Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. In Situ Hybridization
2.3. Histological and Immunohistochemical Evaluation
2.4. Multiplex Immunofluorescence
2.5. Gene Expression Profiling
2.6. Data Analysis and Visualization
2.7. Statistical Evaluation
3. Results
3.1. Patient Characteristics
3.2. mRNA Expression of GATA2 and GATA2 Target Genes RUNX1, EZH2, IKZF1, and LYL1 Is Restored during Disease Progression in GATA2mut Patients
3.3. In GATA2 Haploinsufficient Patients, EZH2 Expression Is Increased in Hematopoietic Progenitors at Advanced Disease Stage
3.4. Robust Expression of the EZH2-Dependent H3K27me3 Gene Silencing Mark in GATA2-EB Patients
3.5. GATA2mut Patients Show Increased BCL2 Expression with Disease Progression
3.6. Profiling of BCL2 Family Members Reveals an Increased MAC Score in GATA2-EB Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostergaard, P.; Simpson, M.A.; Connell, F.C.; Steward, C.G.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.P.; Sampaio, E.P.; Khan, J.; Calvo, K.R.; Lemieux, J.E.; Patel, S.Y.; Frucht, D.M.; Vinh, D.C.; Auth, R.D.; Freeman, A.F.; et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011, 118, 2653–2655. [Google Scholar] [CrossRef]
- Dickinson, R.E.; Griffin, H.; Bigley, V.; Reynard, L.N.; Hussain, R.; Haniffa, M.; Lakey, J.H.; Rahman, T.; Wang, X.N.; McGovern, N.; et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 2011, 118, 2656–2658. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Sanchez, L.A.; Hsu, A.P.; Shaw, P.A.; Zerbe, C.S.; Calvo, K.R.; Arthur, D.C.; Gu, W.; Gould, C.M.; Brewer, C.C.; et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014, 123, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.N.; Chong, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Pasquet, M.; Bellanné-Chantelot, C.; Tavitian, S.; Prade, N.; Beaupain, B.; LaRochelle, O.; Petit, A.; Rohrlich, P.; Ferrand, C.; Van Den Neste, E.; et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood 2013, 121, 822–829. