Endometrial Cancer with and without Endometriosis: Clinicopathological Differences
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
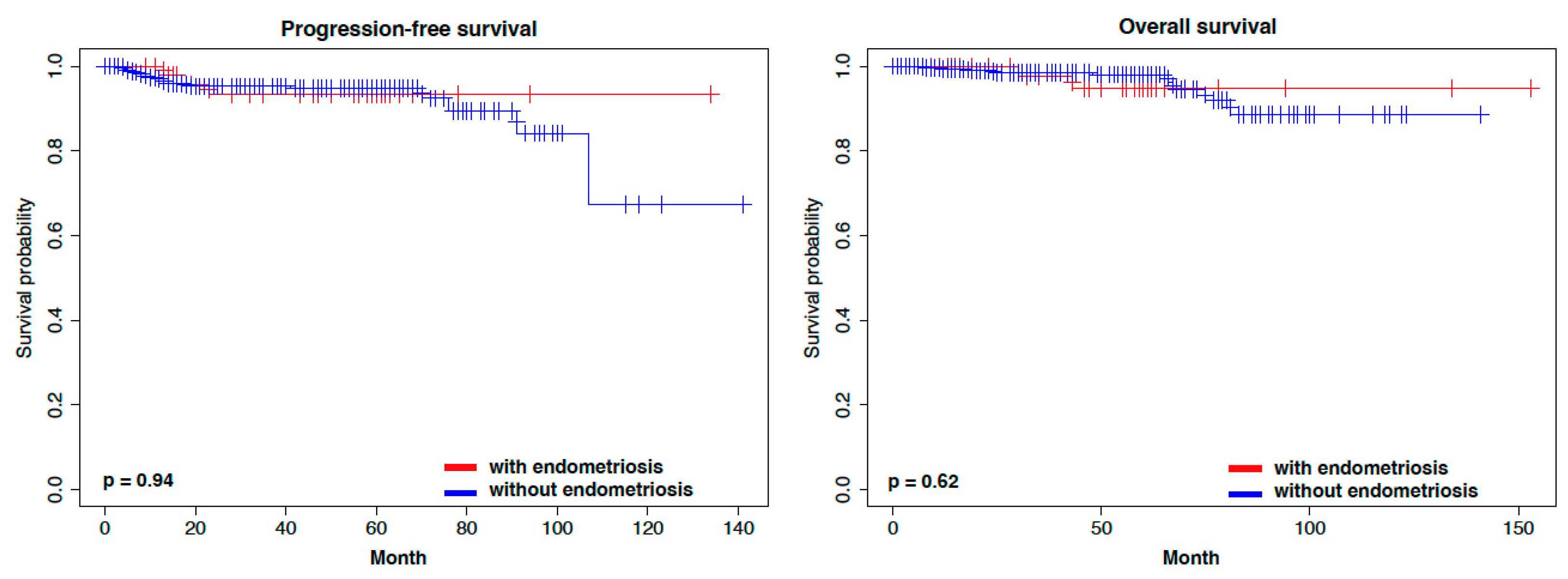
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenberg, V.H.; Weil, C.; Chodick, G.; Shalev, V. Epidemiology of endometriosis: A large population-based database study from a healthcare provider with 2 million members. BJOG 2018, 125, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, D.; Murphy-Barron, C.; Iwasaki, K. Actuarial analysis of private payer administrative claims data for women with endometriosis. J. Manag. Care Pharm. 2007, 13, 262–272. [Google Scholar] [CrossRef]
- Morassutto, C.; Monasta, L.; Ricci, G.; Barbone, F.; Ronfani, L. Incidence and Estimated Prevalence of Endometriosis and Adenomyosis in Northeast Italy: A Data Linkage Study. PLoS ONE 2016, 11, e0154227. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, W.; Mao, W. Malignant changes in adenomyosis in patients with endometrial adenocarcinoma: A case series. Medicine 2017, 96, e8336. [Google Scholar] [CrossRef]
- Pollacco, J.; Sacco, K.; Portelli, M.; Schembri-Wismayer, P.; Calleja-Agius, J. Molecular links between endometriosis and cancer. Gynecol. Endocrinol. 2012, 28, 577–581. [Google Scholar] [CrossRef]
- Aris, A. Endometriosis-associated ovarian cancer: A ten-year cohort study of women living in the Estrie Region of Quebec, Canada. J. Ovarian Res. 2010, 3, 2. [Google Scholar] [CrossRef]
- Kok, V.C.; Tsai, H.J.; Su, C.F.; Lee, C.K. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int. J. Gynecol. Cancer 2015, 25, 968–976. [Google Scholar] [CrossRef]
- Mogensen, J.B.; Kjaer, S.K.; Mellemkjaer, L.; Jensen, A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol. Oncol. 2016, 143, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J. Women’s Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, F.E.; Iliadou, A.N.; Rodriguez-Wallberg, K.; Gemzell-Danielsson, K.; Johansson, A.L.V. The risk of breast and gynecological cancer in women with a diagnosis of infertility: A nationwide population-based study. Eur. J. Epidemiol. 2019, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, L.; Ayansina, D.; Cooper, K.G.; Bhattacharya, S.; Horne, A.W.; Bhattacharya, S. Impact of endometriosis on risk of further gynaecological surgery and cancer: A national cohort study. BJOG 2018, 125, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Jones, M.E.; Swerdlow, A.J.; Botting, B.J.; Davies, M.C.; Jacobs, I.; Bunch, K.J.; Murphy, M.F.G.; Sutcliffe, A.G. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991-2010: Data linkage study including 2.2 million person years of observation. BMJ 2018, 362, k2644. [Google Scholar] [CrossRef]
- Poole, E.M.; Lin, W.T.; Kvaskoff, M.; De Vivo, I.; Terry, K.L.; Missmer, S.A. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control 2017, 28, 437–445. [Google Scholar] [CrossRef]
