Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods:
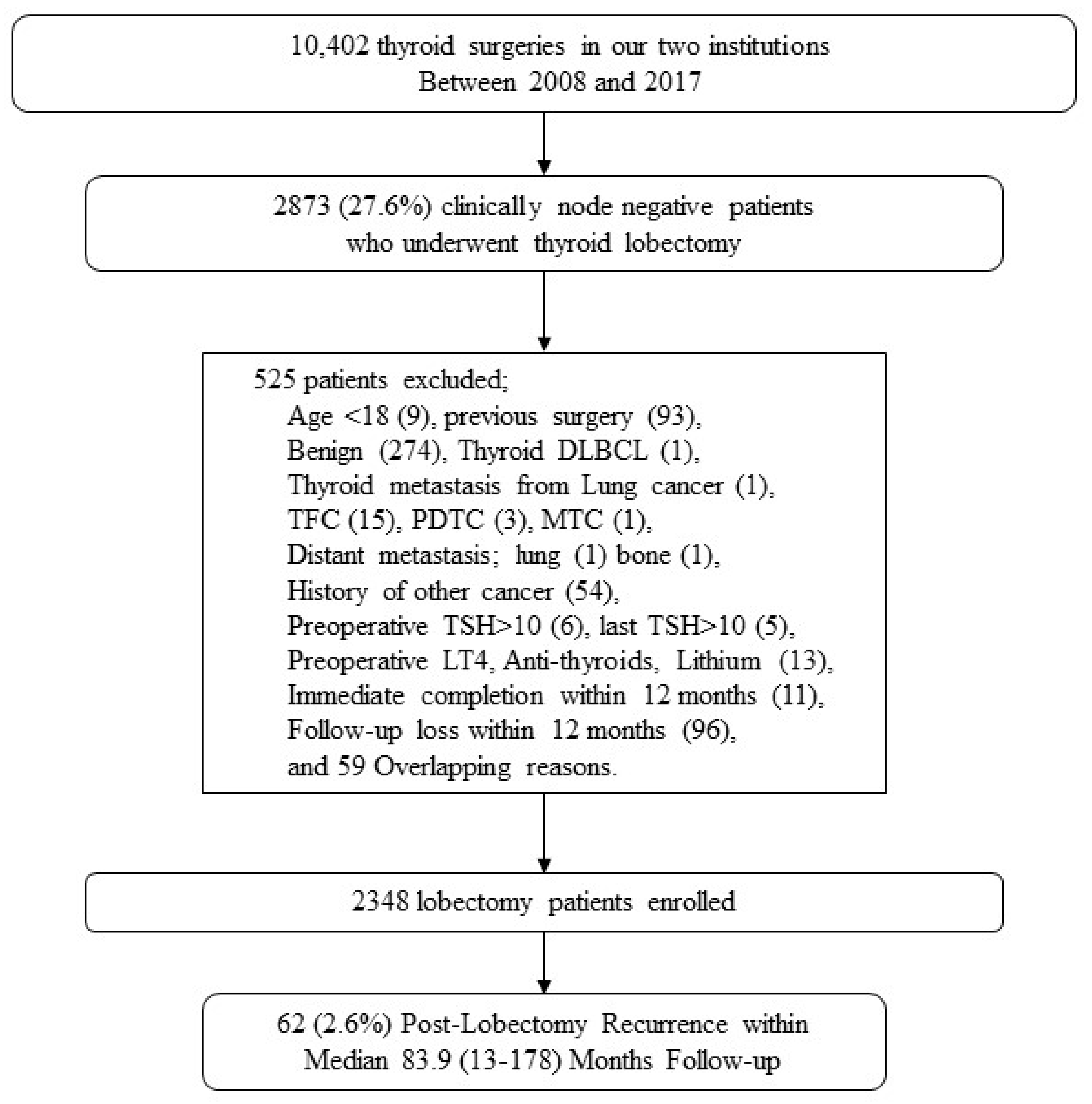
2.1. Patients and Inclusion/Exclusion Criteria
2.2. Surgical Approach
2.3. Central Neck Dissection (CND)
2.4. Risk Stratification
2.5. Immediate vs. Delayed Completion
2.6. Follow-Up
2.7. Statistics
2.8. Ethical Approval
3. Results
3.1. Baseline Characteristics of cN0 PTC Patients
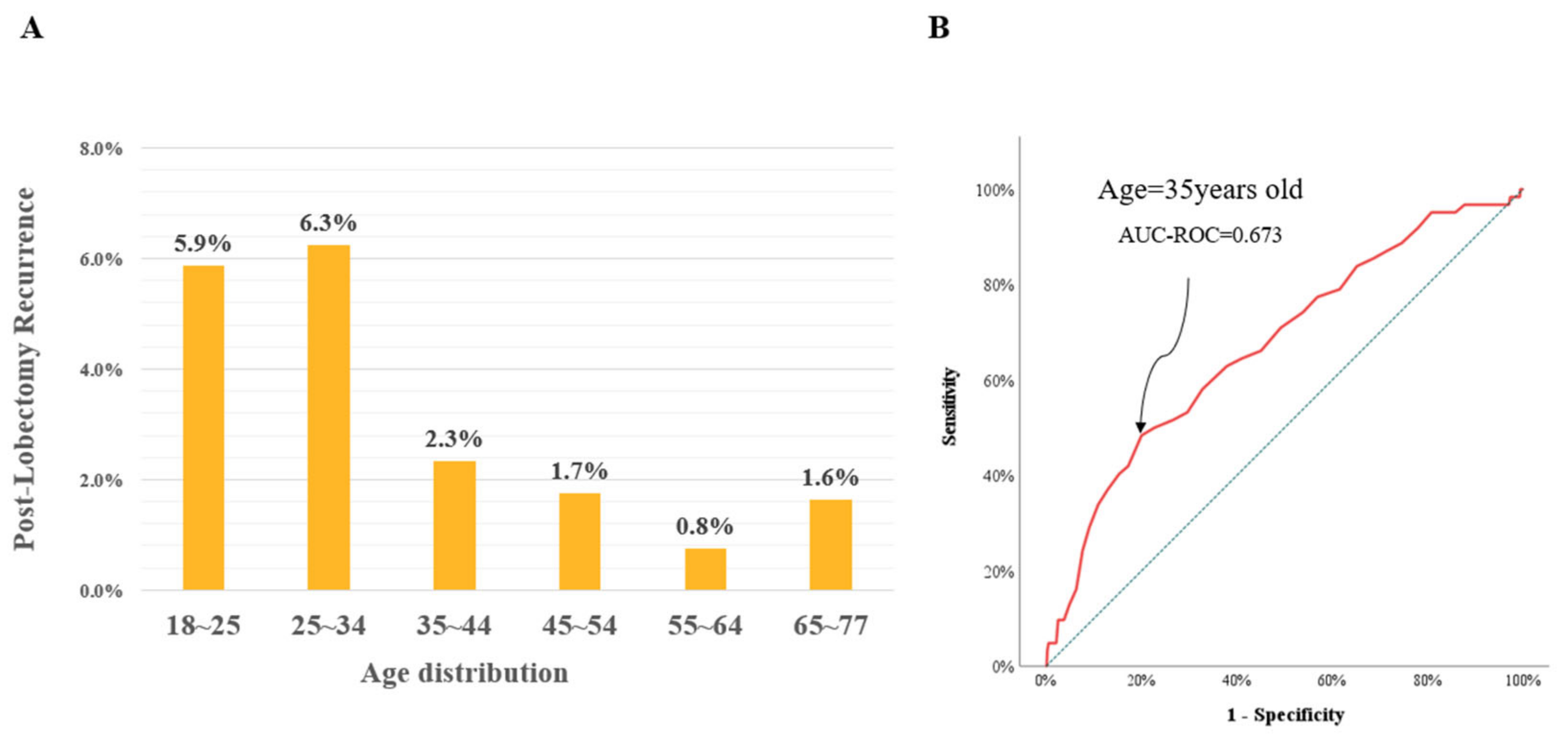
3.2. Characteristics of the Young Age Group
3.3. Cox Analysis
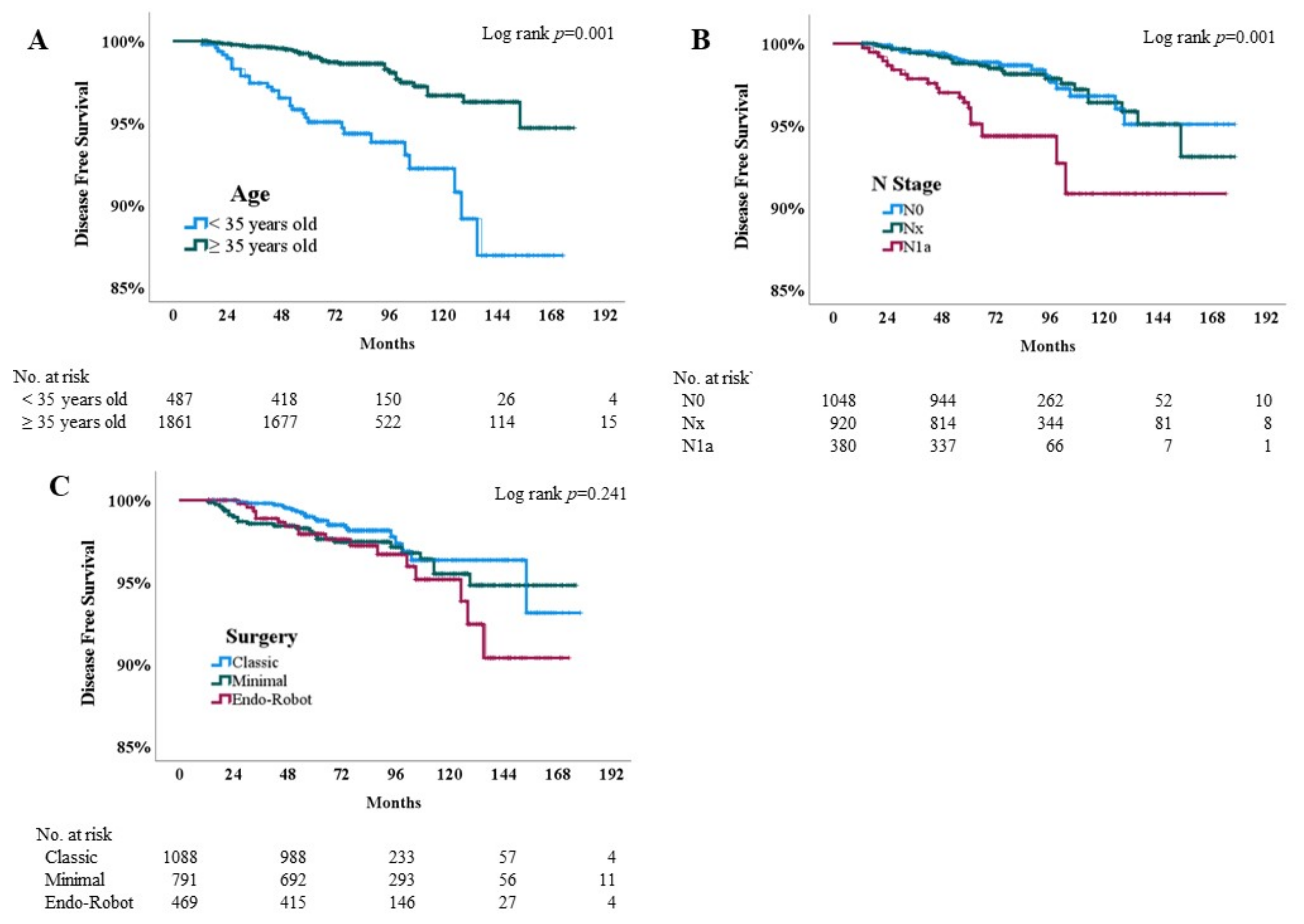
3.4. Kaplan–Meier Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasqual, E.; Sosa, J.A.; Chen, Y.; Schonfeld, S.J.; Berrington de Gonzalez, A.; Kitahara, C.M. Trends in the Management of Localized Papillary Thyroid Carcinoma in the United States (2000–2018). Thyroid 2022, 32, 397–410. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, J.; Kwak, M.K.; Jeon, M.J.; Kim, T.Y.; Hong, E.G.; Kim, W.B.; Kim, W.G. Recent Changes in the Incidence of Thyroid Cancer in Korea between 2005 and 2018: Analysis of Korean National Data. Endocrinol. Metab. 2022, 37, 791–799. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Feurer, I.D.; Griffin, M.R.; Phay, J.E. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008, 144, 1070–1077; discussion 1077–1078. [Google Scholar] [CrossRef]
- Cho, J.S.; Yoon, J.H.; Park, M.H.; Shin, S.H.; Jegal, Y.J.; Lee, J.S.; Kim, H.K. Age and prognosis of papillary thyroid carcinoma: Retrospective stratification into three groups. J. Korean Surg. Soc. 2012, 83, 259–266. [Google Scholar] [CrossRef]
