Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial Design
- Acute and late toxicity according to Common Terminology Criteria for Adverse Events (CTCAE-v5) [19].
- One-, three-, five- and ten-year local control determined using magnetic resonance (MRI) with gadolinium and overall survival, comparing the results with historic standard PBRT cohorts.
- The evaluation of the quality of life of the patients 3 months after the end of the treatment, using a specific questionnaire EORTC-QLQC30 and BN20.
- The evaluation of the dosimetric benefits using techniques that allow an improvement in the dose gradient, achieving a better coverage of the clinical target volume (CTV) and decreasing the dose in surrounding risk organs.
- For chordomas: 37.5 GyRBE in 5 consecutive sessions of 7.5 GyRBE per fraction.
- For chondrosarcomas: 35 GyRBE in 5 consecutive sessions of 7 GyRBE per fraction.
- Patients ≥ 18 years old.
- With a baseline classification on the Karnofsky performance status scale (KPS) ≥ 70%.
- With confirmed histological diagnosis of chordoma or chondrosarcoma of the skull base.
- With a maximum CTV of 50 cc.
- Whose relationship to organs at risk (OARs) allows compliance with the necessary dose restrictions to receive hypofractionated proton therapy in 5 fractions.
- Tumor CTV coverage of at least D95 > 90%.
- Correct compliance with the dose restrictions, at least in the nominal scenario, for critical organs (optic pathway, brain stem, and spinal cord) according to the guidelines published and available in the literature:
- ○
- -
- Optic nerves and chiasm: D0.03 cc ≤ 25 GyRBE, V23.5 < 0.5 cc.
- -
- Brainstem: D0.03 cc ≤ 31 GyRBE, V23 < 0.5 cc.
- -
- Spinal cord: D0.03 cc ≤ 30 GyRBE, V23 < 035 cc.
- ○
- -
- Right and left temporal lobe: V35 < 1.7 cc, V30 < 5.5 cc, V28 < 7 cc.
- -
- Right and left cochlea: D0.03 cc < 25 GyRBE
- Patients with distant metastases.
- Patients who have received previous irradiation in the same location.
- Patients whose clinical or dosimetric characteristics do not meet the inclusion criteria.
- Patients who are simultaneously participating in another study that may affect the results of this protocol.
2.2. Clinical and Physical Aspects
- -
- Computerized tomography (CT) is required to have a field of view of 32 cm and a matrix size of 500 × 500 voxels. The same acquisition with and without iodinated contrast is performed in the supine position with a thermoplastic mask for immobilization. The slice thickness is selected to be lower or equal to 1.25 mm, so enough spatial resolution is guaranteed.
- -
- Thin-cut (1.25 mm) 3 Tesla MRI with 3D T1, T1 postgadolinium and T2 is obtained.
- -
- In the case of previous surgery, the preoperative MRI will be fused.
- -
- The GTV (gross tumor volume) is contoured including the macroscopic tumor based on CT and MRI with and without contrast.
- -
- The CTV is contoured on MRI and CT (bone window), including the current GTV, the pre-surgical GTV considering the corresponding anatomical modifications after surgery (surgical bed), the surrounding areas suspected of containing microscopic disease (the relevant regions of the clivus, adjacent anatomical compartments at risk of direct spread such as cavernous sinus and the surgical approach) with a margin of 5 mm limited by anatomy. This CTV must respect anatomical boundaries, such as unaffected bone, dura mater, and cerebrospinal fluid.
