Bladder Cancer and Probiotics: What Do We Know So Far?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Urinary Microbiota and Bladder Cancer
3. Bladder Cancer and Probiotics
3.1. Bladder Cancer and BCG-Based Immunotherapy
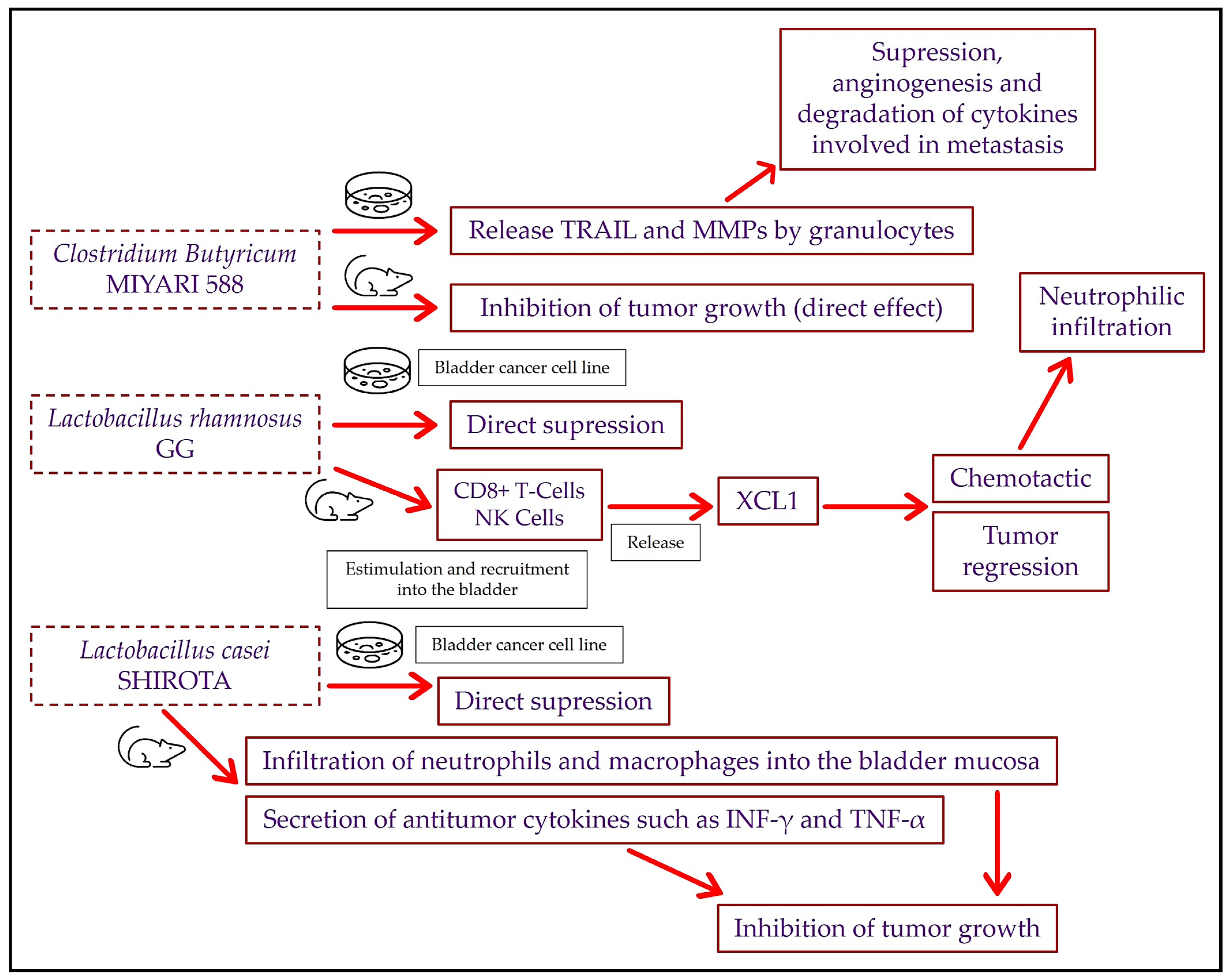
3.2. Bladder Cancer and Intravesical Probiotics: Preclinical Studies
3.3. Bladder Cancer and Oral Probiotics: Murine Assays and Human Clinical Trials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmadi, H.; Duddalwar, V.; Daneshmand, S. Diagnosis and Staging of Bladder Cancer. Hematol. Oncol. Clin. N. Am. 2021, 35, 531–541. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Aron, M. Variant Histology in Bladder Cancer—Current Understanding of Pathologic Subtypes. Curr. Urol. Rep. 2019, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor, J.A., 3rd; et al. What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur. Urol. Focus 2020, 6, 653–663. [Google Scholar] [CrossRef]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Jalloh, M.; Cassell, A.; Diallo, T.; Gaye, O.; Ndoye, M.; Mbodji, M.M.; Mahamat, M.A.; Diallo, A.; Dial, C.; Labou, I.; et al. Is Schistosomiasis a Risk Factor for Bladder Cancer? Evidence-Based Facts. J. Trop. Med. 2020, 2020, 8270810. [Google Scholar] [CrossRef]
- Souaid, T.; Hindy, J.-R.; Diab, E.; Kourie, H.R. Are There Monogenic Hereditary Forms of Bladder Cancer or Only Genetic Susceptibilities? Pharmacogenomics 2021, 22, 619–628. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Potential Impacts of Prebiotics and Probiotics on Cancer Prevention. Anticancer Agents Med. Chem. 2022, 22, 605–628. [Google Scholar] [CrossRef]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Chai, T.C. The Bladder Is Not Sterile: An Update on the Urinary Microbiome. Curr. Bladder Dysfunct. Rep. 2019, 14, 331–341. [Google Scholar] [CrossRef]
- Karstens, L.; Asquith, M.; Caruso, V.; Rosenbaum, J.T.; Fair, D.A.; Braun, J.; Gregory, W.T.; Nardos, R.; McWeeney, S.K. Community Profiling of the Urinary Microbiota: Considerations for Low-Biomass Samples. Nat. Rev. Urol. 2018, 15, 735–749. [Google Scholar] [CrossRef]
- Magistro, G.; Stief, C.G. The Urinary Tract Microbiome: The Answer to All Our Open Questions? Eur. Urol. Focus 2019, 5, 36–38. [Google Scholar] [CrossRef]
- Brubaker, L.; Gourdine, J.-P.F.; Siddiqui, N.Y.; Holland, A.; Halverson, T.; Limeria, R.; Pride, D.; Ackerman, L.; Forster, C.S.; Jacobs, K.M.; et al. Forming Consensus to Advance Urobiome Research. mSystems 2021, 6, e0137120. [Google Scholar] [CrossRef]
- Popović, V.B.; Šitum, M.; Chow, C.-E.T.; Chan, L.S.; Roje, B.; Terzić, J. The Urinary Microbiome Associated with Bladder Cancer. Sci. Rep. 2018, 8, 12157. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the Urinary Microbiota in Male Patients with Bladder Cancer in China. Front. Cell. Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, G.; Chen, C.; Li, K.; Wen, Y.; Zhao, J.; Wu, P. Alterations in Urobiome in Patients with Bladder Cancer and Implications for Clinical Outcome: A Single-Institution Study. Front. Cell Infect. Microbiol. 2020, 10, 555508. [Google Scholar] [CrossRef]