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Wlodarski, M.W.; Kozyra, E.; Niemeyer, C.M. Heterogeneity of GATA2-related myeloid neoplasms. Int. J. Hematol. 2017, 106, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, M.W.; Hirabayashi, S.; Pastor, V.; Starý, J.; Hasle, H.; Masetti, R.; Dworzak, M.; Schmugge, M.; van den Heuvel-Eibrink, M.; Ussowicz, M.; et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 2016, 127, 1387–1397. [Google Scholar] [CrossRef]
- Nováková, M.; Žaliová, M.; Suková, M.; Wlodarski, M.; Janda, A.; Froňková, E.; Campr, V.; Lejhancová, K.; Zapletal, O.; Pospíšilová, D.; et al. Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica 2016, 101, 707–716. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Kozyra, E.J.; Wlodarski, M.W. Germline predisposition in myeloid neoplasms: Unique genetic and clinical features of GATA2 deficiency and SAMD9/SAMD9L syndromes. Best Pract. Res. Clin. Haematol. 2020, 33, 101197. [Google Scholar] [CrossRef]
- Bresnick, E.H.; Jung, M.M.; Katsumura, K.R. Human GATA2 mutations and hematologic disease: How many paths to pathogenesis? Blood Adv. 2020, 4, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Calvo, K.R.; Vinh, D.C.; Maric, I.; Wang, W.; Noel, P.; Stetler-Stevenson, M.; Arthur, D.C.; Raffeld, M.; Dutra, A.; Pak, E.; et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: Diagnostic features and clinical implications. Haematologica 2011, 96, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Rudelius, M.; Weinberg, O.K.; Niemeyer, C.M.; Shimamura, A.; Calvo, K.R. The International Consensus Classification (ICC) of hematologic neoplasms with germline predisposition, pediatric myelodysplastic syndrome, and juvenile myelomonocytic leukemia. Virchows Arch. 2023, 482, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Viré, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef]