2.3. Preliminary Cohort Results
2.3.1. Description of the Prospective Cohort
2.3.2. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Prospective Series
3.2. Treatment Delivery Characteristics
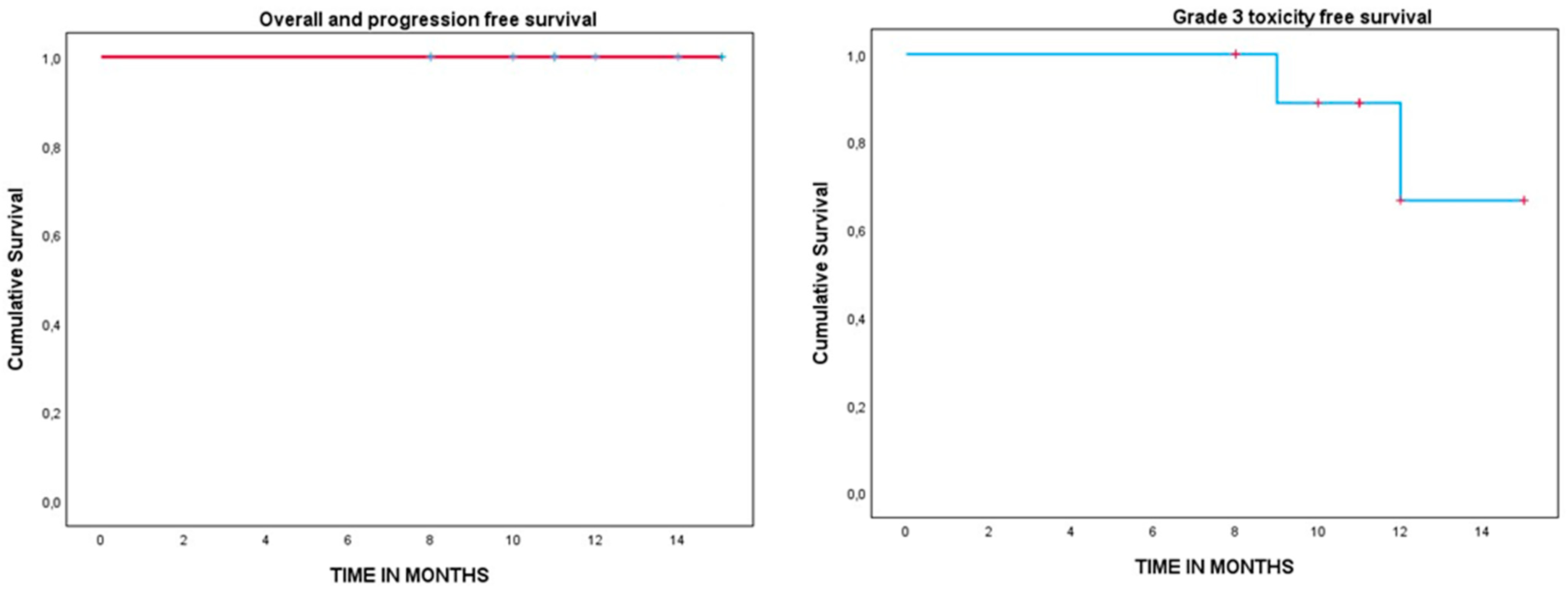
3.3. Survival and Toxicity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, S.H.; Jacobs, W.C.H.; Pondaag, W.; Gelderblom, H.; Nout, R.A.; Dijkstra, P.D.S.; Peul, W.C.; Vleggeert-Lankamp, C.L.A. Chordoma: A systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur. Spine J. 2018, 27, 3043–3058. [Google Scholar] [CrossRef]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.-V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Gelderblom, H.; Hogendoorn, P.C.W.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.; Bovée, J.V. The clinical approach towards chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef]
- Bloch, O.G.; Jian, B.J.; Yang, I.; Han, S.J.; Aranda, D.; Ahn, B.J.; Parsa, A.T. A systematic review of intracranial chondrosarcoma and survival. J. Clin. Neurosci. 2009, 16, 1547–1551. [Google Scholar] [CrossRef]
- Jiang, B.; Veeravagu, A.; Feroze, A.H.; Lee, M.; Harsh, G.R.; Soltys, S.G.; Gibbs, I.C.; Adler, J.R.; Chang, S.D. Cyberknife radiosurgery for the management of skull base and spinal chondrosarcomas. J. Neuro-Oncol. 2013, 114, 209–218. [Google Scholar] [CrossRef]
- Austin, J.P.; Urie, M.M.; Cardenosa, G.; Munzenrider, J. Probable Causes of recurrence in patients with chordoma and chondrosarcoma of the base of the skull and cervical spine. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 439–444. [Google Scholar] [CrossRef]
- Bohman, L.-E.; Koch, M.; Bailey, R.L.; Alonso-Basanta, M.; Lee, J.Y. Skull base chordoma and chondrosarcoma: Influence of clinical and demographic factors on prognosis: A SEER analysis. World Neurosurg. 2014, 82, 806–814. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Raleigh, D.R.; Johnson, J.; Garsa, A.A.; Theodosopoulos, P.V.; Aghi, M.K.; Ames, C.; McDermott, M.W.; Barani, I.J.; Braunstein, S.E. Management of Chordoma and Chondrosarcoma with Fractionated Stereotactic Radiotherapy. Front. Surg. 2017, 4, 35. [Google Scholar] [CrossRef]
- Sallabanda, M.; Garcia, R.; Lorenzana, L.; Santaolalla, I.; Abarca, J.; Sallabanda, K. Treatment of Chordomas and Chondrosarcomas With CyberKnife Robotic Hypofractionated Radiosurgery: A Single Institution Experience. Cureus 2021, 13, e17012. [Google Scholar] [CrossRef]
- Kremenevski, N.; Schlaffer, S.-M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef]
- Gwak, H.-S.; Yoo, H.-J.; Youn, S.-M.; Chang, U.; Lee, D.H.; Yoo, S.-Y.; Rhee, C.H. Hypofractionated stereotactic radiation therapy for skull base and upper cervical chordoma and chondrosarcoma: Preliminary results. Stereotact. Funct. Neurosurg. 2005, 83, 233–243. [Google Scholar] [CrossRef]
- Connors, S.W.; Aoun, S.G.; Shi, C.; Peinado-Reyes, V.; Hall, K.; Bagley, C.A. Recent advances in understanding and managing chordomas: An update. F1000Research 2020, 9, 713. [Google Scholar] [CrossRef]