- Tuttle, M.; Morris, L.; Haugen, B.; Shah, J.; Sosa, J.; Rohren, E.; Subramaniam, R.; Hunt, J.; Perrier, N.; Amin, M.; et al. Thyroid-Differentiated and Anaplastic Carcinoma (Chapter 73); Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Tam, S.; Boonsripitayanon, M.; Amit, M.; Fellman, B.M.; Li, Y.; Busaidy, N.L.; Cabanillas, M.E.; Dadu, R.; Sherman, S.; Waguespack, S.G.; et al. Survival in Differentiated Thyroid Cancer: Comparing the AJCC Cancer Staging Seventh and Eighth Editions. Thyroid 2018, 28, 1301–1310. [Google Scholar] [CrossRef]
- Adam, M.A.; Pura, J.; Goffredo, P.; Dinan, M.A.; Reed, S.D.; Scheri, R.P.; Hyslop, T.; Roman, S.A.; Sosa, J.A. Presence and Number of Lymph Node Metastases Are Associated with Compromised Survival for Patients Younger Than Age 45 Years with Papillary Thyroid Cancer. J. Clin. Oncol. 2015, 33, 2370–2375. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhao, K.; Li, D.; Chen, Z.; Jiang, R.; Wang, X.; He, X. Lymph node metastasis in young and middle-aged papillary thyroid carcinoma patients: A SEER-based cohort study. BMC Cancer 2020, 20, 181. [Google Scholar] [CrossRef]
- Yoo, H.; Chae, B.J.; Park, H.S.; Kim, K.H.; Kim, S.H.; Song, B.J.; Jung, S.S.; Bae, J.S. Comparison of surgical outcomes between endoscopic and robotic thyroidectomy. J. Surg. Oncol. 2012, 105, 705–708. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.J.; Park, C.S.; Kim, B.W. Frozen section analysis of central lymph nodes in papillary thyroid cancer: The significance in determining the extent of surgery. Gland Surg. 2022, 11, 640–650. [Google Scholar] [CrossRef]