- Saavalainen, L.; Lassus, H.; But, A.; Tiitinen, A.; Harkki, P.; Gissler, M.; Pukkala, E.; Heikinheimo, O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet. Gynecol. 2018, 131, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M. Environmental (nongenetic) factors in gynecological cancers: Update and future perspectives. Future Oncol. 2015, 11, 295–307. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Yong, P.J. Endometriosis-associated Ovarian Cancers. Clin. Obstet. Gynecol. 2017, 60, 711–727. [Google Scholar] [CrossRef]
- Sato, N.; Tsunoda, H.; Nishida, M.; Morishita, Y.; Takimoto, Y.; Kubo, T.; Noguchi, M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000, 60, 7052–7056. [Google Scholar] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Suda, K.; Cruz Diaz, L.A.; Yoshihara, K.; Nakaoka, H.; Yachida, N.; Motoyama, T.; Inoue, I.; Enomoto, T. Clonal lineage from normal endometrium to ovarian clear cell carcinoma through ovarian endometriosis. Cancer Sci. 2020, 111, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Mao, T.L.; Seckin, T.; Wu, C.H.; Guan, B.; Ogawa, H.; Futagami, M.; Mizukami, H.; Yokoyama, Y.; Kurman, R.J.; et al. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int. J. Gynecol. Cancer 2012, 22, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrigton, C.S.; Young, R.H. WHO Classification of Tumors of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014; pp. 121–167. [Google Scholar]
- Ulbright, T.M.; Roth, L.M. Metastatic and independent cancers of the endometrium and ovary: A clinicopathologic study of 34 cases. Hum. Pathol. 1985, 16, 28–34. [Google Scholar] [CrossRef]
- Scully, R.; Young, R. Metastatic Tumors of the Ovary; Springer-Verlag: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Prat, J.; Gallardo, A.; Cuatrecasas, M.; Catasus, L. Endometrial carcinoma: Pathology and genetics. Pathology 2007, 39, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ortmann, O. Endocrine Risk Factors of Endometrial Cancer: Polycystic Ovary Syndrome, Oral Contraceptives, Infertility, Tamoxifen. Cancers 2020, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.S.; Weissfeld, J.L.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Linkov, F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010, 21, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Nakaoka, H.; Suda, K.; Yoshihara, K.; Ishiguro, T.; Yachida, N.; Saito, K.; Ueda, H.; Sugino, K.; Mori, Y.; et al. Spatiotemporal dynamics of clonal selection and diversification in normal endometrial epithelium. Nat. Commun. 2022, 13, 943. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Raffaelli, R.; Garzon, S.; Baggio, S.; Genna, M.; Pomini, P.; Lagana, A.S.; Ghezzi, F.; Franchi, M. Mesenteric vascular and nerve sparing surgery in laparoscopic segmental intestinal resection for deep infiltrating endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 214–219. [Google Scholar] [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Michels, K.B.; Hunter, D.J. In utero exposures and the incidence of endometriosis. Fertil. Steril. 2004, 82, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Worley, M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.W. Endometriosis-associated ovarian cancer: A review of pathogenesis. Int. J. Mol. Sci. 2013, 14, 5367–5379. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Watanabe, K.; Sato, N.; Ichikawa, Y. Malignant transformation of ovarian endometriosis. Gynecol. Obstet. Investig. 2000, 50 (Suppl. S1), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Gridley, G.; Persson, I.; Baron, J.; Bergqvist, A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am. J. Obstet. Gynecol. 1997, 176, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Zaino, R.; Whitney, C.; Brady, M.F.; DeGeest, K.; Burger, R.A.; Buller, R.E. Simultaneously detected endometrial and ovarian carcinomas—A prospective clinicopathologic study of 74 cases: A gynecologic oncology group study. Gynecol. Oncol. 2001, 83, 355–362. [Google Scholar] [CrossRef]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388.e381. [Google Scholar] [CrossRef]
- Hermens, M.; van Altena, A.M.; Velthuis, I.; van de Laar, D.C.M.; Bulten, J.; van Vliet, H.; Siebers, A.G.; Bekkers, R.L.M. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers 2021, 13, 4592. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Wang, Y.K.; Maassen, M.; Horlings, H.M.; Bashashati, A.; Senz, J.; Mackenzie, R.; Grewal, D.S.; Li-Chang, H.; Karnezis, A.N.; et al. Synchronous Endometrial and Ovarian Carcinomas: Evidence of Clonality. J. Natl. Cancer Inst. 2016, 108, djv428. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Ng, C.K.; De Filippo, M.R.; Piscuoglio, S.; Macedo, G.S.; Gatius, S.; Perez Mies, B.; Soslow, R.A.; Lim, R.S.; Viale, A.; et al. Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. J. Natl. Cancer Inst. 2016, 108, djv427. [Google Scholar] [CrossRef]
- Reijnen, C.; Kusters-Vandevelde, H.V.N.; Ligtenberg, M.J.L.; Bulten, J.; Oosterwegel, M.; Snijders, M.; Sweegers, S.; de Hullu, J.A.; Vos, M.C.; van der Wurff, A.A.M.; et al. Molecular profiling identifies synchronous endometrial and ovarian cancers as metastatic endometrial cancer with favorable clinical outcome. Int. J. Cancer 2020, 147, 478–489. [Google Scholar] [CrossRef]
- Hsu Lin, L.; Allison, D.H.R.; Turashvili, G.; Vasudevaraja, V.; Tran, I.; Serrano, J.; Weigelt, B.; Ladanyi, M.; Abu-Rustum, N.R.; Snuderl, M.; et al. DNA Methylation Signature of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. Mod. Pathol. 2023, 36, 100321. [Google Scholar] [CrossRef] [PubMed]

| Site of Endometriosis | Case * |
|---|---|
| Ovary | 48.5% (49/101) |
| Uterus | 48.5% (49/101) |
| Fallopian tube | 4.0% (4/101) |
| Peritoneum | 2.0% (2/101) |
| Lymph node | 2.0% (2/101) |
| Colorectal | 1.0% (1/101) |
| With Endometriosis (101) | Without Endometriosis (619) | p-Value | |
|---|---|---|---|
| Age | 54.0 (31.0–76.0) | 58.0 (22.0–94.0) | 0.002 |
| BMI | 24.6 ± 5.6 | 24.6 ± 5.6 | 0.852 |
| CA125 [IU] | 219.1 ± 778.8 | 98.9 ± 344.6 | 0.172 |
| Pregnancy | 1.58 ± 1.36 | 1.99 ± 1.54 | 0.019 |
| Delivery | 1.25 ± 1.09 | 1.56 ± 1.15 | 0.012 |
| Hypertension | 23.8% (24/101) | 26.7% (166/618) | 0.722 |
| Diabetes Mellitus | 15.8% (16/101) | 12.9% (80/618) | 0.534 |