- Mammar, H.; Kerrou, K.; Nataf, V.; Pontvert, D.; Clemenceau, S.; Lot, G.; George, B.; Polivka, M.; Mokhtari, K.; Ferrand, R.; et al. Positron emission tomography/computed tomography imaging of residual skull base chordoma before radiotherapy using fluoromisonidazole and fluorodeoxyglucose: Potential consequences for dose painting. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 681–687. [Google Scholar] [CrossRef]
- Palm, R.F.; Oliver, D.E.; Yang, G.Q.; Abuodeh, Y.; Naghavi, A.O.; Johnstone, P.A.S. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: An analysis of the National Cancer Data Base. Cancer 2019, 125, 642–651. [Google Scholar] [CrossRef]
- Basler, L.; Poel, R.; Schröder, C.; Bolsi, A.; Lomax, A.; Tanadini-Lang, S.; Guckenberger, M.; Weber, D.C. Dosimetric analysis of local failures in skull-base chordoma and chondrosarcoma following pencil beam scanning proton therapy. Radiat. Oncol. 2020, 15, 266. [Google Scholar] [CrossRef]
- Henderson, F.C.; McCool, K.; Seigle, J.; Jean, W.; Harter, W.; Gagnon, G.J. Treatment of chordomas with CyberKnife: Georgetown University experience and treatment recommendations. Neurosurgery 2009, 64, A44–A53. [Google Scholar] [CrossRef]
- Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Website. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05861245 (accessed on 13 October 2023).
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE-Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Timmerman, R. A Story of Hypofractionation and the Table on the Wall. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 4–21. [Google Scholar] [CrossRef]
- Diez, P.; Hanna, G.G.; Aitken, K.; van As, N.; Carver, A.; Colaco, R.; Conibear, J.; Dunne, E.; Eaton, D.; Franks, K.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Grimm, J.; LaCouture, T.; Croce, R.; Yeo, I.; Zhu, Y.; Xue, J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J. Appl. Clin. Med. Phys. 2011, 12, 267–292. [Google Scholar] [CrossRef]
- Bertolet, A.; Abolfath, R.; Carlson, D.J.; Lustig, R.A.; Hill-Kayser, C.; Alonso-Basanta, M.; Carabe, A. Correlation of LET With MRI Changes in Brain and Potential Implications for Normal Tissue Complication Probability for Patients With Meningioma Treated With Pencil Beam Scanning Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 112, 237–246. [Google Scholar] [CrossRef]
- McNamara, A.L.; Schuemann, J.; Paganetti, H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys. Med. Biol. 2015, 60, 8399–8416. [Google Scholar] [CrossRef]
- Konieczkowski, D.J.; DeLaney, T.F.; Yamada, Y. Radiation Strategies for Spine Chordoma: Proton Beam, Carbon Ions, and Stereotactic Body Radiation Therapy. Neurosurg. Clin. N. Am. 2020, 31, 263–288. [Google Scholar] [CrossRef]
- Fossati, P.; Vavassori, A.; Deantonio, L.; Ferrara, E.; Krengli, M.; Orecchia, R. Review of photon and proton radiotherapy for skull base tumours. Rep. Pract. Oncol. Radiother. 2016, 21, 336–355. [Google Scholar] [CrossRef]
