Urethral Mesh Assessment in Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
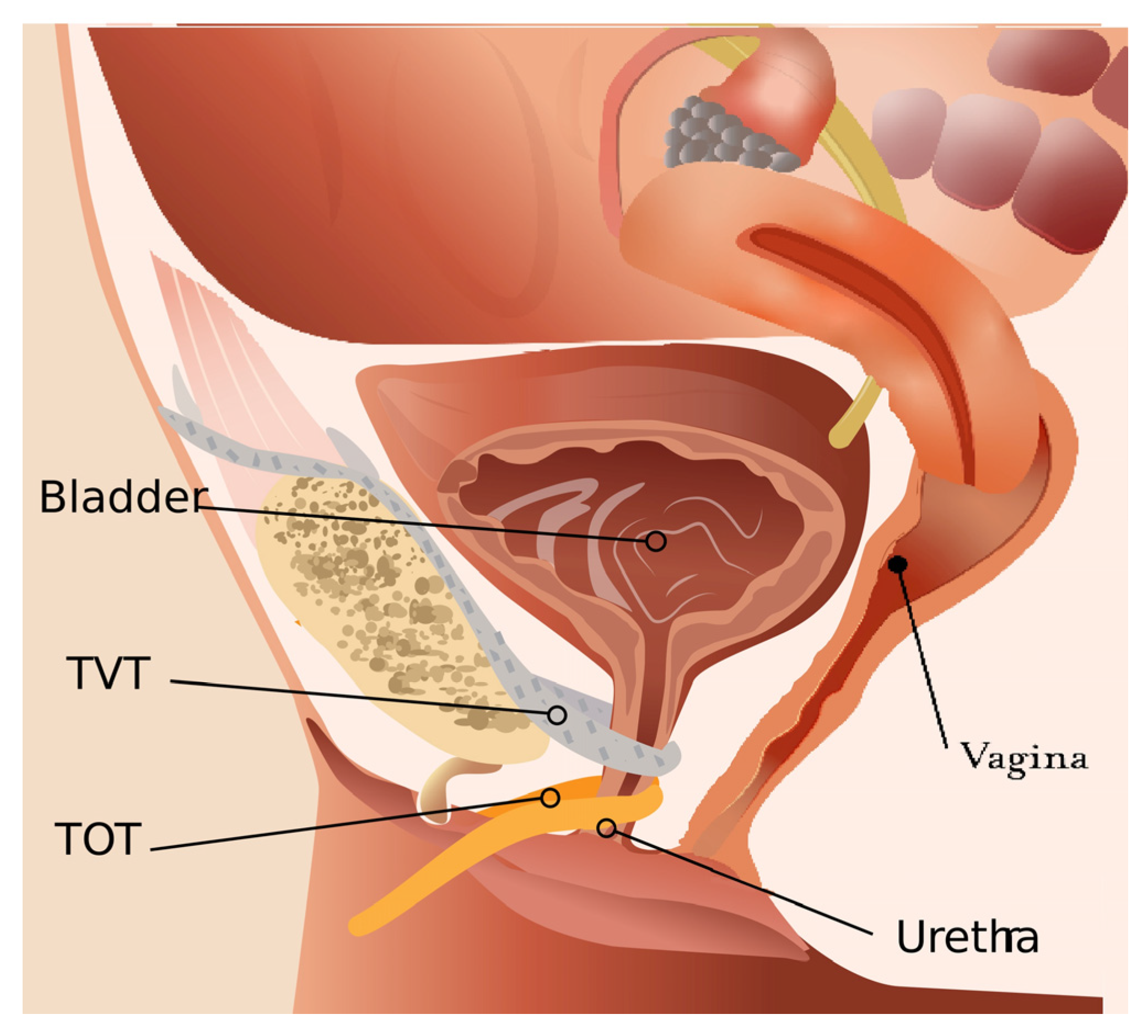
2. Types of Urethral Meshes
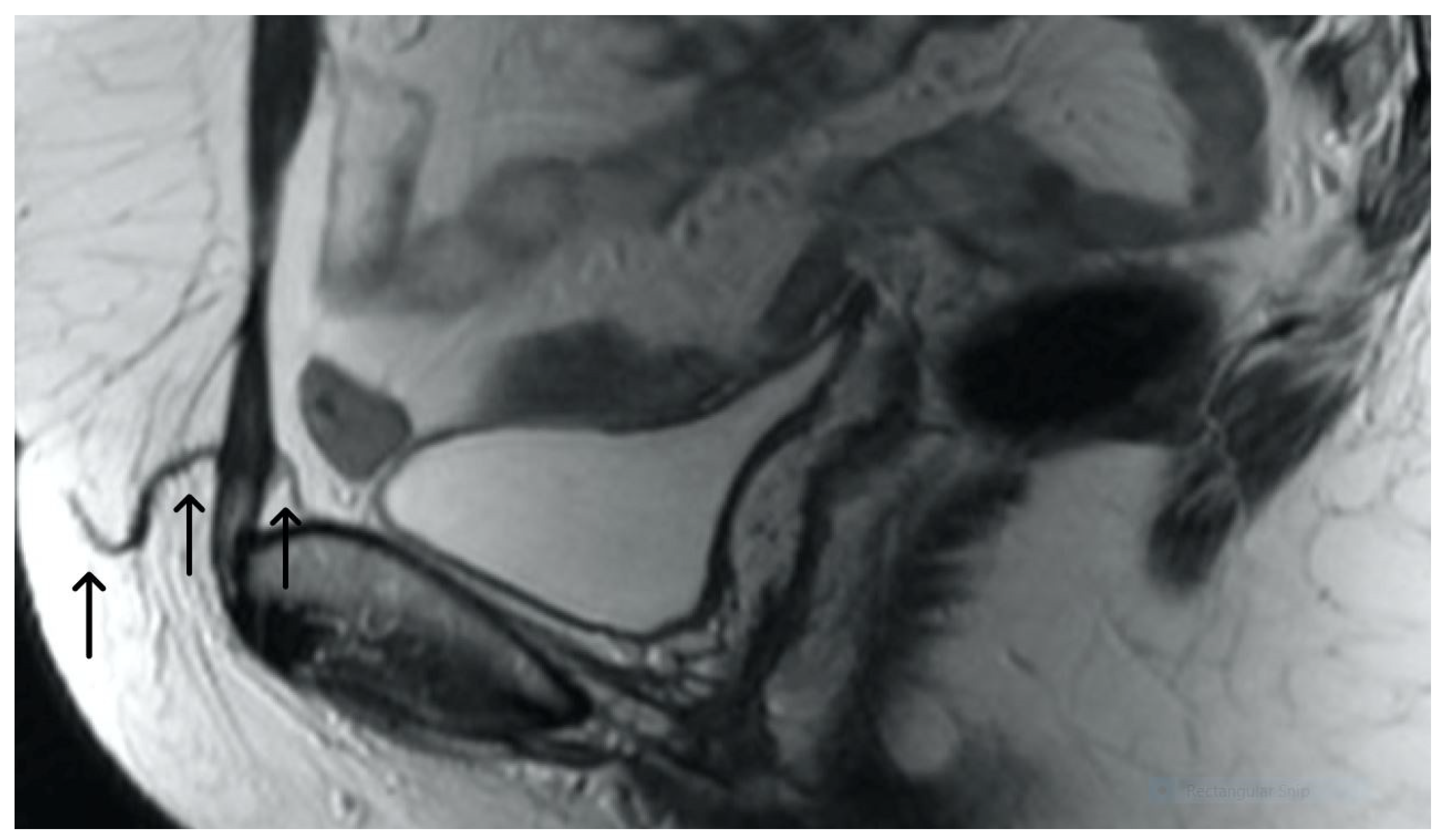
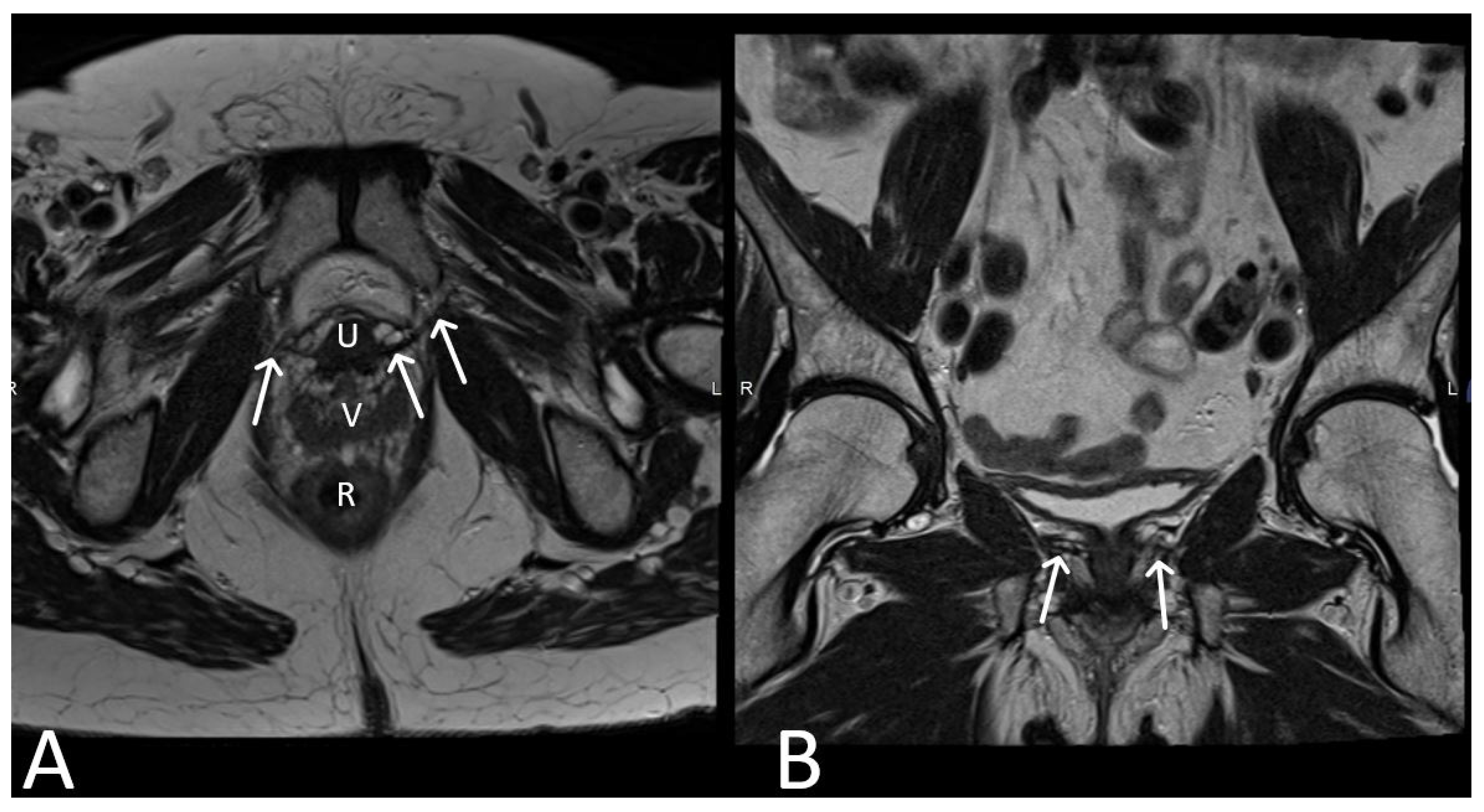
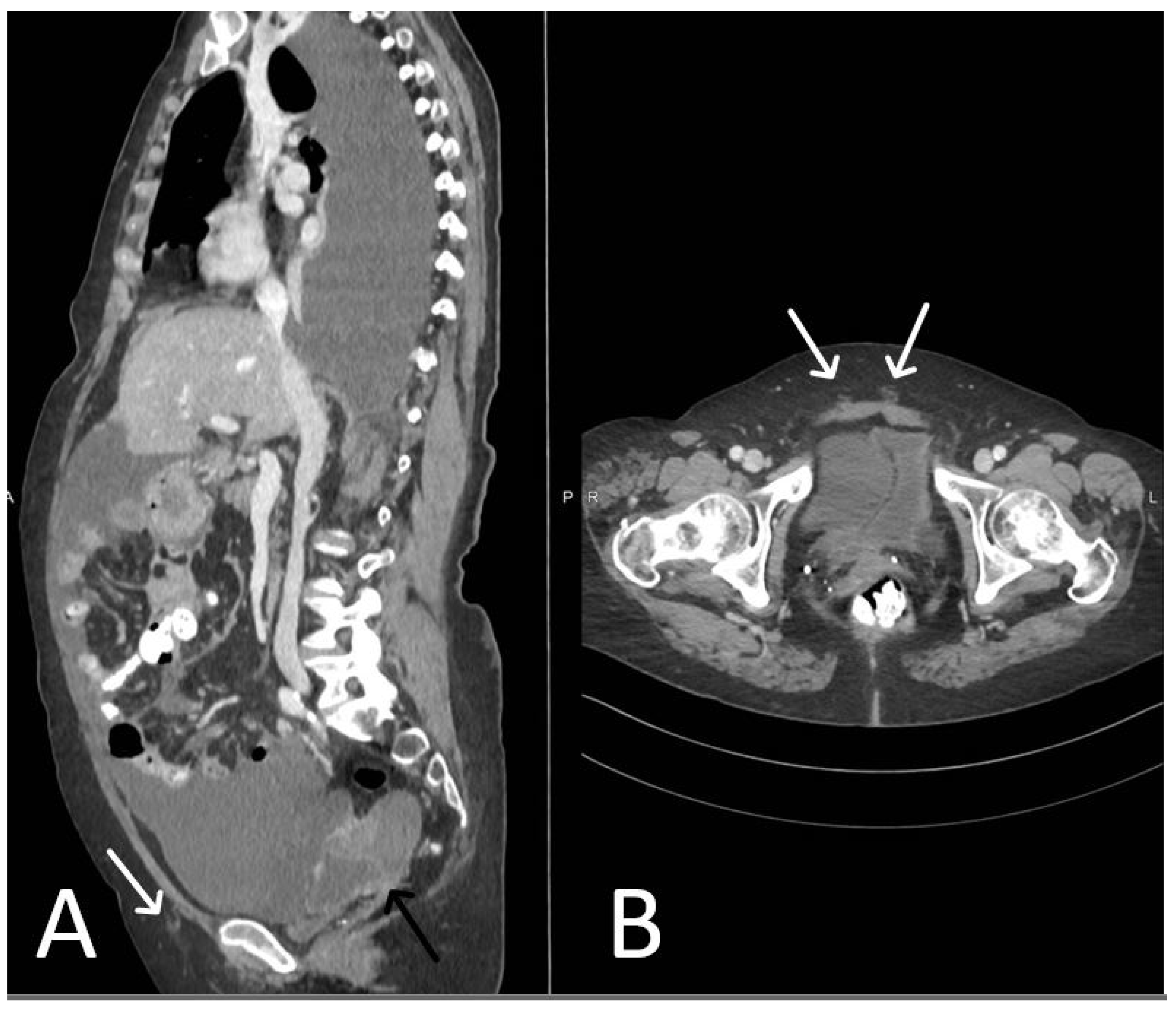
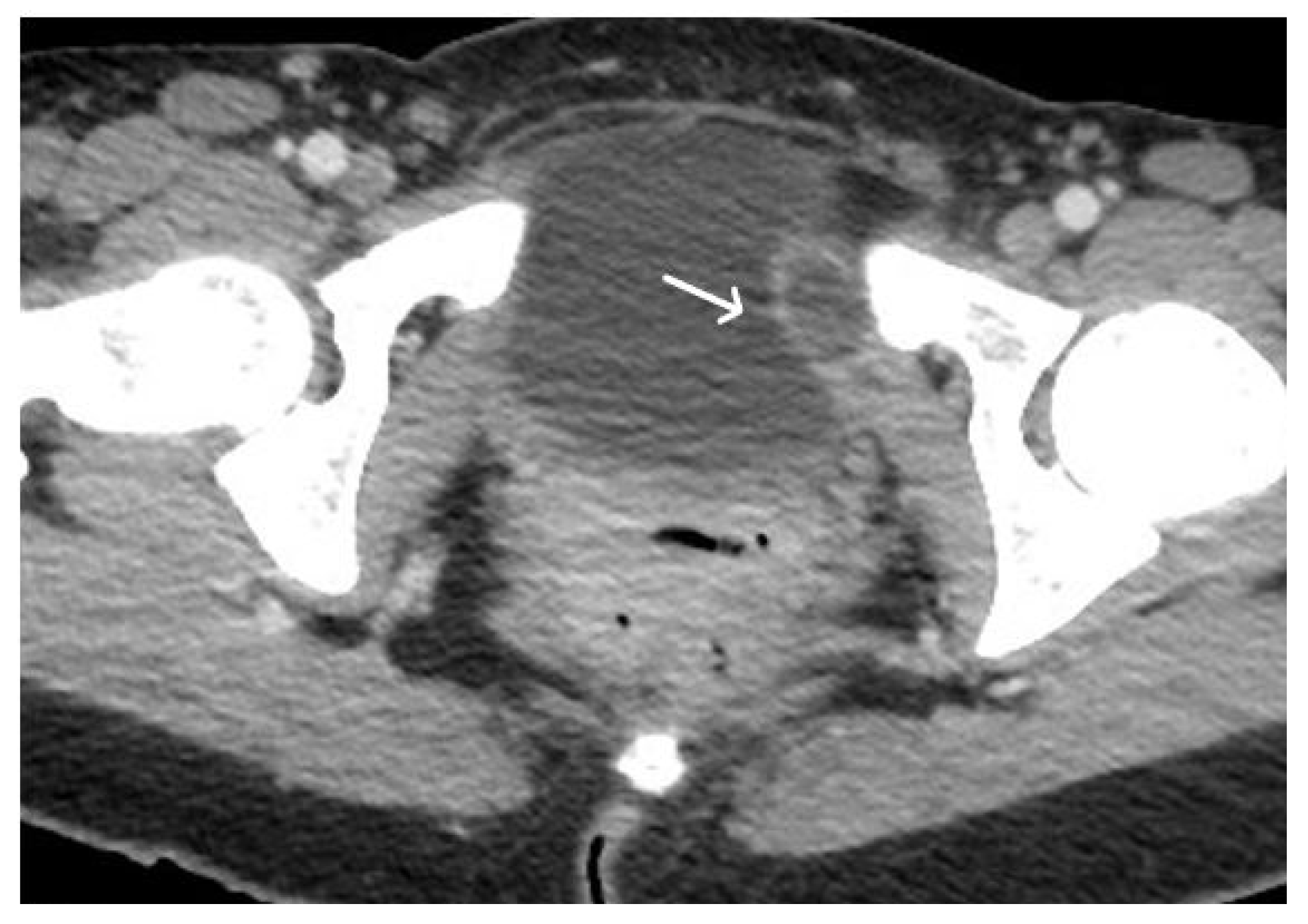
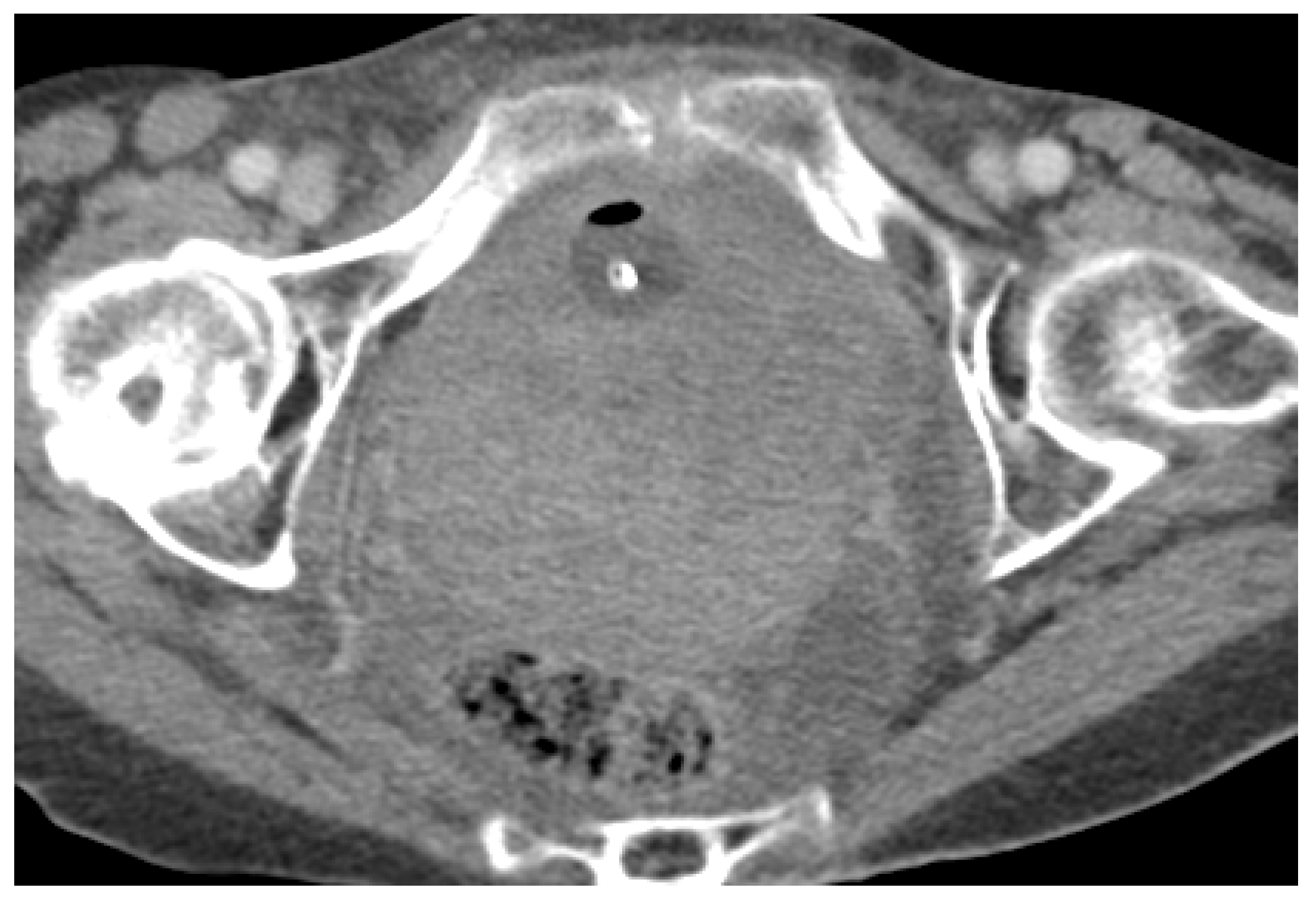
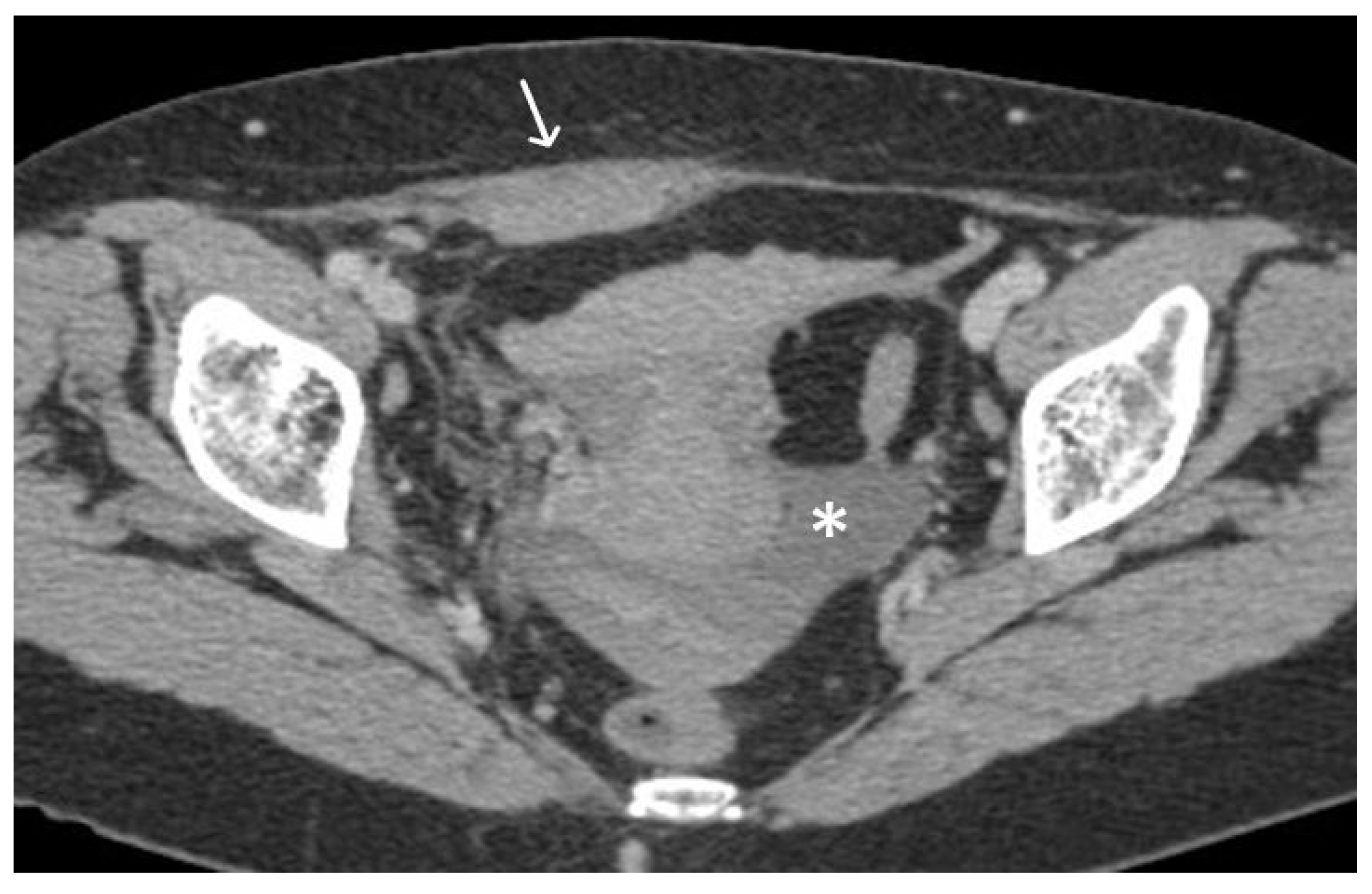
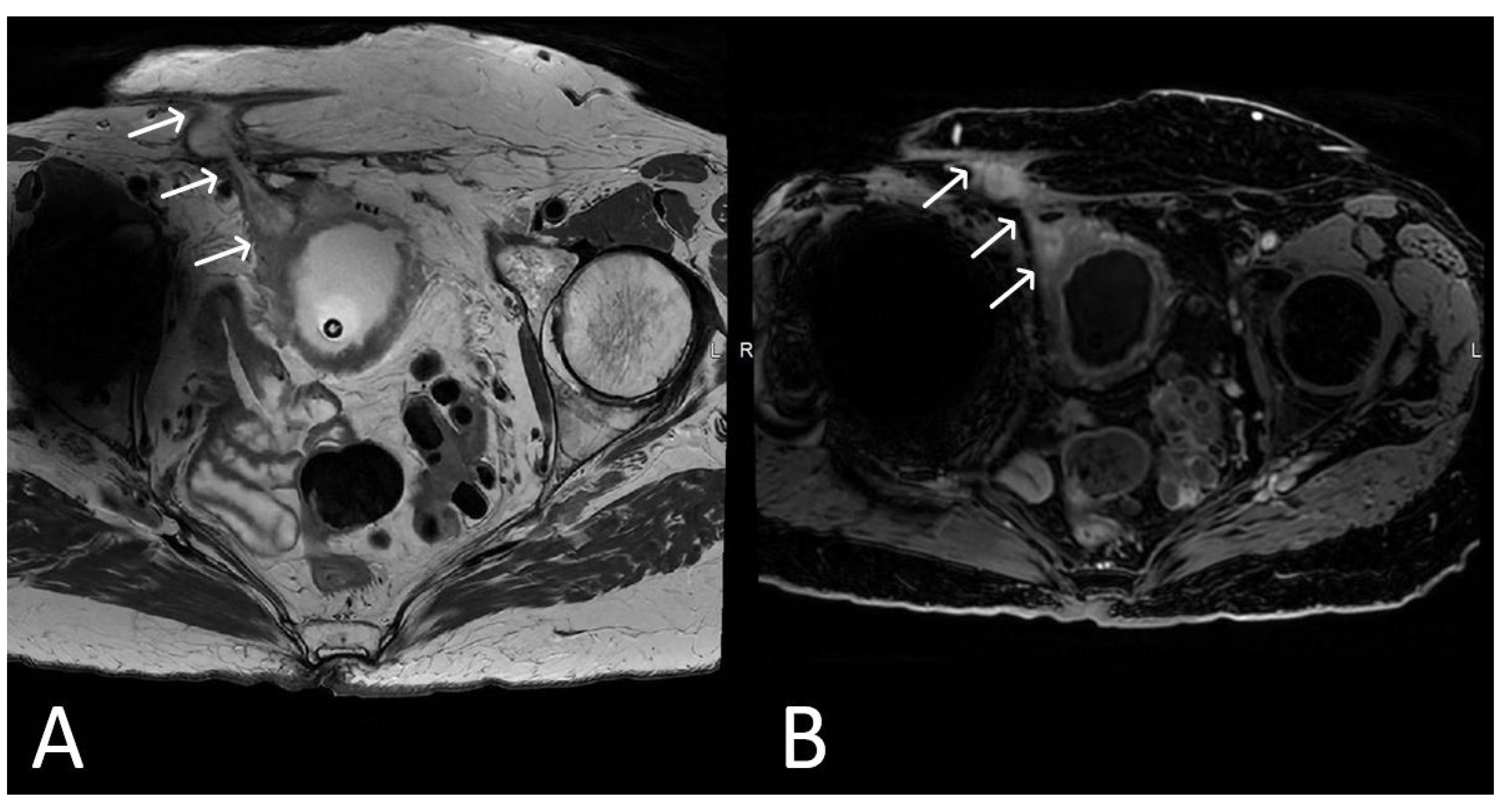
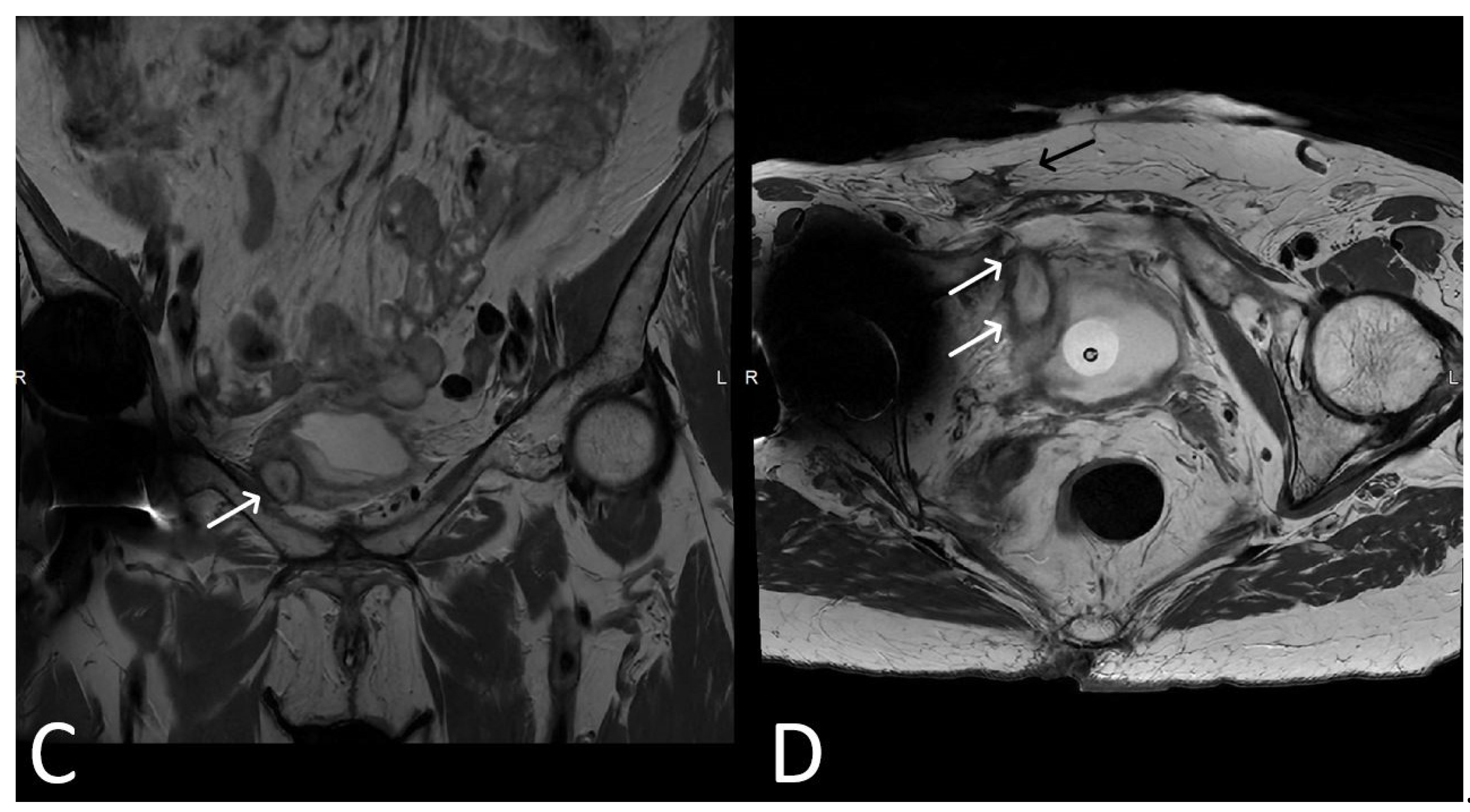
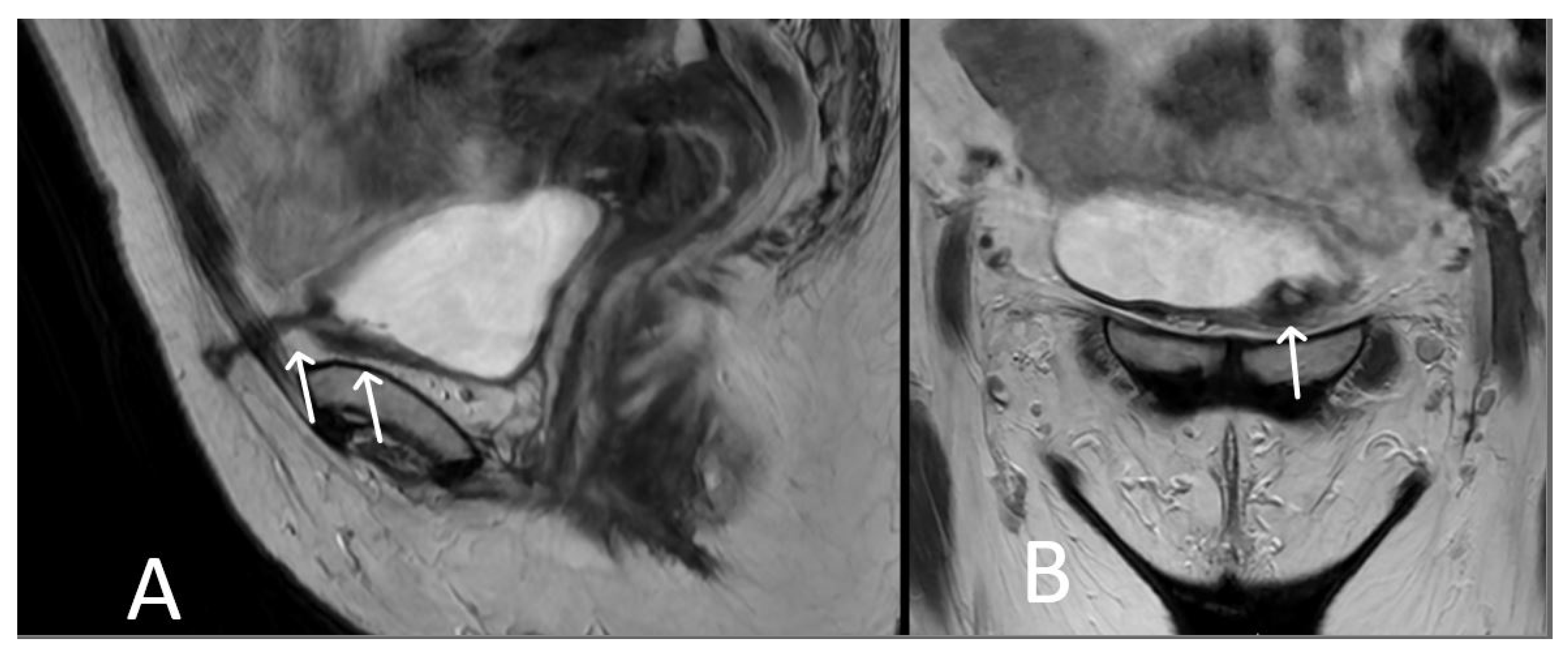
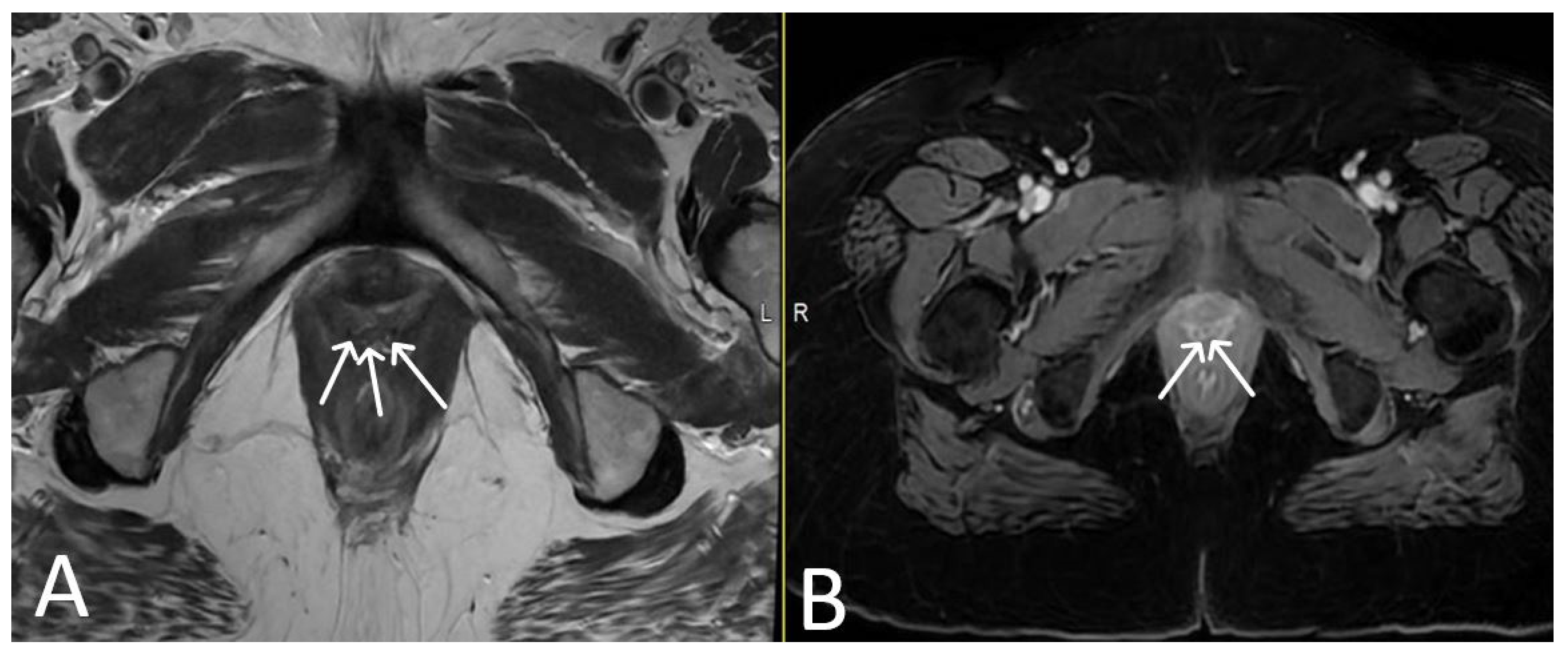
3. Normal Appearance of Urethral Meshes on MRI/CT
4. Urethral Mesh in Oncological Patients
5. Urethral Mesh Complications
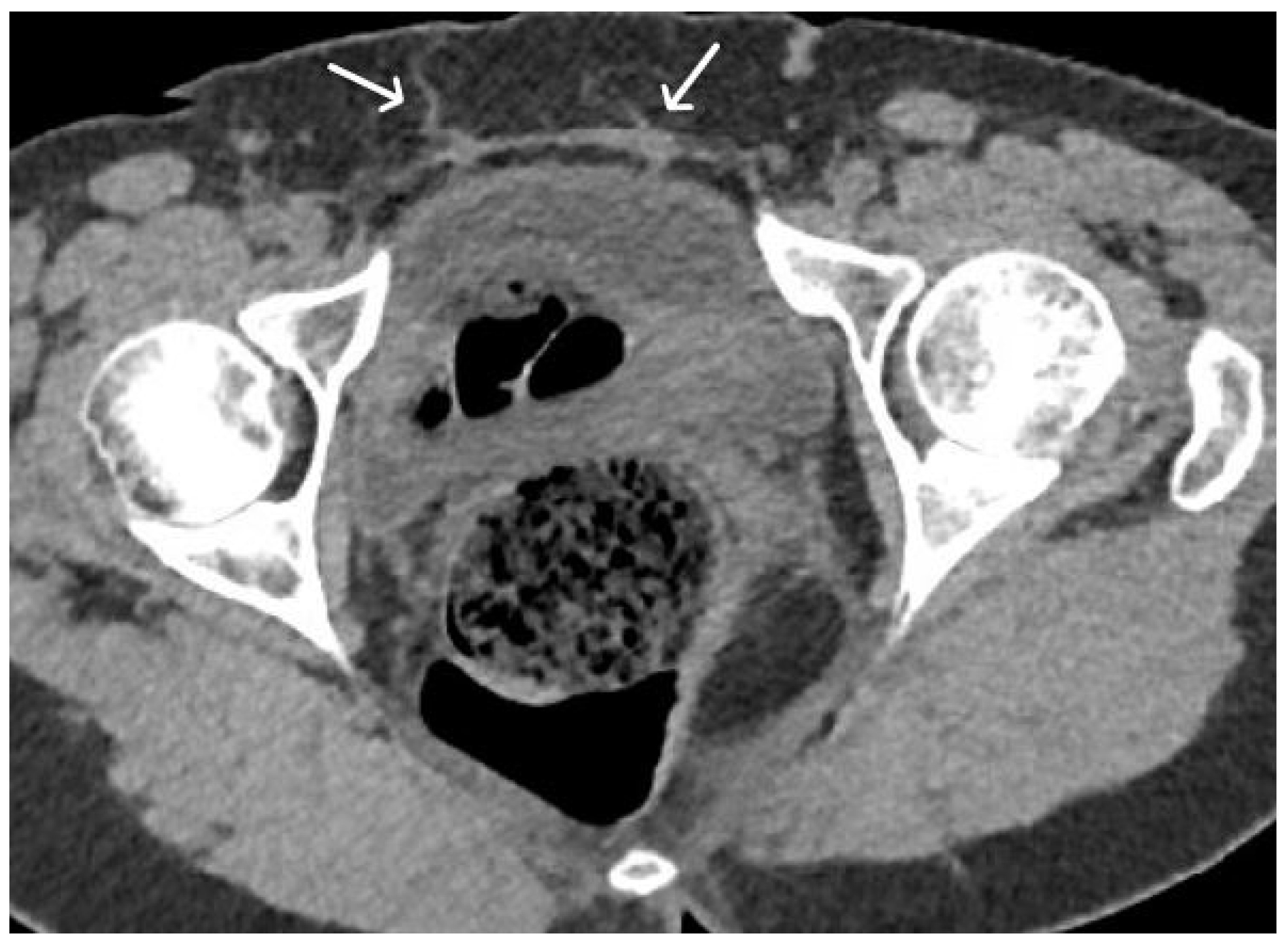
5.1. Short-Term Complications
5.2. Long-Term Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Afonso, J.S.; Jorge, R.M.; Martins, P.S.; Soldi Mda, S.; Alves, O.L.; Patricio, B.; Mascarenhas, T.; Sartori, M.G.; Girao, M.J. Structural and thermal properties of polypropylene mesh used in treatment of stress urinary incontinence. Acta Bioeng. Biomech. 2009, 11, 27–33. [Google Scholar] [PubMed]