- Raffaelli, M.; Sessa, L.; De Crea, C.; Fadda, G.; Princi, P.; Rossi, E.D.; Traini, E.; Revelli, L.; Pennestri, F.; Gallucci, P.; et al. Is it possible to intraoperatively modulate the extent of thyroidectomy in small papillary thyroid carcinoma? Surgery 2021, 169, 77–81. [Google Scholar] [CrossRef]
- Pasieka, J.L.; Thompson, N.W.; McLeod, M.K.; Burney, R.E.; Macha, M. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J. Surg. 1992, 16, 711–716; discussion 716–717. [Google Scholar] [CrossRef]
- Na, Y.M.; Cho, J.S.; Park, M.H. Levothyroxine Cessation After Thyroid Lobectomy for Papillary Thyroid Cancer Can Be Achieved at the Same Rate as that for Benign Tumors Regardless of the Duration of Thyroid-stimulating Hormone Suppression. Anticancer Res. 2021, 41, 5713–5721. [Google Scholar] [CrossRef]
- Won, H.R.; Jeon, E.; Chang, J.W.; Kang, Y.E.; Song, K.; Kim, S.W.; Lim, D.M.; Ha, T.K.; Chung, K.W.; Kim, H.J.; et al. Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1470. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Kloos, R.T. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 2001, 86, 1447–1463. [Google Scholar] [CrossRef]
- Conroy, P.C.; Wilhelm, A.; Calthorpe, L.; Ullmann, T.M.; Davis, S.; Huang, C.Y.; Shen, W.T.; Gosnell, J.; Duh, Q.Y.; Roman, S.; et al. Endocrine surgeons are performing more thyroid lobectomies for low-risk differentiated thyroid cancer since the 2015 ATA guidelines. Surgery 2022, 172, 1392–1400. [Google Scholar] [CrossRef]
- Kuo, L.E.; Angell, T.E.; Pandian, T.K.; Moore, A.L.; Alexander, E.K.; Barletta, J.A.; Gawande, A.A.; Lorch, J.H.; Marqusee, E.; Moore, F.D., Jr.; et al. Completion Thyroidectomy is Less Common Following Updated 2015 American Thyroid Association Guidelines. Ann. Surg. Oncol. 2021, 28, 484–491. [Google Scholar] [CrossRef]