- Ares, C.; Hug, E.B.; Lomax, A.J.; Bolsi, A.; Timmermann, B.; Rutz, H.P.; Schuller, J.C.; Pedroni, E.; Goitein, G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosacomas of the skull base: First long-term report. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1111–1118. [Google Scholar] [CrossRef]
- Kilby, W.; Dooley, J.R.; Kuduvalli, G.; Sayeh, S.; Maurer, C.R. The CyberKnife Robotic Radiosurgery System in 2010. Technol. Cancer Res. Treat. 2010, 9, 433–452. [Google Scholar] [CrossRef]
- Liu, A.-L.; Wang, Z.-C.; Sun, S.-B.; Wang, M.-H.; Luo, B.; Liu, P. Gamma knife radiosurgery for residual skull base chordomas. Neurol. Res. 2008, 30, 557–561. [Google Scholar] [CrossRef]
- Kano, H.; Iqbal, F.O.; Sheehan, J.; Mathieu, D.; A Seymour, Z.; Niranjan, A.; Flickinger, J.C.; Kondziolka, D.; E Pollock, B.; Rosseau, G.; et al. Stereotactic radiosurgery for chordoma: A report from the North American Gamma Knife Consortium. Neurosurgery 2011, 68, 379–389. [Google Scholar] [CrossRef]
- Iyer, A.; Kano, H.; Kondziolka, D.; Liu, X.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for intracranial chondrosarcoma. J. Neuro-Oncol. 2012, 108, 535–542. [Google Scholar] [CrossRef]
- Kano, H.; Sheehan, J.; Sneed, P.K.; McBride, H.L.; Young, B.; Duma, C.; Mathieu, D.; Seymour, Z.; McDermott, M.W.; Kondziolka, D.; et al. Skull base chondrosarcoma radiosurgery: Report of the North American Gamma Knife Consortium. J. Neurosurg. 2015, 123, 1268–1275. [Google Scholar] [CrossRef]
- Yamada, Y.; Laufer, I.; Cox, B.W.; Lovelock, D.M.; Maki, R.G.; Zatcky, J.M.; Boland, P.J.; Bilsky, M.H. Preliminary results of high-dose single-fraction radiotherapy for the management of chordomas of the spine and sacrum. Neurosurgery 2013, 73, 673–680. [Google Scholar] [CrossRef]
- Jiang, B.; Veeravagu, A.; Lee, M.; Harsh, G.R.; Lieberson, R.E.; Bhatti, I.; Soltys, S.G.; Gibbs, I.C.; Adler, J.R.; Chang, S.D. Management of intracranial and extracranial chordomas with CyberKnife stereotactic radiosurgery. J. Clin. Neurosci. 2012, 19, 1101–1106. [Google Scholar] [CrossRef]
- Brand, D.H.; Kirby, A.M.; Yarnold, J.; Somaiah, N. How Low Can You Go? The Radiobiology of Hypofractionation. Clin. Oncol. 2022, 34, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Speckter, H.; Santana, J.; Miches, I.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Garcia, J.; Stoeter, P. Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J. Radiat. Oncol. 2019, 8, 279–289. [Google Scholar] [CrossRef]
- Mahadevan, A.; Floyd, S.; Wong, E.; Chen, C.; Kasper, E. Clinical outcome after hypofractionated stereotactic radiotherapy (HSRT) for benign skull base tumors. Comput. Aided Surg. 2011, 16, 112–120. [Google Scholar] [CrossRef]
- McDonald, M.W.; Linton, O.R.; Calley, C.S. Dose–volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 261–267. [Google Scholar] [CrossRef] [PubMed]
- PTCOG Website. Available online: https://www.ptcog.site/index.php/facilities-in-operation-public (accessed on 11 October 2023).