- Nolfi, A.L.; Brown, B.N.; Liang, R.; Palcsey, S.L.; Bonidie, M.J.; Abramowitch, S.D.; Moalli, P.A. Host response to synthetic mesh in women with mesh complications. Am. J. Obstet. Gynecol. 2016, 215, 206.e1–206.e8. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1720; discussion 1720. [Google Scholar] [CrossRef] [PubMed]
- Delorme, E. Trans-obturator urethral suspension: A minimally invasive procedure to treat female stress urinary incontinence. Prog. Urol. 2001, 11, 1306–1313. [Google Scholar] [PubMed]
- Abdel-Fattah, M.; Cooper, D.; Davidson, T.; Kilonzo, M.; Hossain, M.; Boyers, D.; Bhal, K.; Wardle, J.; N’Dow, J.; MacLennan, G.; et al. Single-Incision Mini-Slings for Stress Urinary Incontinence in Women. N. Engl. J. Med. 2022, 386, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.D.; Serels, S.R.; Davila, G.W.; Settle, P. Minimally invasive treatment for female stress urinary incontinence (SUI): A review including TVT, TOT, and mini-sling. Surg. Technol. Int. 2009, 18, 157–173. [Google Scholar] [PubMed]
- Unger, C.A.; Rizzo, A.E.; Ridgeway, B. Indications and risk factors for midurethral sling revision. Int. Urogynecol. J. 2016, 27, 117–122. [Google Scholar] [CrossRef]
- Wu, J.M.; Gandhi, M.P.; Shah, A.D.; Shah, J.Y.; Fulton, R.G.; Weidner, A.C. Trends in inpatient urinary incontinence surgery in the USA, 1998–2007. Int. Urogynecol. 2011, 22, 1437–1443. [Google Scholar] [CrossRef]