- Huang, K.; O’Neill, K.L.; Li, J.; Zhou, W.; Han, N.; Pang, X.; Wu, W.; Struble, L.; Borgstahl, G.; Liu, Z.; et al. BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis. Cell Res. 2019, 29, 942–952. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Waclawiczek, A.; Leppä, A.M.; Renders, S.; Stumpf, K.; Reyneri, C.; Betz, B.; Janssen, M.; Shahswar, R.; Donato, E.; Karpova, D.; et al. Combinatorial BCL-2 family expression in Acute Myeloid Leukemia Stem Cells predicts clinical response to Azacitidine/Venetoclax. Cancer Discov. 2023, 13, 1408–1427. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Bresnick, E.H.; GATA Factor Mechanisms Group. The GATA factor revolution in hematology. Blood 2017, 129, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, L.; Li, G.; Zhang, Y.; Dong, C.; Ren, F.; Chen, W.; Ma, Y. EZH2/EHMT2 Histone Methyltransferases Inhibit the Transcription of DLX5 and Promote the Transformation of Myelodysplastic Syndrome to Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2021, 9, 619795. [Google Scholar] [CrossRef] [PubMed]
- Stomper, J.; Meier, R.; Ma, T.; Pfeifer, D.; Ihorst, G.; Blagitko-Dorfs, N.; Greve, G.; Zimmer, D.; Platzbecker, U.; Hagemeijer, A.; et al. Integrative study of EZH2 mutational status, copy number, protein expression and H3K27 trimethylation in AML/MDS patients. Clin. Epigenetics 2021, 13, 77. [Google Scholar] [CrossRef]
- Göllner, S.; Oellerich, T.; Agrawal-Singh, S.; Schenk, T.; Klein, H.U.; Rohde, C.; Pabst, C.; Sauer, T.; Lerdrup, M.; Tavor, S.; et al. Loss of the histone methyltransferase EZH2 induces resistance to multiple drugs in acute myeloid leukemia. Nat. Med. 2017, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.L.; Nguyen, J.; Al Ali, N.H.; Komrokji, R.S.; Sallman, D.; Zhang, X.; Song, J.; Padron, E.; Lancet, J.E.; Moscinski, L.C.; et al. Association of EZH2 protein expression by immunohistochemistry in myelodysplasia related neoplasms with mutation status, cytogenetics and clinical outcomes. Br. J. Haematol. 