Trend and Joinpoint Analysis of Cancer Incidence and 1-Year Mortality in North-East Spain 2005–2020
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data
2.2. Osona Tumor Registry
2.3. Registration in the Osona Tumor Registry
2.4. Events of Interest
2.5. Statistical Analysis
3. Results
3.1. Number of Cases
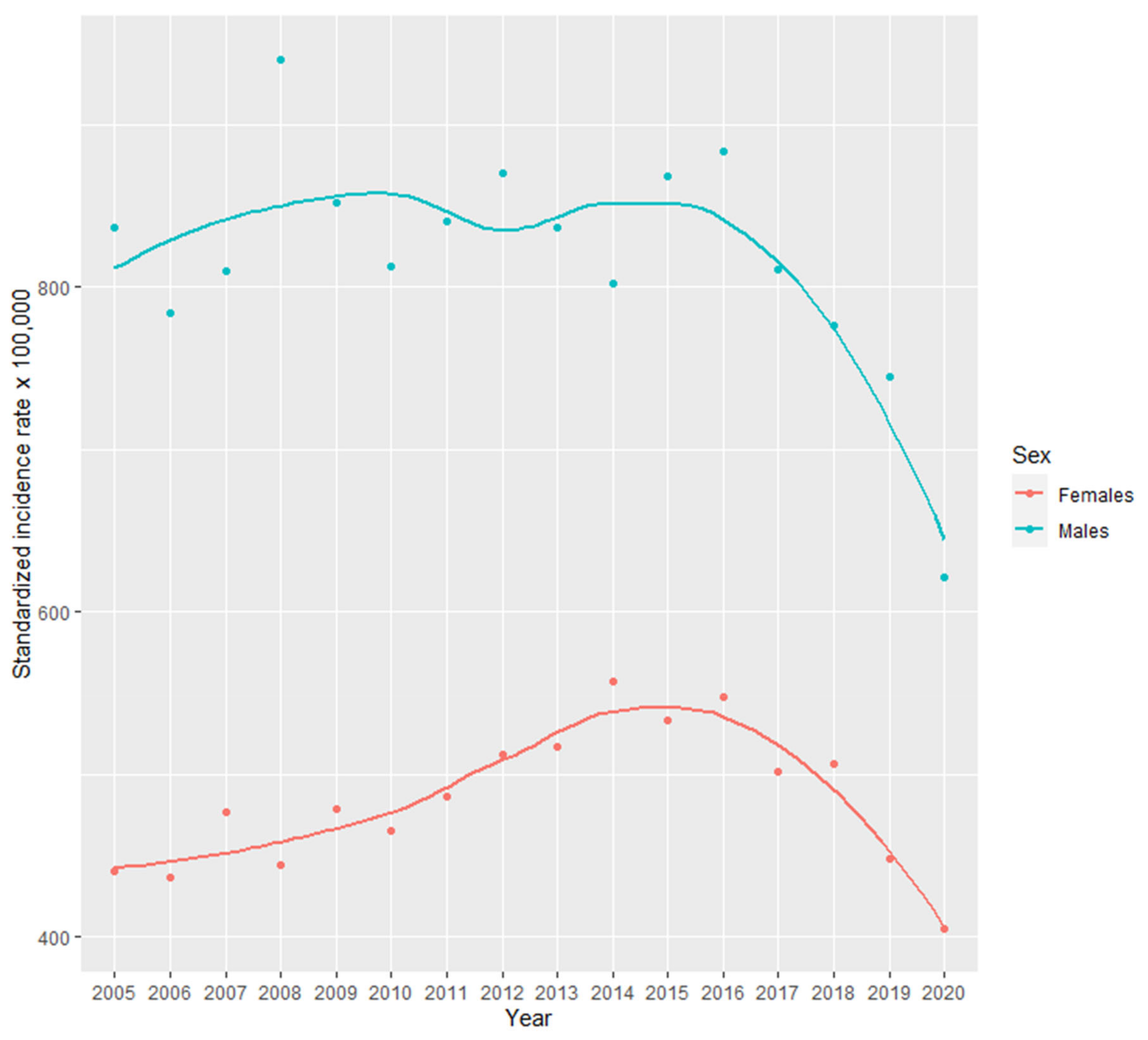
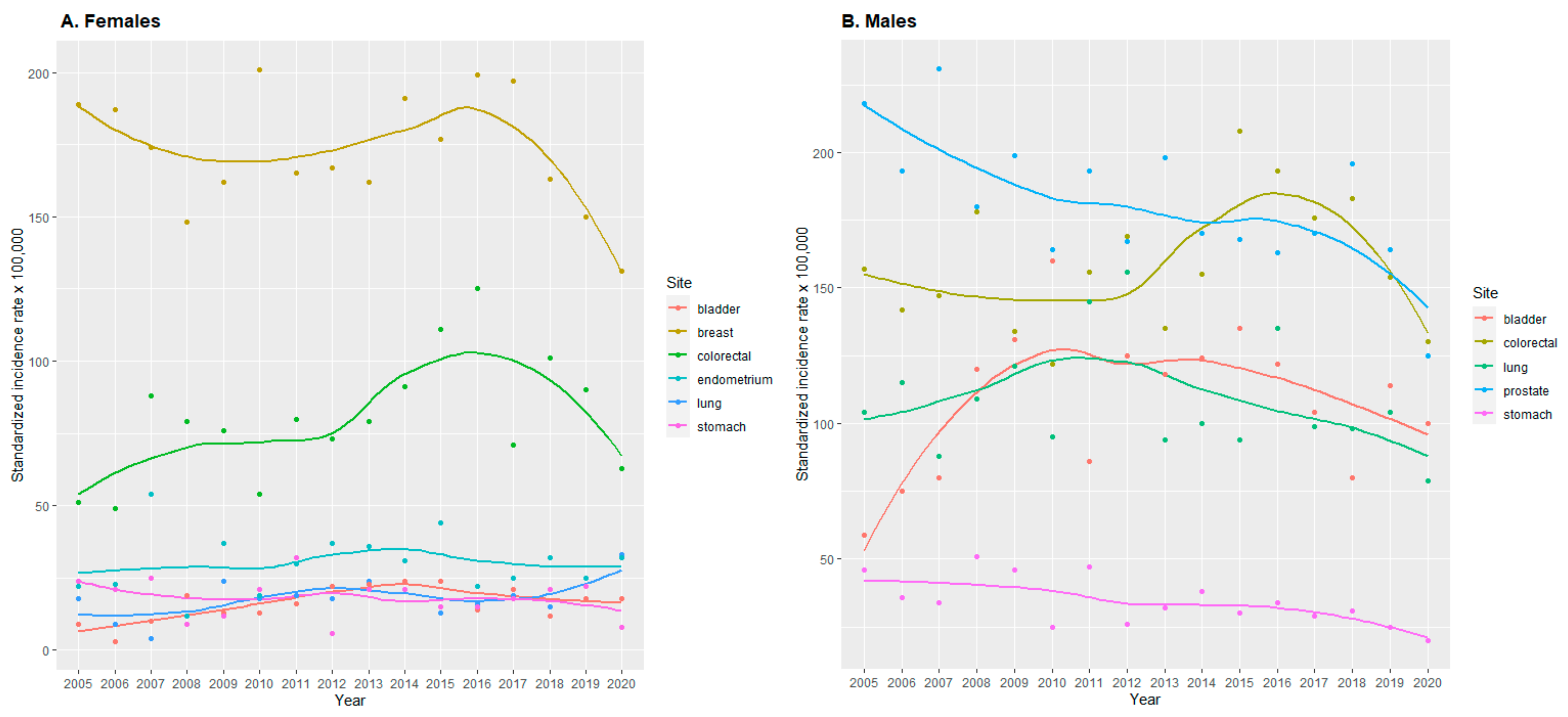
3.2. Incidence Rates