- Hussein, A.A.; Elsayed, A.S.; Durrani, M.; Jing, Z.; Iqbal, U.; Gomez, E.C.; Singh, P.K.; Liu, S.; Smith, G.; Tang, L.; et al. Investigating the Association between the Urinary Microbiome and Bladder Cancer: An Exploratory Study. Urol. Oncol. 2021, 39, 370.e9–370.e19. [Google Scholar] [CrossRef]
- Bi, H.; Tian, Y.; Song, C.; Li, J.; Liu, T.; Chen, Z.; Chen, C.; Huang, Y.; Zhang, Y. Urinary Microbiota—A Potential Biomarker and Therapeutic Target for Bladder Cancer. J. Med. Microbiol. 2019, 68, 1471–1478. [Google Scholar] [CrossRef]
- Chipollini, J.; Wright, J.R.; Nwanosike, H.; Kepler, C.Y.; Batai, K.; Lee, B.R.; Spiess, P.E.; Stewart, D.B.; Lamendella, R. Characterization of Urinary Microbiome in Patients with Bladder Cancer: Results from a Single-Institution, Feasibility Study. Urol. Oncol. 2020, 38, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis Signatures of the Microbial Profile in Tissue from Bladder Cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef]
- Pederzoli, F.; Ferrarese, R.; Amato, V.; Locatelli, I.; Alchera, E.; Lucianò, R.; Nebuloni, M.; Briganti, A.; Gallina, A.; Colombo, R.; et al. Sex-Specific Alterations in the Urinary and Tissue Microbiome in Therapy-Naïve Urothelial Bladder Cancer Patients. Eur. Urol. Oncol. 2020, 3, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Yacouba, A.; Alou, M.T.; Lagier, J.-C.; Dubourg, G.; Raoult, D. Urinary Microbiota and Bladder Cancer: A Systematic Review and a Focus on Uropathogens. Semin. Cancer Biol. 2022, 86, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, L.; Lee, P.; Huang, W.C.; Nossa, C.; Ma, Y.; Deng, F.-M.; Zhou, M.; Melamed, J.; Pei, Z. Mini-Review: Perspective of the Microbiome in the Pathogenesis of Urothelial Carcinoma. Am. J. Clin. Exp. Urol. 2014, 2, 57–61. [Google Scholar] [PubMed]
- Mai, G.; Chen, L.; Li, R.; Liu, Q.; Zhang, H.; Ma, Y. Common Core Bacterial Biomarkers of Bladder Cancer Based on Multiple Datasets. BioMed Res. Int. 2019, 2019, 4824909. [Google Scholar] [CrossRef]
- Mansour, B.; Monyók, Á.; Makra, N.; Gajdács, M.; Vadnay, I.; Ligeti, B.; Juhász, J.; Szabó, D.; Ostorházi, E. Bladder Cancer-Related Microbiota: Examining Differences in Urine and Tissue Samples. Sci. Rep. 2020, 10, 11042. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Woolbright, B.L.; Umar, S.; Ingersoll, M.A.; Taylor, J.A., 3rd. Bladder Cancer, Inflammageing and Microbiomes. Nat. Rev. Urol. 2022, 19, 495–509. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Ku, W.-C.; Liu, C.-Y.; Cheng, Y.-C.; Chien, C.-C.; Chang, K.-W.; Huang, C.-J. Supplementation of Probiotic Butyricicoccus Pullicaecorum Mediates Anticancer Effect on Bladder Urothelial Cells by Regulating Butyrate-Responsive Molecular Signatures. Diagnostics 2021, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Wissel, E.; Leon, L.; Tipton, L. Opportunities for Growth in the Growing Field of Psychobiotics. Benef. Microbes 2022, 13, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Morales, A. History of Bacillus Calmette-Guerin and Bladder Cancer: An Immunotherapy Success Story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef]