2019, 184, 450–455. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Skrabanek, L.; Li, Y.; Jiemjit, A.; Fandy, T.E.; Paietta, E.; Fernandez, H.; Tallman, M.S.; Greally, J.M.; Carraway, H.; et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood 2009, 114, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Wu, L.; Zhang, Q.; Yang, R.; Yang, Y.; Zhang, Z.; He, Q.; Chang, C. Overexpression of the EZH2, RING1 and BMI1 genes is common in myelodysplastic syndromes: Relation to adverse epigenetic alteration and poor prognostic scoring. Ann. Hematol. 2011, 90, 643–653. [Google Scholar] [CrossRef]
- Issa, J.P. The myelodysplastic syndrome as a prototypical epigenetic disease. Blood 2013, 121, 3811–3817. [Google Scholar] [CrossRef]
- Karoopongse, E.; Yeung, C.; Byon, J.; Ramakrishnan, A.; Holman, Z.J.; Jiang, P.Y.; Yu, Q.; Deeg, H.J.; Marcondes, A.M. The KDM2B- let-7b -EZH2 axis in myelodysplastic syndromes as a target for combined epigenetic therapy. PLoS ONE 2014, 9, e107817. [Google Scholar] [CrossRef]
- De Souza Fernandez, T.; Fonseca Alvarenga, T.; Almeida Antônio de Kós, E.; Lamim Lovatel, V.; Tavares, R.C.; da Costa, E.S.; de Souza Fernandez, C.; Abdelhay, E. Aberrant Expression of EZH2 in Pediatric Patients with Myelodysplastic Syndrome: A Potential Biomarker of Leukemic Evolution. Biomed. Res. Int. 2019, 2019, 3176565. [Google Scholar] [CrossRef]
- Raza, A.; Gezer, S.; Mundle, S.; Gao, X.Z.; Alvi, S.; Borok, R.; Rifkin, S.; Iftikhar, A.; Shetty, V.; Parcharidou, A.; et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood 1995, 86, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Mufti, G.J.; Rasool, F.; Mijovic, A.; Devereux, S.; Pagliuca, A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood 2000, 96, 3932–3938. [Google Scholar] [CrossRef] [PubMed]