- Hartl, D.M.; Guerlain, J.; Breuskin, I.; Hadoux, J.; Baudin, E.; Al Ghuzlan, A.; Terroir-Cassou-Mounat, M.; Lamartina, L.; Leboulleux, S. Thyroid Lobectomy for Low to Intermediate Risk Differentiated Thyroid Cancer. Cancers 2020, 12, 3282. [Google Scholar] [CrossRef]
- Addasi, N.; Fingeret, A.; Goldner, W. Hemithyroidectomy for Thyroid Cancer: A Review. Medicina 2020, 56, 586. [Google Scholar] [CrossRef]
- Sung, T.Y.; Yoon, J.H.; Han, M.; Lee, Y.H.; Lee, Y.M.; Song, D.E.; Chung, K.W.; Kim, W.B.; Shong, Y.K.; Hong, S.J. Oncologic Safety of Robot Thyroid Surgery for Papillary Thyroid Carcinoma: A Comparative Study of Robot versus Open Thyroid Surgery Using Inverse Probability of Treatment Weighting. PLoS ONE 2016, 11, e0157345. [Google Scholar] [CrossRef]
- Tae, K.; Song, C.M.; Ji, Y.B.; Sung, E.S.; Jeong, J.H.; Kim, D.S. Oncologic outcomes of robotic thyroidectomy: 5-year experience with propensity score matching. Surg. Endosc. 2016, 30, 4785–4792. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Pasternak, J.D.; Lim, J.; Kwon, J.S.; Vriens, M.R.; Clark, O.H.; Shen, W.T.; Gosnell, J.E.; Suh, I.; Duh, Q.Y. Frequency of High-Risk Characteristics Requiring Total Thyroidectomy for 1–4 cm Well-Differentiated Thyroid Cancer. Thyroid 2016, 26, 820–824. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Srivastava, A.; Yu, W.; Liu, C.; Wei, D.; Li, Y.; Yang, J. Risk Factors That Influence Surgical Decision-Making for Patients with Low-Risk Differentiated Thyroid Cancer with Tumor Diameters of 1–4 cm. Cancer Manag. Res. 2020, 12, 12423–12428. [Google Scholar] [CrossRef]