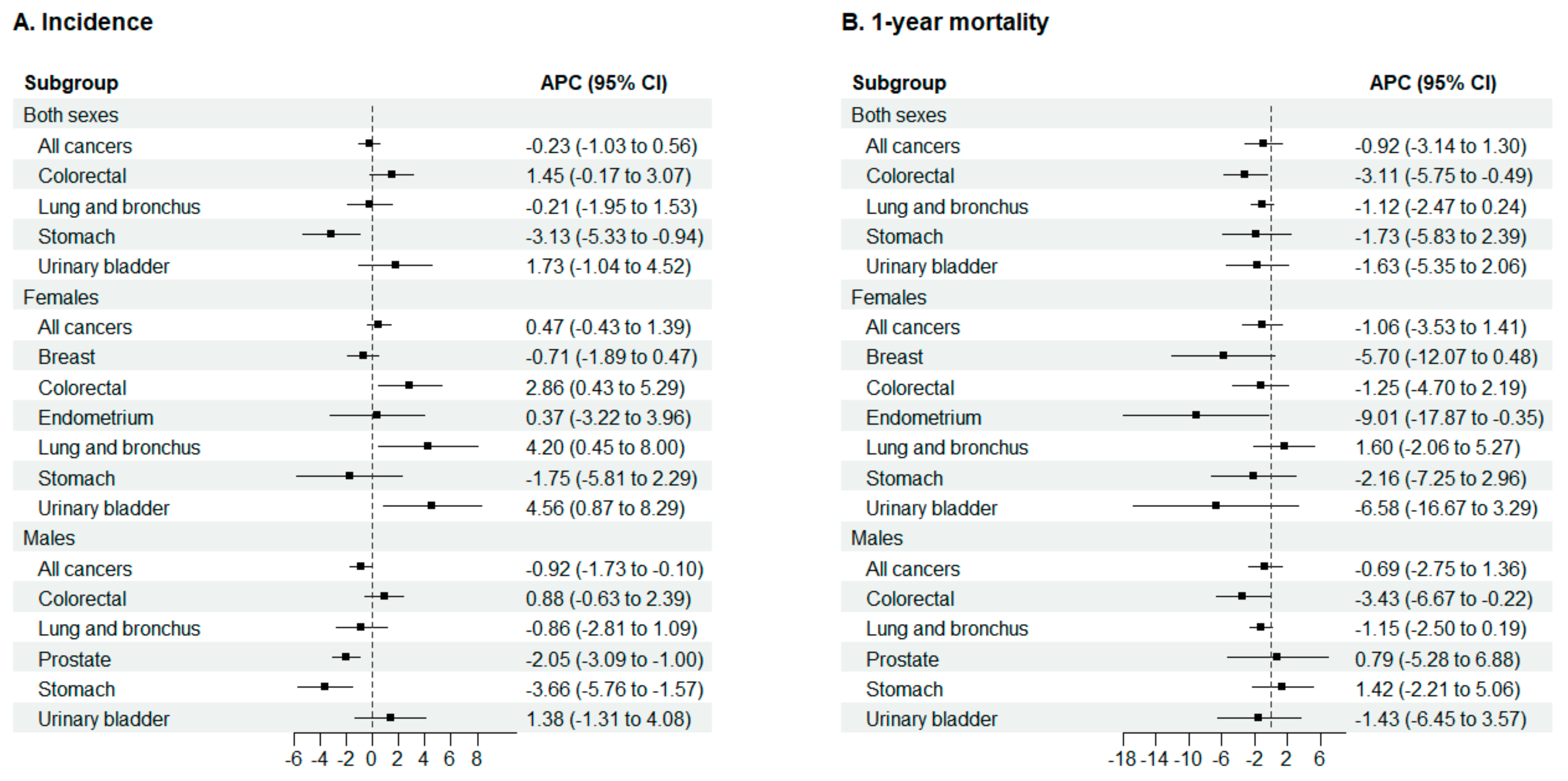
3.3. Incidence Rate Trends
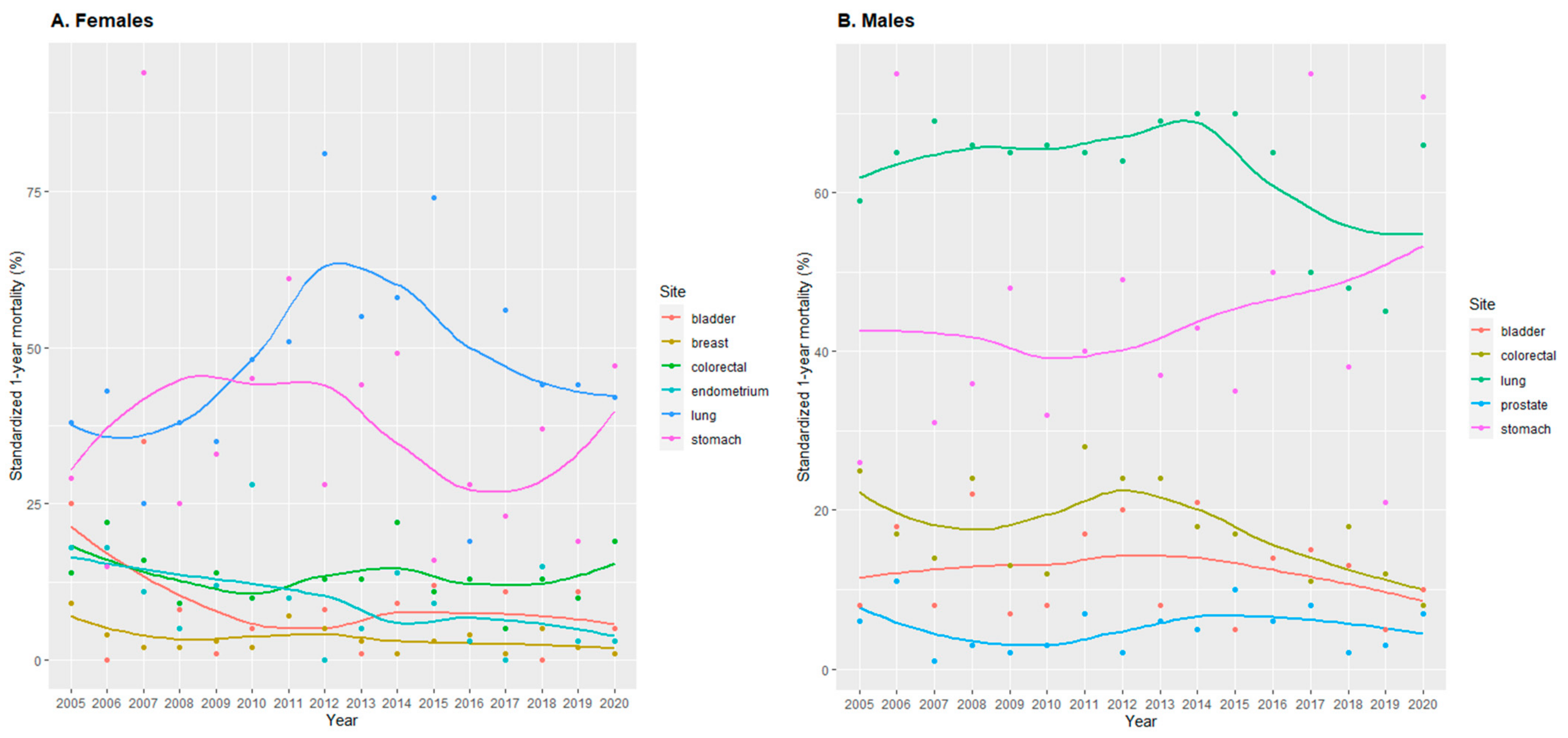
3.4. 1-Year Mortality
3.5. Incidence and 1-Year Mortality of Cancer during the COVID-19 Pandemic
3.6. Sensitivity Analyses
4. Discussion
4.1. Trends in Cancer Incidence
4.2. Trends in Cancer Mortality
4.3. Incidence and 1-Year Mortality of Cancer during the COVID-19 Pandemic
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar]
- Zanetti, R.; Sacchetto, L.; Coebergh, J.W.; Rosso, S. To accelerate cancer prevention in Europe: Challenges for cancer registries. Eur. J. Cancer. 2018, 104, 141–159. [Google Scholar] [CrossRef]
- European Network of Cancer Registries. ENCR Recommendations. Available online: https://encr.eu/ENCR-Recommendations (accessed on 28 September 2023).
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. Cancer Incidence in Five Continents; International Agency for Research on Cancer: Lyon, France, 2021; Volume XI.
- Cancer Epidemiology: Principles and Methods. Edited by I Dos Santos Silva. Chapter 17. The Role of Cancer Registries. 1999. Available online: https://www.iarc.who.int/news-events/iarc-publications-cancer-epidemiology-principles-and-methods/ (accessed on 28 September 2023).