| Menopause | 56.4% (57/101) | 63.3% (392/619) | 0.539 |
| With Endometriosis | Without Endometriosis | p-Value | |
|---|---|---|---|
| Number of cases | 101 | 619 | - |
| Stage | |||
| IA | 68.3% (69/101) | 56.4% (349/619) | 0.029 |
| IB | 8.0% (8/101) | 14.9% (92/619) | |
| II | 6.0% (6/101) | 7.8% (48/619) | |
| IIIA | 5.0% (5/101) | 4.0% (25/619) | |
| IIIB | 2.0% (2/101) | 0.5% (3/619) | |
| IIIC1 | 3.0% (3/101) | 5.7% (35/619) | |
| IIIC2 | 1.0% (1/101) | 4.0% (25/619) | |
| IVA | 0 (0/101) | 0.2% (1/619) | |
| IVB | 7.0% (7/101) | 7.0% (41/619) | |
| Histology | |||
| Endometrioid | 83.1% (84/101) | 83.0% (514/619) | 1.0 |
| grade 1 | 58.4% (59/101) | 52.8% (327/619) | |
| grade 2 | 18.8% (19/101) | 22.0% (136/619) | |
| grade 3 | 5.9% (6/101) | 8.2% (51/619) | |
| Clear | 5.9% (6/101) | 3.2% (20/619) | |
| Serous | 4.0% (4/101) | 4.7% (29/619) | |
| Mucinous | 1.0% (1/101) | 0.2% (1/619) | |
| Mixed | 4.0% (4/101) | 4.0% (25/619) | |
| Carcinosarcoma | 1.0% (1/101) | 3.1% (19/619) | |
| Others | 1.0% (1/101) | 1.8% (11/619) |
| Synchronous Ovarian Cancer | With Endometriosis (101) | Without Endometriosis (619) | p-Value |
|---|---|---|---|
| Metastasis | 6.0% (6/101) | 6.1% (38/619) | 1.0 |
| Dual primary | 14.9% (15/101) | 1.6% (10/619) | <0.001 |
| Endometrial Cancer | Ovarian Cancer | With Endometriosis (n = 15) | Without Endometriosis (n = 10) |
|---|---|---|---|
| Endometrioid | Endometrioid | 73.3% (11/15) | 50.0% (5/10) |
| Endometrioid | High-grade serous | 13.3% (2/15) | 10.0% (1/10) |
| Endometrioid | Clear | 6.6% (1/15) | 10.0% (1/10) |
| Endometrioid | Mixed | 6.6% (1/15) | 10.0% (1/10) |
| Mixed | High-grade serous | 0.0% (0/15) | 10.0% (1/10) |
| Mixed | Mucinous | 0.0% (0/15) | 10.0% (1/10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minamikawa, T.; Yachida, N.; Takahashi, K.; Saito, K.; Sekizuka, T.; Akashi, H.; Suzuki, M.; Mori, Y.; Yamawaki, K.; Suda, K.; et al. Endometrial Cancer with and without Endometriosis: Clinicopathological Differences. Cancers 2023, 15, 5635. https://doi.org/10.3390/cancers15235635
Minamikawa T, Yachida N, Takahashi K, Saito K, Sekizuka T, Akashi H, Suzuki M, Mori Y, Yamawaki K, Suda K, et al. Endometrial Cancer with and without Endometriosis: Clinicopathological Differences. Cancers. 2023; 15(23):5635. https://doi.org/10.3390/cancers15235635
Chicago/Turabian StyleMinamikawa, Takahiro, Nozomi Yachida, Kotaro Takahashi, Kyota Saito, Tomoyuki Sekizuka, Hidehiko Akashi, Miho Suzuki, Yutaro Mori, Kaoru Yamawaki, Kazuaki Suda, and et al. 2023. "Endometrial Cancer with and without Endometriosis: Clinicopathological Differences" Cancers 15, no. 23: 5635. https://doi.org/10.3390/cancers15235635
APA StyleMinamikawa, T., Yachida, N., Takahashi, K., Saito, K., Sekizuka, T., Akashi, H., Suzuki, M., Mori, Y., Yamawaki, K., Suda, K., Tamura, R., Adachi, S., & Yoshihara, K. (2023). Endometrial Cancer with and without Endometriosis: Clinicopathological Differences. Cancers, 15(23), 5635. https://doi.org/10.3390/cancers15235635