- Jilg, S.; Reidel, V.; Müller-Thomas, C.; König, J.; Schauwecker, J.; Höckendorf, U.; Huberle, C.; Gorka, O.; Schmidt, B.; Burgkart, R.; et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leukemia 2016, 30, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Boudard, D.; Vasselon, C.; Berthéas, M.F.; Jaubert, J.; Mounier, C.; Reynaud, J.; Viallet, A.; Chautard, S.; Guyotat, D.; Campos, L. Expression and prognostic significance of Bcl-2 family proteins in myelodysplastic syndromes. Am. J. Hematol. 2002, 70, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Short, N.J.; Montalban-Bravo, G.; Huang, X.; Bueso-Ramos, C.; Qiao, W.; Yang, H.; Zhao, C.; Kadia, T.; Borthakur, G.; et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood 2017, 130, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Garcia, J.S.; Fenaux, P.; Platzbecker, U.; Miyazaki, Y.; Xiao, Z.-J.; Zhou, Y.; Naqvi, K.; Kye, S.; Garcia-Manero, G. Phase 3 VERONA study of venetoclax with azacitidine to assess change in complete remission and overall survival in treatment-naïve higher-risk myelodysplastic syndromes. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS7054. [Google Scholar] [CrossRef]
- Masetti, R.; Baccelli, F.; Leardini, D.; Gottardi, F.; Vendemini, F.; Di Gangi, A.; Becilli, M.; Lodi, M.; Tumino, M.; Vinci, L.; et al. Venetoclax-based therapies in pediatric advanced MDS and relapsed/refractory AML: A multicenter retrospective analysis. Blood Adv. 2023, 7, 4366–4370. [Google Scholar] [CrossRef]
- Winters, A.C.; Maloney, K.W.; Treece, A.L.; Gore, L.; Franklin, A.K. Single-center pediatric experience with venetoclax and azacitidine as treatment for myelodysplastic syndrome and acute myeloid leukemia. Pediatr. Blood Cancer 2020, 67, e28398. [Google Scholar] [CrossRef] [PubMed]