- Larsen, E.S.; Joensen, U.N.; Poulsen, A.M.; Goletti, D.; Johansen, I.S. Bacillus Calmette-Guérin Immunotherapy for Bladder Cancer: A Review of Immunological Aspects, Clinical Effects and BCG Infections. APMIS 2020, 128, 92–103. [Google Scholar] [CrossRef]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal Activity of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef]
- Ludwig, A.T.; Moore, J.M.; Luo, Y.; Chen, X.; Saltsgaver, N.A.; O’Donnell, M.A.; Griffith, T.S. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand: A Novel Mechanism for Bacillus Calmette-Guérin-Induced Antitumor Activity. Cancer Res. 2004, 64, 3386–3390. [Google Scholar] [CrossRef]
- Kemp, T.J.; Ludwig, A.T.; Earel, J.K.; Moore, J.M.; Vanoosten, R.L.; Moses, B.; Leidal, K.; Nauseef, W.M.; Griffith, T.S. Neutrophil Stimulation with Mycobacterium Bovis Bacillus Calmette-Guerin (BCG) Results in the Release of Functional Soluble TRAIL/Apo-2L. Blood 2005, 106, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Teppema, J.S.; de Boer, E.C.; Steerenberg, P.A.; van der Meijden, A.P. Morphological Aspects of the Interaction of Bacillus Calmette-Guérin with Urothelial Bladder Cells in Vivo and in Vitro: Relevance for Antitumor Activity? Urol. Res. 1992, 20, 219–228. [Google Scholar] [CrossRef]
- Mitropoulos, D.N. Novel Insights into the Mechanism of Action of Intravesical Immunomodulators. In Vivo 2005, 19, 611–621. [Google Scholar] [PubMed]
- van der Meijden, A.P.M.; Sylvester, R.J.; Oosterlinck, W.; Hoeltl, W.; Bono, A.V. Maintenance Bacillus Calmette-Guerin for Ta T1 Bladder Tumors Is Not Associated with Increased Toxicity: Results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur. Urol. 2003, 44, 429–434. [Google Scholar] [CrossRef]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M.; Yamada, T.; Miki, T.; Sakai, T. Clostridium Butyricum MIYAIRI 588 Shows Antitumor Effects by Enhancing the Release of TRAIL from Neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Kleifeld, O. Tumour Microenvironment—Opinion: Validating Matrix Metalloproteinases as Drug Targets and Anti-Targets for Cancer Therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-Producing Human Gut Symbiont, Clostridium Butyricum, and Its Role in Health and Disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Seow, S.W.; Rahmat, J.N.B.; Mohamed, A.A.K.; Mahendran, R.; Lee, Y.K.; Bay, B.H. Lactobacillus Species Is More Cytotoxic to Human Bladder Cancer Cells than Mycobacterium Bovis (Bacillus Calmette-Guerin). J. Urol. 2002, 168, 2236–2239. [Google Scholar] [CrossRef]
- Takahashi, T.; Kushiro, A.; Nomoto, K.; Uchida, K.; Morotomi, M.; Yokokura, T.; Akaza, H. Antitumor Effects of the Intravesical Instillation of Heat Killed Cells of the Lactobacillus Casei Strain Shirota on the Murine Orthotopic Bladder Tumor MBT-2. J. Urol. 2001, 166, 2506–2511. [Google Scholar] [CrossRef]
- Seow, S.W.; Rahmat, J.N.; Bay, B.H.; Lee, Y.K.; Mahendran, R. Expression of Chemokine/Cytokine Genes and Immune Cell Recruitment Following the Instillation of Mycobacterium Bovis, Bacillus Calmette-Guérin or Lactobacillus Rhamnosus Strain GG in the Healthy Murine Bladder. Immunology 2008, 124, 419–427. [Google Scholar] [CrossRef]