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Tichanek, F.; Försti, A.; Hemminki, O.; Hemminki, A.; Hemminki, K. Survival, Incidence, and Mortality Trends in Female Cancers in the Nordic Countries. Obstet. Gynecol. Int. 2023, 2023, 6909414. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Defossez, G.; Uhry, Z.; Delafosse, P.; Dantony, E.; d’Almeida, T.; Plouvier, S.; Bossard, N.; Bouvier, A.M.; Molinié, F.; Woronoff, A.S.; et al. French Network of Cancer Registries (FRANCIM). Cancer incidence and mortality trends in France over 1990–2018 for solid tumors: The sex gap is narrowing. BMC Cancer 2021, 21, 726. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Traini, E.; Alam, T.; Allen, C.A.; Carreras, G.; Compton, K.; Fitzmaurice, C.; Force, L.M.; Gallus, S.; Gorini, G.; et al. National burden of cancer in Italy, 1990–2017: A systematic analysis for the global burden of disease study 2017. Sci. Rep. 2020, 10, 22099. [Google Scholar] [PubMed]
- International Agency for Research on Cancer; World Health Organization. Cancer Incidence in Five Continents. Cancer Registry List. Available online: https://ci5.iarc.fr/CI5I-X/Pages/database.aspx (accessed on 28 September 2023).
- Sato, Y.; Fujiwara, Y.; Fukuda, N.; Hayama, B.; Ito, Y.; Ohno, S.; Takahashi, S. COVID-19 Working group of The Cancer Institute Hospital of Japanese Foundation for Cancer Research. Changes in Treatment Behavior during the COVID-19 Pandemic among Patients at a Cancer Hospital. Cancer Cell 2021, 39, 130–131. [Google Scholar] [PubMed]
- World Health Organization. International Classification of Diseases for Oncology. Third Edition. Available online: https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology (accessed on 28 September 2023).
- National Institutes of Health; National Cancer Institute. Cancer Staging. Available online: www.cancer.gov/about-cancer/diagnosis-staging/staging (accessed on 28 September 2023).
- Spanish Ministry of Health. National Death Index—Access Request. Available online: https://www.sanidad.gob.es/en/estadEstudios/estadisticas/estadisticas/estMinisterio/IND_TipoDifusion.htm (accessed on 28 September 2023).
- Statistical Institute of Catalonia. Population Census. Available online: https://www.idescat.cat/pub/?id=ep&lang=en (accessed on 28 September 2023).
- European Commission; Eurostat; Pace, M.; Gissler, M.; Lanzieri, G.; Grande, E.; Wojtyniak, B.; Cayotte, E.; Glickman, M.; Agafitei, L.; et al. Revision of the European Standard Population: Report of Eurostat’s Task Force: 2013 edition. Publications Office. 2013; Available online: https://data.europa.eu/doi/10.2785/11470 (accessed on 16 November 2023).
- National Institutes of Health; National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Age Standards for Survival. Available online: https://seer.cancer.gov/stdpopulations/survival.html (accessed on 28 September 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 28 September 2023).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- National Cancer Institute; Division of Cancer Control & Population Sciences. Joinpoint Trend Analysis Software. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 28 September 2023).
- International Agency for Research on Cancer; Global Cancer Observatory. Cancer Over Time. Available online: https://gco.iarc.fr/overtime/en (accessed on 28 September 2023).
- Clèries, R.; Esteban, L.; Borràs, R.; Marcos-Gragera, R.; Freitas, A.; Carulla, M.; Buxó, M.; Puigdefàbregas, A.; Izquierdo, A.; Gispert, R.; et al. Time trends of cancer incidence and mortality in Catalonia during 1993–2007. Clin. Transl. Oncol. 2014, 16, 18–28. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Human Cancer: Known Causes and Prevention by Organ Site. Available online: https://monographs.iarc.who.int/human_cancer_known_causes_and_prevention_organ_site/ (accessed on 28 September 2023).
- Spanish National Statistics Institute. European Health Survey in Spain 2020. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176784&menu=ultiDatos&idp=1254735573175 (accessed on 28 September 2023).
- Encuesta Nacional de Salud. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE17_pres_web.pdf (accessed on 28 September 2023).
- Spanish Ministry of Health. Screening Program of Colorectal Cancer. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/cribado/cancer/colorrectal.htm (accessed on 28 September 2023).