- Nguyen, J.N.; Jakus-Waldman, S.M.; Walter, A.J.; White, T.; Menefee, S.A. Perioperative complications and reoperations after incontinence and prolapse surgeries using prosthetic implants. Obstet. Gynecol. 2012, 3, 539–546. [Google Scholar] [CrossRef]
- Vigil, H.R.; Wallis, C.J.D.; Zhang, B.; LaBossiere, J.R.; Carr, L.K.; Herschorn, S. Stress Incontinence Surgery Does Not Cause Pelvic Malignancy: A Population-Based Cohort Study. J. Urol. 2021, 205, 1725–1732. [Google Scholar] [CrossRef]
- Nougaret, S.; Horta, M.; Sala, E.; Lakhman, Y.; Thomassin-Naggara, I.; Kido, A.; Masselli, G.; Bharwani, N.; Sadowski, E.; Ertmer, A.; et al. Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2019, 29, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 2021, 31, 7802–7816. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Takeuchi, M.; Vargas, H.A.; Muglia, V.F.; Cipollari, S.; Catalano, C.; Panebianco, V. Overview of VI-RADS in Bladder Cancer. AJR Am. J. Roentgenol. 2020, 214, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; Freeman, R.M.; Swift, S.E.; Cosson, M.; Davila, G.W.; Deprest, J.; Dwyer, P.L.; Fatton, B.; Kocjancic, E.; Lee, J.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol. Urodyn. 2011, 30, 2–12. [Google Scholar] [PubMed]