- Seow, S.W.; Cai, S.; Rahmat, J.N.; Bay, B.H.; Lee, Y.K.; Chan, Y.H.; Mahendran, R. Lactobacillus Rhamnosus GG Induces Tumor Regression in Mice Bearing Orthotopic Bladder Tumors. Cancer Sci. 2010, 101, 751–758. [Google Scholar] [CrossRef]
- Lei, Y.; Takahama, Y. XCL1 and XCR1 in the Immune System. Microbes Infect. 2012, 14, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of CDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Asano, M.; Karasawa, E.; Takayama, T. Antitumor Activity of Lactobacillus Casei (LC 9018) against Experimental Mouse Bladder Tumor (MBT-2). J. Urol. 1986, 136, 719–721. [Google Scholar] [CrossRef]
- Lim, B.-K.; Mahendran, R.; Lee, Y.-K.; Bay, B.-H. Chemopreventive Effect of Lactobacillus Rhamnosus on Growth of a Subcutaneously Implanted Bladder Cancer Cell Line in the Mouse. Jpn. J. Cancer Res. 2002, 93, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Oda, Y.; Owari, T.; Iida, K.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Miyamoto, T.; Shimizu, T.; Ohnishi, K.; et al. Probiotics Enhances Anti-Tumor Immune Response Induced by Gemcitabine plus Cisplatin Chemotherapy for Urothelial Cancer. Cancer Sci. 2023, 114, 1118–1130. [Google Scholar] [CrossRef]
- Aso, Y.; Akazan, H. Prophylactic Effect of a Lactobacillus Casei Preparation on the Recurrence of Superficial Bladder Cancer. BLP Study Group. Urol. Int. 1992, 49, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S. Preventive Effect of a Lactobacillus Casei Preparation on the Recurrence of Superficial Bladder Cancer in a Double-Blind Trial. The BLP Study Group. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [CrossRef]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N. Prevention of Recurrence with Epirubicin and Lactobacillus Casei after Transurethral Resection of Bladder Cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef]
- O’Donnell, M.A. Does the Probiotic L. Casei Help Prevent Recurrence after Transurethral Resection for Superficial Bladder Cancer? Nat. Clin. Pract. Urol. 2008, 5, 526–527. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T.; Masumori, N.; Akaza, H.; Miyanaga, N.; Kitamura, T.; Kawabe, K.; Kotake, T.; Kuroda, M.; et al. Habitual Intake of Lactic Acid Bacteria and Risk Reduction of Bladder Cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dai, H.; Liang, W.; Zhang, L.; Deng, Z. Fermented Dairy Foods Intake and Risk of Cancer. Int. J. Cancer 2019, 144, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
| Study | Sample | Population | Key Results |
|---|---|---|---|
| Xu 2014 [30] | Urine | 8 patients with uroepithelial carcinoma and 6 healthy controls |
|
| Popovic 2018 [21] | Urine | 12 BC patients and 11 healthy controls. All participants were men. BC Staging: 83% pTa and 17% pT1. |
|
| Wu 2018 [22] | Urine | 31 BC patients (26 NMIBC and 5 MIBC) and 18 healthy controls. All participants were men. |
|
| Bi 2019 [25] | Urine | 29 BC patients (20 men and 9 women) and 26 healthy controls (15 men and 11 women). BC Staging: 34% pTa, 34% pT1, 18% pT2a, 7% pT2b, and 7% pT3a. |
|
| Mai 2019 [31] | Urine | 24 BC patients (18 men and 6 women). Comparison with 2 previous datasets. |
|
