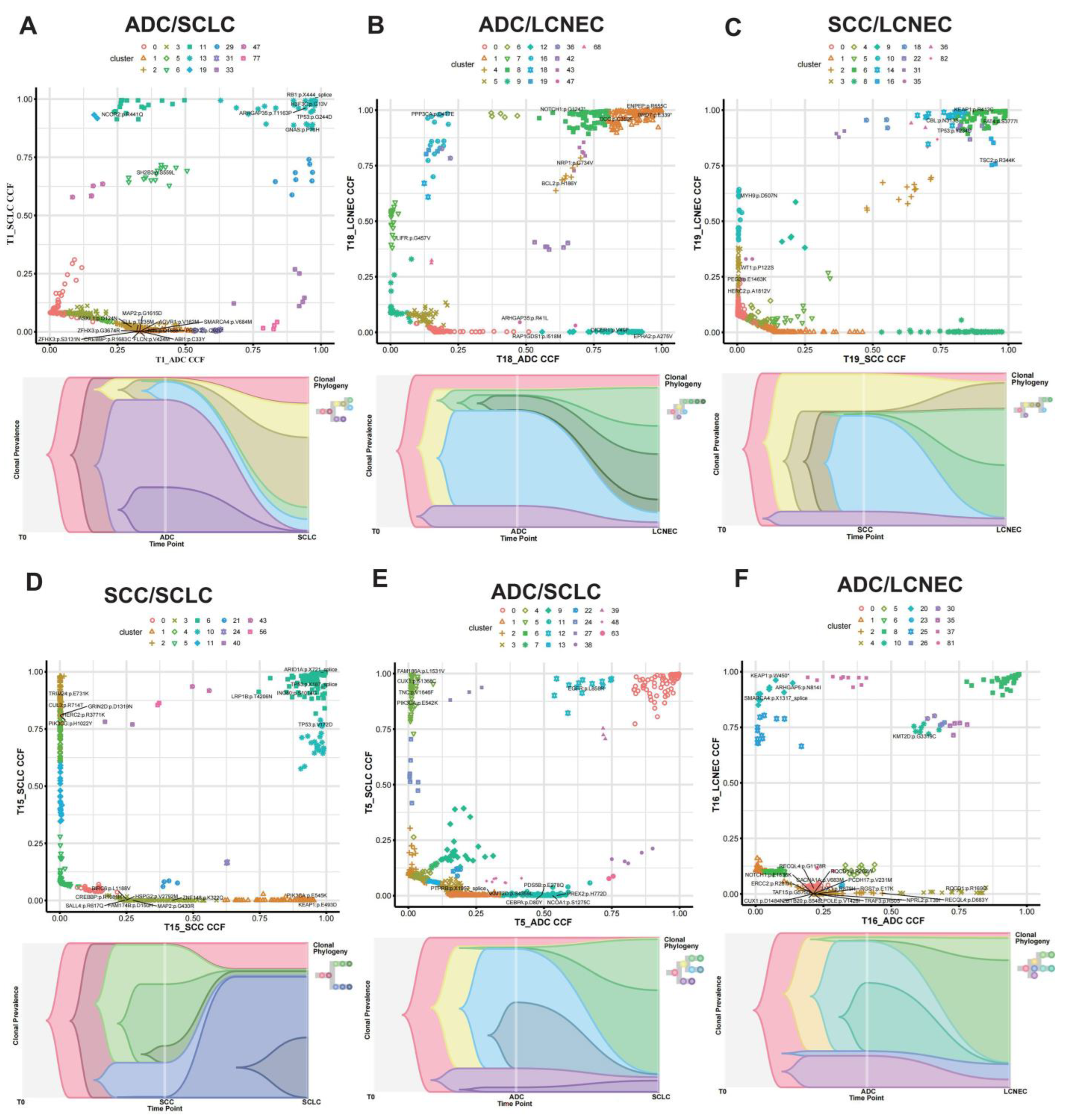

| Variable | N (%) | C-SCLC | C-LCNEC | ||
|---|---|---|---|---|---|
| SCLC/ADC (n = 15) | SCLC/SCC (n = 1) | LCNEC/ADC (n = 3) | LCNEC/SCC (n = 2) | ||
| Gender | |||||
| Male | 18 (85.7) | 13 | 5 | ||
| Female | 3 (14.3) | 3 | 0 | ||
| Age (median) | |||||
| ≤62y | 11 (52.4) | 10 | 1 | ||
| >62y | 10 (47.6) | 6 | 4 | ||
| Mitotic Figure (NE) | |||||
| ≤31 | 11 (52.4) | 9 | 2 | ||
| >31 | 10 (47.6) | 7 | 3 | ||
| Mitotic Figure (non-NE) | |||||
| ≤2 | 14 (66.7) | 11 | 3 | ||
| >2 | 7 (33.3) | 5 | 2 | ||
| Tumor size (cm) | |||||
| ≤3.4 | 10 (47.6) | 8 | 2 | ||
| >3.4 | 11 (52.4) | 8 | 3 | ||
| Tumor location | |||||
| Peripheral | 10 (47.6) | 9 | 1 | ||
| Central | 11 (52.4) | 7 | 4 | ||
| Necrosis | |||||
| Presence | 12 (57.1) | 7 | 5 | ||
| Absence | 9 (42.9) | 9 | 0 | ||
| Pleural invasion | |||||
| Yes | 12 (57.1) | 9 | 3 | ||
| No | 9 (42.9) | 7 | 2 | ||
| Vascular invasion | |||||
| Yes | 8 (38.1) | 6 | 2 | ||
| No | 13 (61.9) | 10 | 3 | ||
| Nerve invasion | |||||
| Yes | 0 (0.0) | 0 | 0 | ||
| No | 21 (100.0) | 16 | 5 | ||
| STAS | |||||
| Yes | 2 (9.5) | 2 | 0 | ||
| No | 19 (90.5) | 14 | 5 | ||
| Neoadjuvant therapy | |||||
| Yes | 6 (28.6) | 4 | 2 | ||
| No | 15 (71.4) | 12 | 3 | ||
| Smoke | |||||
| Yes | 1 (4.8) | 1 | 0 | ||
| Pre-smoking | |||||
| Within 1 year | 3 (14.3) | 2 | 1 | ||
| Within 1–3 years | 3 (14.3) | 1 | 2 | ||
| Above 3 years | 4 (19.0) | 3 | 1 | ||
| Never | 10 (47.6) | 9 | 1 | ||
| Family history | |||||
| Yes | 5 (23.8) | 3 | 2 | ||
| No | 16 (76.2) | 13 | 3 | ||
| T stage | |||||
| T1a | 1 (4.8) | 1 | 0 | ||
| T1b | 1 (4.8) | 1 | 0 | ||
| T1c | 7 (33.3) | 6 | 1 | ||
| T2a | 8 (38.1) | 5 | 3 | ||
| T2b | 1 (4.8) | 1 | 0 | ||
| T3 | 2 (9.4) | 1 | 1 | ||
| T4 | 1 (4.8) | 1 | 0 | ||
| n | |||||
| 0 | 15 (71.4) | 12 | 3 | ||
| N1a | 1 (4.8) | 0 | 1 | ||
| N1b | 2 (9.4) | 2 | 0 | ||
| N2a1 | 1 (4.8) | 0 | 1 | ||
| N2a2 | 1 (4.8) | 1 | 0 | ||
| N2b | 1 (4.8) | 1 | 0 | ||
| Clinical stage | |||||
| IA1 | 0 (0.0) | 0 | 0 | ||
| IA2 | 1 (4.8) | 1 | 0 | ||
| IA3 | 3 (14.3) | 3 | 0 | ||
| IB | 7 (33.3) | 5 | 2 | ||
| IIA | 1 (4.8) | 1 | 0 | ||
| IIB | 5 (23.8) | 3 | 2 | ||
| IIIA | 3 (14.3) | 2 | 1 | ||
| IV | 1 (4.8) | 1 | 0 | ||
| Rb IHC Expression | |||||
|---|---|---|---|---|---|