- Ford, A.A.; Rogerson, L.; Cody, J.D.; Aluko, P.; Ogah, J.A. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst. Rev. 2017, 7, Cd006375. [Google Scholar] [CrossRef]
- Ross, S.; Robert, M.; Lier, D.; Eliasziw, M.; Jacobs, P. Surgical management of stress urinary incontinence in women: Safety, effectiveness and cost-utility of trans-obturator tape (TOT) versus tension-free vaginal tape (TVT) five years after a randomized surgical trial. BMC Women’s Health 2011, 11, 34. [Google Scholar] [CrossRef]
- Naumann, G.; Albrich, S.; Skala, C.; Laterza, R.; Kölbl, H. Single-Incision Slings (SIS)—A New Option for the Surgical Treatment of Female Stress Urinary Incontinence. Geburtshilfe Frauenheilkd. 2012, 72, 125–131. [Google Scholar] [CrossRef]
- Blewniewski, M.; Markowski, M.; Kliś, R.; Różański, W. Mini-slings—An option in stress urinary incontinence treatment. Case studies. Cent. Eur. J. Urol. 2015, 68, 68–71. [Google Scholar] [CrossRef]
- Patel, T.; Sugandh, F.; Bai, S.; Varrassi, G.; Devi, A.; Khatri, M.; Kumar, S.; Dembra, D.; Dahri, S. Single Incision Mini-Sling Versus Mid-Urethral Sling (Transobturator/Retropubic) in Females with Stress Urinary Incontinence: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e37773. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; De Rosa, N.; Tornesello, M.L.; Piccoli, R.; Bertrando, A.; Lavitola, G.; Morra, I.; Di Spiezio Sardo, A.; Buonaguro, F.M.; Nappi, C. Quality of life, lifestyle behavior and employment experience: A comparison between young and midlife survivors of gynecology early stage cancers. Gynecol. Oncol. 2012, 124, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, R.; Steffens, D.; Solomon, M.J.; Lee, P.J.; Austin, K.K.S.; Koh, C.E. Elderly Patients Have Better Quality of Life but Worse Survival Following Pelvic Exenteration: A 25-Year Single-Center Experience. Ann. Surg. Oncol. 2021, 28, 5226–5235. [Google Scholar] [CrossRef] [PubMed]
- Stanca, M.; Căpîlna, D.M.; Căpîlna, M.E. Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers 2022, 14, 2346. [Google Scholar] [CrossRef]
- Aoki, Y.; Brown, H.W.; Brubaker, L.; Cornu, J.N.; Daly, J.O.; Cartwright, R. Urinary incontinence in women. Nat. Rev. Dis. Prim. 2017, 3, 17042. [Google Scholar] [CrossRef]
- Radzimińska, A.; Strączyńska, A.; Weber-Rajek, M.; Styczyńska, H.; Strojek, K.; Piekorz, Z. The impact of pelvic floor muscle training on the quality of life of women with urinary incontinence: A systematic literature review. Clin. Interv. Aging 2018, 13, 957–965. [Google Scholar] [CrossRef]