| Liu 2019 [27] | Tumoral tissue | 22 samples of tumoral tissue and 12 samples of adjacent nontumoral tissue. 5 NMIBC and 17 MIBC patients. |
|
| Mansour 2020 [32] | Urine and tumoral tissue | 10 BC patients (5 men and 5 women). 6 NMIBC and 4 MIBC patients. |
|
| Pederzoli 2020 [28] | Urine and tumoral tissue | 49 BC patients (36 men and 13 women) and 59 healthy controls (34 men and 25 women) |
|
| Zeng 2020 [23] | Urine | 62 male BC patients (51 NMIBC and 11 MIBC) and 19 healthy controls. Follow-ups in 40 NMIBC patients. |
|
| Chipollini 2020 [26] | Urine | 25 BC patients (17 MIBC and 12 NMIBC) and 10 healthy controls (globally 87% men). |
|
| Hussein 2021 [24] | Urine | 43 BC patients (84% men and 67% NMIBC) and 10 healthy controls |
|
| Study | Type | Probiotic | Key Results |
|---|---|---|---|
| Shinnoh 2013 [45] | Mice and in vitro | Clostridium butyricum MIYARI 588 |
|
| Seow 2002 [48] | In vitro | Lactobacillus rhamnosus GG and Lactobacillus casei Shirota |
|
| Takahashi 2001 [49] | Mice | Lactobacillus casei Shirota |
|
| Seow 2008 [50] | Mice | Lactobacillus rhamnosus GG |
|
| Seow 2010 [51] | Mice | Lactobacillus rhamnosus GG |
|
| Study | Type | Probiotic | Key Results |
|---|---|---|---|
| Asano 1986 [54] | Mice | Lactobacillus casei (LC9018) |
|
| Lim 2002 [55] | Mice | Lactobacillus rhamnosus GG |
|
| Miyake 2023 [56] | Mice | Lactobacillus casei Shirota and Bifidobacterium breve |
|
| Aso 1992 [57] | Clinical trial | Lactobacillus casei 1010 CFU/g (1 g 3 times a week for1 year) |
|
| Aso 1995 [58] | Clinical trial | Lactobacillus casei 1010 CFU/g (1 g 3 times a week for1 year) |
|
| Naito 2008 [59] | Clinical trial | Lactobacillus casei 1010 CFU/g (1 g 3 times a week for 1 year) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Pellicer, P.; Boix-Rodríguez, C.; Hernández-Belmonte, A.; Encarnación-Castellano, C.d.l.; Mendiola-López, A.; Núñez-Delegido, E.; Navarro-Moratalla, L.; Agüera-Santos, J.; Navarro-López, V.; Galán-Llopis, J.A. Bladder Cancer and Probiotics: What Do We Know So Far? Cancers 2023, 15, 5551. https://doi.org/10.3390/cancers15235551
Sánchez-Pellicer P, Boix-Rodríguez C, Hernández-Belmonte A, Encarnación-Castellano Cdl, Mendiola-López A, Núñez-Delegido E, Navarro-Moratalla L, Agüera-Santos J, Navarro-López V, Galán-Llopis JA. Bladder Cancer and Probiotics: What Do We Know So Far? Cancers. 2023; 15(23):5551. https://doi.org/10.3390/cancers15235551
Chicago/Turabian StyleSánchez-Pellicer, Pedro, Claudia Boix-Rodríguez, Adriana Hernández-Belmonte, Cristina de la Encarnación-Castellano, Alberto Mendiola-López, Eva Núñez-Delegido, Laura Navarro-Moratalla, Juan Agüera-Santos, Vicente Navarro-López, and Juan Antonio Galán-Llopis. 2023. "Bladder Cancer and Probiotics: What Do We Know So Far?" Cancers 15, no. 23: 5551. https://doi.org/10.3390/cancers15235551
APA StyleSánchez-Pellicer, P., Boix-Rodríguez, C., Hernández-Belmonte, A., Encarnación-Castellano, C. d. l., Mendiola-López, A., Núñez-Delegido, E., Navarro-Moratalla, L., Agüera-Santos, J., Navarro-López, V., & Galán-Llopis, J. A. (2023). Bladder Cancer and Probiotics: What Do We Know So Far? Cancers, 15(23), 5551. https://doi.org/10.3390/cancers15235551