- Santos, A.; Penfold, S.; Gorayski, P.; Le, H. The Role of Hypofractionation in Proton Therapy. Cancers 2022, 14, 2271. [Google Scholar] [CrossRef]
- Cao, H.; Xiao, Z.; Zhang, Y.; Kwong, T.; Danish, S.F.; Weiner, J.; Wang, X.; Yue, N.; Dai, Z.; Kuang, Y.; et al. Dosimetric comparisons of different hypofractionated stereotactic radiotherapy techniques in treating intracranial tumors > 3 cm in longest diameter. J. Neurosurg. 2020, 132, 1024–1032. [Google Scholar] [CrossRef]
- Friedrich, T. Proton RBE Dependence on Dose in the Setting of Hypofractionation. Br. J. Radiol. 2020, 93, 20190291. [Google Scholar] [CrossRef]
- Paganetti, H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys. Med. Biol. 2014, 59, R419. [Google Scholar] [CrossRef]
- Sgalambro, F.; Zugaro, L.; Bruno, F.; Palumbo, P.; Salducca, N.; Zoccali, C.; Barile, A.; Masciocchi, C.; Arrigoni, F. Interventional Radiology in the Management of Metastases and Bone Tumors. J. Clin. Med. 2022, 11, 3265. [Google Scholar] [CrossRef]
- Mercatali, L.; Vanni, S.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Cocchi, C.; Calabrese, C.; Gurrieri, L.; Fausti, V.; Riva, N.; et al. The emerging role of cancer nanotechnology in the panorama of sarcoma. Front. Bioeng. Biotechnol. 2022, 10, 953555. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Plitt, A.R.; Lim, M.; Garzon-Muvdi, T. Immunotherapy for Chordoma and Chondrosarcoma: Current Evidence. Cancers 2021, 13, 2408. [Google Scholar] [CrossRef]

| Chordomas (n = 6) | Chondrosarcomas (n = 5) | |
|---|---|---|
| Total dose | 37.5 GyRBE | 35 GyRBE |
| Dose per fraction | 7.5 GyRBE | 7 GyRBE |
| Median CTV (range) | 32.2 cc (13.7–43.5 cc) | 32.5 cc (10.6–47.5 cc) |
| Median number of beams (range) | 4 (4–6) | 4 (4–5) |
| Use of apertures | 4 patients | 3 patients |
| Median CTV coverage (range) | D95 = 95% (92.2–99.8%) | D95 = 96% (94.2–98.7%) |
| Median CTV D99 (range) | 39 Gy (39.9–38.8 GyRBE) | 36.4 Gy (37–36 GyRBE) |
| Median D0.03 cc at brainstem (range) | 26 Gy (30–24.7 GyRBE) | 26.5 Gy (29.1–25.2 GyRBE) |
| Median D0.03 cc at optic pathway (range) | 20.2 Gy (23–8.9 GyRBE) | 20.7 Gy (23.6–16.3 GyRBE) |
| Chordomas (n = 6) | Chondrosarcomas (n = 5) | |
|---|---|---|
| Acute toxicity (0–3 months) | ||
| Headache grade I–II | 4 patients (67%) | 3 patients (60%) |
| Asthenia grade I | 3 patients (50%) | 2 patients (40%) |
| Reversible alopecia | 2 patients (33%) | 3 patients (60%) |
| Nausea grade I | 1 patient (17%) | 2 patients (40%) |
| Dysphagia grade I | 2 patients (33%) | |
| Subacute toxicity (3–6 months) | ||
| IV cranial nerve palsy | 1 patient (17%) | |
| Late toxicity (6–15 months) | ||
| Temporal lobe necrosis grade III | 1 patient (17%) | 1 patient (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallabanda, M.; Vera, J.A.; Pérez, J.M.; Matute, R.; Montero, M.; de Pablo, A.; Cerrón, F.; Valero, M.; Castro, J.; Mazal, A.; et al. Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial. Cancers 2023, 15, 5579. https://doi.org/10.3390/cancers15235579
Sallabanda M, Vera JA, Pérez JM, Matute R, Montero M, de Pablo A, Cerrón F, Valero M, Castro J, Mazal A, et al. Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial. Cancers. 2023; 15(23):5579. https://doi.org/10.3390/cancers15235579
Chicago/Turabian StyleSallabanda, Morena, Juan Antonio Vera, Juan María Pérez, Raúl Matute, Marta Montero, Ana de Pablo, Fernando Cerrón, Mireia Valero, Juan Castro, Alejandro Mazal, and et al. 2023. "Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial" Cancers 15, no. 23: 5579. https://doi.org/10.3390/cancers15235579
APA StyleSallabanda, M., Vera, J. A., Pérez, J. M., Matute, R., Montero, M., de Pablo, A., Cerrón, F., Valero, M., Castro, J., Mazal, A., & Miralbell, R. (2023). Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial. Cancers, 15(23), 5579. https://doi.org/10.3390/cancers15235579