- Luque-Fernandez, M.A.; Gonçalves, K.; Salamanca-Fernández, E.; Redondo-Sanchez, D.; Leem, S.F.; Rodríguez-Barranco, M.; Carmona-García, M.C.; Marcos-Gragera, R.; Sánchez, M.J. Multimorbidity and short-term overall mortality among colorectal cancer patients in Spain: A population-based cohort study. Eur. J. Cancer. 2020, 129, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Cowling, T.E.; Bellot, A.; Boyle, J.; Walker, K.; Kuryba, A.; Galbraith, S.; Aggarwal, A.; Braun, M.; Sharples, L.D.; van der Meulen, J. One-year mortality of colorectal cancer patients: Development and validation of a prediction model using linked national electronic data. Br. J. Cancer. 2020, 123, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Breugom, A.J.; Bastiaannet, E.; Dekker, J.W.T.; Wouters, M.W.J.M.; van de Velde, C.J.H.; Liefers, G.J. Decrease in 30-day and one-year mortality over time in patients aged ≥75 years with stage I-III colon cancer: A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Spanish Society of Medical Oncology. Cancer Figures in Spain 2023. Available online: https://seom.org/publicaciones/el-cancer-en-espanyacom (accessed on 28 September 2023).
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10, e043828. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.A.; Goldacre, R.; Spata, E.; Mafham, M.; Finan, P.J.; Shelton, J.; Richards, M.; Spencer, K.; Emberson, J.; Hollings, S. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study. Lancet Gastroenterol. Hepatol. 2021, 6, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nonboe, M.H.; Napolitano, G.; Schroll, J.B.; Vejborg, I.; Waldstrøm, M.; Lynge, E. Impact of COVID-19 pandemic on breast and cervical cancer screening in Denmark: A register-based study. eLife 2023, 12, e81605. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Asociación Española Contra el Cáncer; Observatorio del Cáncer. Dimensiones del Cáncer. Available online: https://observatorio.contraelcancer.es/explora/dimensiones-del-cancer (accessed on 28 September 2023).

| Period | APC (95% CI) | Period | APC (95% CI) | Period | APC (95% CI) | |
|---|---|---|---|---|---|---|
| Incidence | ||||||
| Both sexes | ||||||
| All cancers | 2005–2016 | 1.3 (0.6, 2.0) | 2016–2020 | −6.7 (−9.6, −3.6) | ||
| Urinary bladder | 2005–2009 | 25.3 (4.2, 50.5) | 2009–2020 | −2.6 (−5.7, 0.5) | ||
| Females | ||||||
| All cancers | 2005–2016 | 2.3 (1.5, 3.0) | 2016–2020 | −6.9 (−10.1, −3.7) | ||
| Breast | 2005–2017 | 0.6 (−1.0, 2.3) | 2017–2020 | −10.8 (−23.1, 3.4) | ||
| Males | ||||||
| All cancers | 2005–2017 | 0.2 (−0.8, 1.1) | 2017–2020 | −8.8 (−16.0, −1.1) | ||
| Urinary bladder | 2005–2009 | 23.6 (1.8, 49.9) | 2009–2020 | −2.8 (−6.0, 0.5) | ||
| 1-year mortality | ||||||
| Both sexes | ||||||
| Lung and bronchus | 2005–2015 | 1.0 (−1.6, 3.7) | 2015–2020 | −6.9 (−13.6, 0.3) | ||
| Males | ||||||
| Lung and bronchus | 2005–2015 | 1.3 (0.2, 2.4) | 2015–2018 | −13.7 (−24.3, −1.7) | 2018–2020 | 15.0 (−1.9, 34.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roura, P.; Puigoriol, E.; Altimiras, J.; Batiste-Alentorn, E.; Dégano, I.R. Trend and Joinpoint Analysis of Cancer Incidence and 1-Year Mortality in North-East Spain 2005–2020. Cancers 2023, 15, 5527. https://doi.org/10.3390/cancers15235527
Roura P, Puigoriol E, Altimiras J, Batiste-Alentorn E, Dégano IR. Trend and Joinpoint Analysis of Cancer Incidence and 1-Year Mortality in North-East Spain 2005–2020. Cancers. 2023; 15(23):5527. https://doi.org/10.3390/cancers15235527
Chicago/Turabian StyleRoura, Pere, Emma Puigoriol, Jacint Altimiras, Eduard Batiste-Alentorn, and Irene R. Dégano. 2023. "Trend and Joinpoint Analysis of Cancer Incidence and 1-Year Mortality in North-East Spain 2005–2020" Cancers 15, no. 23: 5527. https://doi.org/10.3390/cancers15235527
APA StyleRoura, P., Puigoriol, E., Altimiras, J., Batiste-Alentorn, E., & Dégano, I. R. (2023). Trend and Joinpoint Analysis of Cancer Incidence and 1-Year Mortality in North-East Spain 2005–2020. Cancers, 15(23), 5527. https://doi.org/10.3390/cancers15235527