- Hazewinkel, M.H.; Sprangers, M.A.; van der Velden, J.; van der Vaart, C.H.; Stalpers, L.J.; Burger, M.P.; Roovers, J.P. Long-term cervical cancer survivors suffer from pelvic floor symptoms: A cross-sectional matched cohort study. Gynecol. Oncol. 2010, 117, 281–286. [Google Scholar] [CrossRef]
- Nakayama, N.; Tsuji, T.; Aoyama, M.; Fujino, T.; Liu, M. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: A questionnaire survey. BMC Women’s Health 2020, 20, 148. [Google Scholar] [CrossRef]
- Novara, G.; Galfano, A.; Boscolo-Berto, R.; Secco, S.; Cavalleri, S.; Ficarra, V.; Artibani, W. Complication rates of tension-free midurethral slings in the treatment of female stress urinary incontinence: A systematic review and meta-analysis of randomized controlled trials comparing tension-free midurethral tapes to other surgical procedures and different devices. Eur. Urol. 2008, 53, 288–308. [Google Scholar]
- Gomes, C.M.; Carvalho, F.L.; Bellucci, C.H.S.; Hemerly, T.S.; Baracat, F.; de Bessa, J., Jr.; Srougi, M.; Bruschini, H. Update on complications of synthetic suburethral slings. Int. Braz. J. Urol. 2017, 43, 822–834. [Google Scholar] [CrossRef]
- Barisiene, M.; Cerniauskiene, A.; Matulevicius, A. Complications and their treatment after midurethral tape implantation using retropubic and transobturator approaches for treatment of female stress urinary incontinence. Videosurg. Other Miniinvasive Tech. 2018, 13, 501–506. [Google Scholar] [CrossRef] [PubMed]