| p53 IHC expression | Inconsistent expression | Consistent mutant expression | Consistent wild-type expression | Total | |
| Inconsistent expression | 1 | 2 | 1 | 4 | |
| Consistent mutant expression | 2 | 9 | 3 | 14 | |
| Consistent wild-type expression | 0 | 1 | 2 | 3 | |
| Total | 3 | 12 | 6 | 21 | |
| RB1 Status | |||||
|---|---|---|---|---|---|
| TP53 status | Inconsistent status | Consistent mutant type | Consistent wild type | Total | |
| Inconsistent status | 1 | 0 | 1 | 2 | |
| Consistent mutant type | 3 | 3 | 10 | 16 | |
| Consistent wild type | 2 | 0 | 1 | 3 | |
| Total | 6 | 3 | 12 | 21 | |
| Category | Combined NECs (%) | Concordance Rate (%) | |
|---|---|---|---|
| C-SCLC | C-LCNEC | ||
| TP53 | 18/21 (85.7) | 17/21 (81.0) | |
| 14/16 (87.5) | 4/5 (80.0) | ||
| p53 IHC | 18/21 (85.7) | ||
| 14/16 (87.5) | 4/5 (80.0) | ||
| RB1 | 9/21 (42.9) | 13/21 (61.9) | |
| 7/16 (43.8) | 2/5 (40.0) | ||
| Rb IHC | 15/21 (71.4) | ||
| 13/16 (81.3) | 2/5 (40.0) | ||
| EGFR | 9/21 (42.9) | / | |
| 8/16 (50.0) | 1/5 (20.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhu, Y.; Sun, W.; Yang, X.; Liu, X.; Chi, K.; Huang, X.; Zhou, L.; Cai, W.; Lin, D. Clonality Analysis for the Relationship between the Pulmonary Combined Neuroendocrine Carcinoma and “the So-Called Reported Histologic Transformation”. Cancers 2023, 15, 5649. https://doi.org/10.3390/cancers15235649
Wang H, Zhu Y, Sun W, Yang X, Liu X, Chi K, Huang X, Zhou L, Cai W, Lin D. Clonality Analysis for the Relationship between the Pulmonary Combined Neuroendocrine Carcinoma and “the So-Called Reported Histologic Transformation”. Cancers. 2023; 15(23):5649. https://doi.org/10.3390/cancers15235649
Chicago/Turabian StyleWang, Haiyue, Yanli Zhu, Wei Sun, Xin Yang, Xinying Liu, Kaiwen Chi, Xiaozheng Huang, Lixin Zhou, Weijing Cai, and Dongmei Lin. 2023. "Clonality Analysis for the Relationship between the Pulmonary Combined Neuroendocrine Carcinoma and “the So-Called Reported Histologic Transformation”" Cancers 15, no. 23: 5649. https://doi.org/10.3390/cancers15235649
APA StyleWang, H., Zhu, Y., Sun, W., Yang, X., Liu, X., Chi, K., Huang, X., Zhou, L., Cai, W., & Lin, D. (2023). Clonality Analysis for the Relationship between the Pulmonary Combined Neuroendocrine Carcinoma and “the So-Called Reported Histologic Transformation”. Cancers, 15(23), 5649. https://doi.org/10.3390/cancers15235649