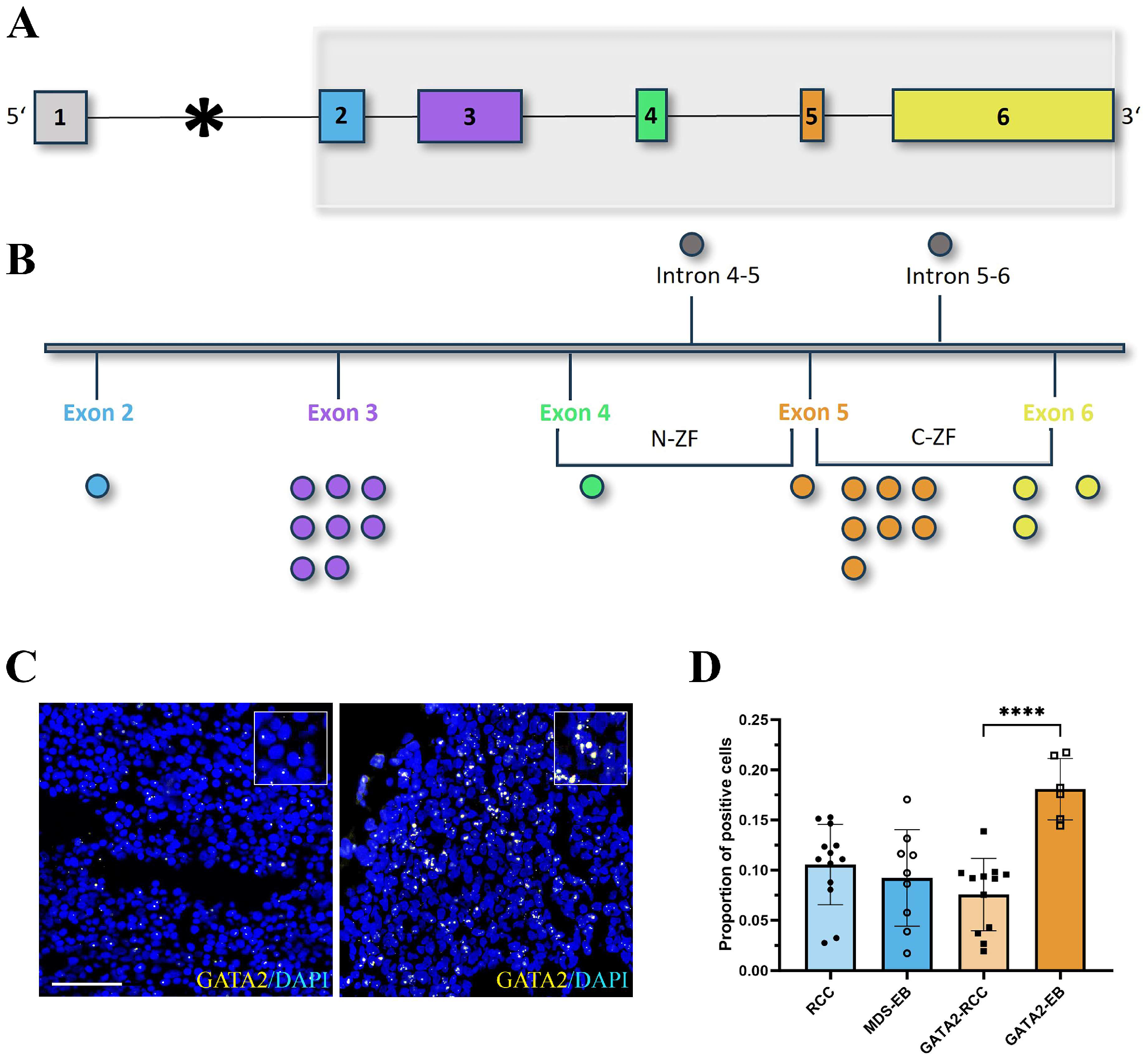


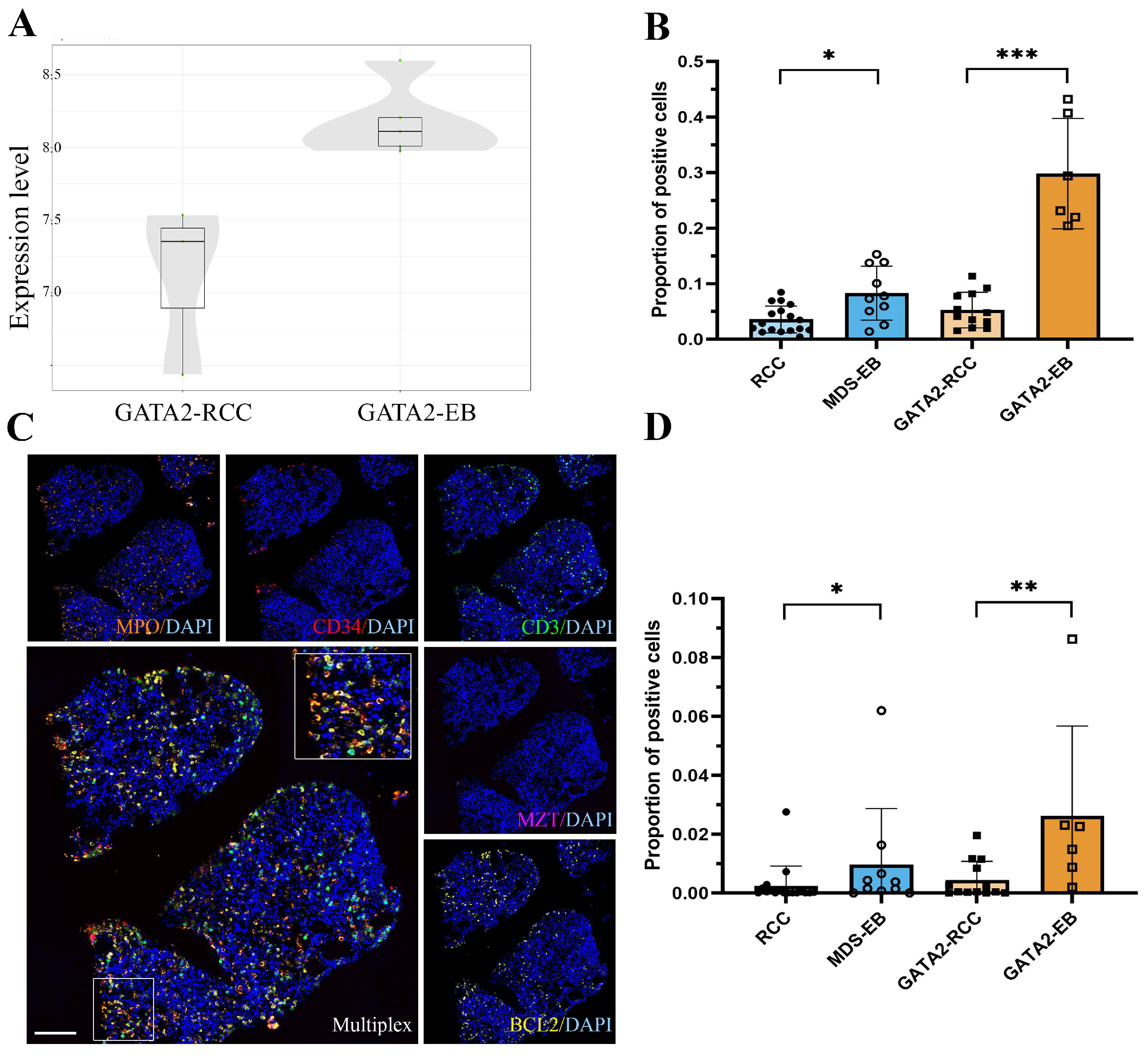
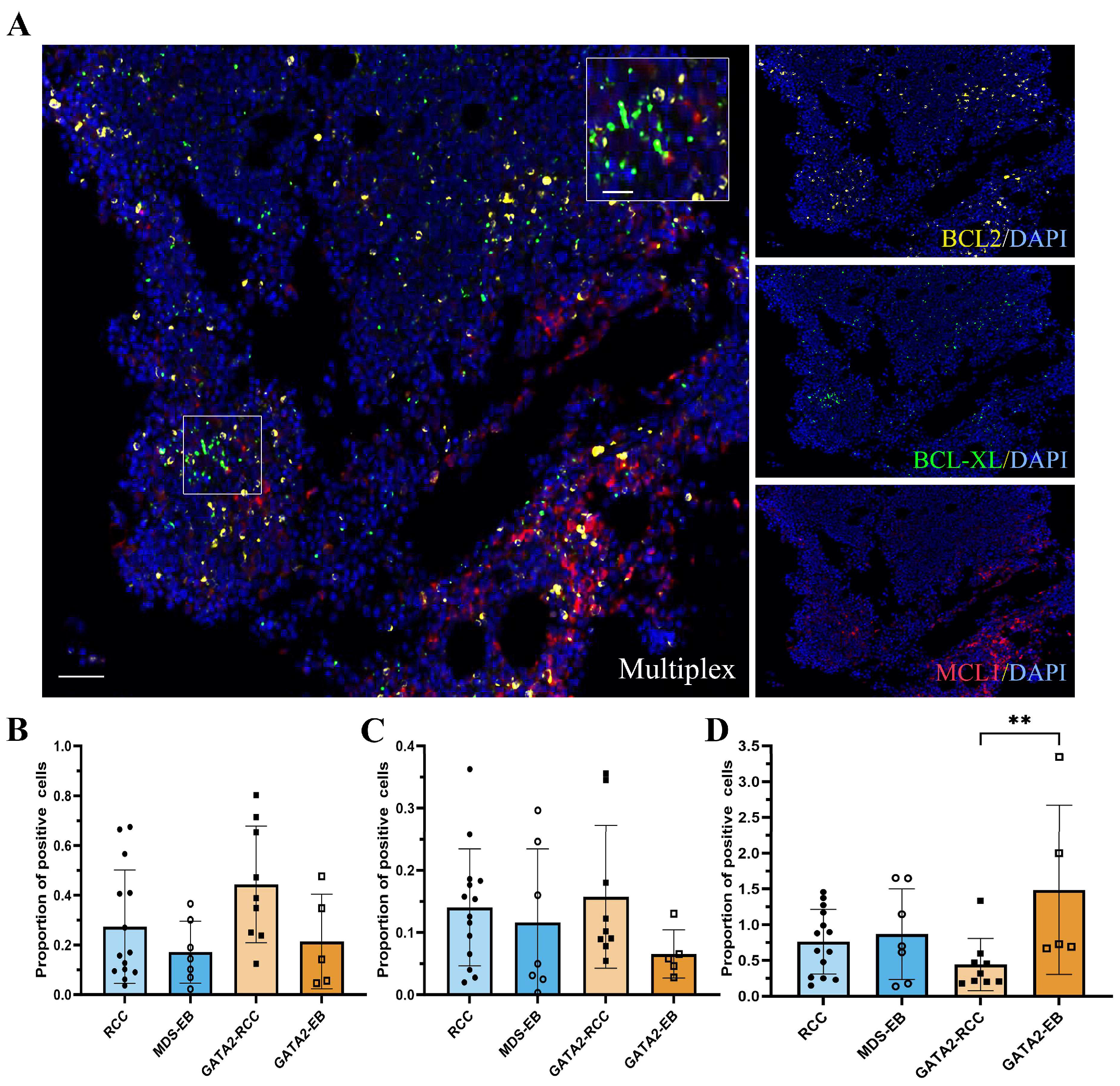
| Parameter | GATA2mut n = 30 | GATA2WT n = 27 | |
|---|---|---|---|
| Age at onset | Years, median (range) | 13 (4–20) | 9 (2–19) |
| Sex | Males, n (%) | 16 (53) | 16 (59) |
| Females, n (%) | 14 (47) | 11 (41) | |
| Diagnosis | RCC, n (%) | 24 (80) | 17 (63) |
| MDS-EB, n (%) | 6 (20) | 10 (37) | |
| Normal, n (%) | 9 (33) | 15 (56) | |
| Karyotype 1 | Monosomy 7, n (%) 2 | 11 (41) | 8 (30) |
| Trisomy 8, n (%) | 7 (26) | 3 (11) | |
| Other 3, n (%) | 0 (0) | 1 (3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, F.; Piontek, G.; Schneider-Kimoto, Y.; Schwarz-Furlan, S.; De Vito, R.; Locatelli, F.; Gengler, C.; Yoshimi, A.; Jung, A.; Klauschen, F.; et al. Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation. Cancers 2023, 15, 5594. https://doi.org/10.3390/cancers15235594
Schreiber F, Piontek G, Schneider-Kimoto Y, Schwarz-Furlan S, De Vito R, Locatelli F, Gengler C, Yoshimi A, Jung A, Klauschen F, et al. Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation. Cancers. 2023; 15(23):5594. https://doi.org/10.3390/cancers15235594
Chicago/Turabian StyleSchreiber, Franziska, Guido Piontek, Yuki Schneider-Kimoto, Stephan Schwarz-Furlan, Rita De Vito, Franco Locatelli, Carole Gengler, Ayami Yoshimi, Andreas Jung, Frederick Klauschen, and et al. 2023. "Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation" Cancers 15, no. 23: 5594. https://doi.org/10.3390/cancers15235594
APA StyleSchreiber, F., Piontek, G., Schneider-Kimoto, Y., Schwarz-Furlan, S., De Vito, R., Locatelli, F., Gengler, C., Yoshimi, A., Jung, A., Klauschen, F., Niemeyer, C. M., Erlacher, M., & Rudelius, M. (2023). Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation. Cancers, 15(23), 5594. https://doi.org/10.3390/cancers15235594