- LaSala, C.A. Incomplete bladder emptying after the tension-free vaginal tape procedure, necessitating release of the mesh. A report of three cases. J. Reprod. Med. 2003, 48, 387–390. [Google Scholar] [PubMed]
- Klutke, C.; Siegel, S.; Carlin, B.; Paszkiewicz, E.; Kirkemo, A.; Klutke, J. Urinary retention after tension-free vaginal tape procedure: Incidence and treatment. Urology 2001, 58, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Petri, E.; Ashok, K. Complications of synthetic slings used in female stress urinary incontinence and applicability of the new IUGA-ICS classification. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Boman, J.; Elzayat, E.; Lemieux, M.C.; Corcos, J. Severe soft tissue infection of the thigh after vaginal erosion of transobturator tape for stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007, 18, 207–212. [Google Scholar] [CrossRef]
- Lee, D.; Zimmern, P.E. Management of complications of mesh surgery. Curr. Opin. Urol. 2015, 25, 284–291. [Google Scholar] [CrossRef]
- Committee on Gynecologic Practice American Urogynecologic Society. Committee Opinion No. 694: Management of Mesh and Graft Complications in Gynecologic Surgery. Obstet. Gynecol. 2017, 129, e102–e108. [Google Scholar] [CrossRef]







| Short-Term | Long-Term | ||
|---|---|---|---|
| TVT | TOT | TVT and TOT | |
| Injuries | Bladder | Obturator nerve | Bladder outlet syndrome |
| Urethra | Obturator vessels (artery and vein with medial and lateral branches) | Urge-incontinence Recurrent urinary infections Acute urinary retention Mesh exposure Mesh extrusion Mesh infection | |
| Bowel | |||
| Vessels | |||
| Acute urinary retention | |||
| Mesh Infections | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintican, R.; Buckley, A.; Feier, D.; Freeman, S. Urethral Mesh Assessment in Cancer Patients. Cancers 2023, 15, 5599. https://doi.org/10.3390/cancers15235599
Pintican R, Buckley A, Feier D, Freeman S. Urethral Mesh Assessment in Cancer Patients. Cancers. 2023; 15(23):5599. https://doi.org/10.3390/cancers15235599
Chicago/Turabian StylePintican, Roxana, Anne Buckley, Diana Feier, and Susan Freeman. 2023. "Urethral Mesh Assessment in Cancer Patients" Cancers 15, no. 23: 5599. https://doi.org/10.3390/cancers15235599
APA StylePintican, R., Buckley, A., Feier, D., & Freeman, S. (2023). Urethral Mesh Assessment in Cancer Patients. Cancers, 15(23), 5599. https://doi.org/10.3390/cancers15235599