- Song, E.; Han, M.; Oh, H.S.; Kim, W.W.; Jeon, M.J.; Lee, Y.M.; Kim, T.Y.; Chung, K.W.; Kim, W.B.; Shong, Y.K.; et al. Lobectomy Is Feasible for 1–4 cm Papillary Thyroid Carcinomas: A 10-Year Propensity Score Matched-Pair Analysis on Recurrence. Thyroid 2019, 29, 64–70. [Google Scholar] [CrossRef]
- Kwon, H.; Lim, W.; Moon, B.I. Number of Tumor Foci as a Risk Factor for Recurrence in Papillary Thyroid Carcinoma: Does It Improve Predictability? Cancers 2022, 14, 4141. [Google Scholar] [CrossRef]
- Yang, Z.; Heng, Y.; Qiu, W.; Tao, L.; Cai, W. Cervical Lymph Node Metastasis Differences in Patients with Unilateral or Bilateral Papillary Thyroid Microcarcinoma: A Multi-Center Analysis. J. Clin. Med. 2022, 11, 4929. [Google Scholar] [CrossRef]
- Woo, J.; Kim, H.; Kwon, H. Impact of Multifocality on the Recurrence of Papillary Thyroid Carcinoma. J. Clin. Med. 2021, 10, 5144. [Google Scholar] [CrossRef]
- Kim, K.; Bae, J.S.; Kim, J.S. Clinical Implication of Mutifocality for Risk of Recurrence in Patients with Papillary Thyroid Carcinoma. J. Endocr. Surg. 2022, 22, 10–17. [Google Scholar] [CrossRef]
- Bouliotis, G.; Billingham, L. Crossing survival curves: Alternatives to the log-rank test. Trials 2011, 12, A137. [Google Scholar] [CrossRef]
| Characteristics | Total n = 2348 |
|---|---|
| Age | |
| Median (range), years | 44 (18–77) |
| Sex | |
| Male | 534 (22.7%) |
| Surgical approach | |
| Conventional | 1088 (46.3%) |
| Minimally invasive | 791 (33.7%) |
| RET | 469 (20.0%) |
| T stage | |
| T1a | 1932 (82.3%) |
| T1b | 144 (6.1%) |
| T2 | 24 (1.0%) |
| T3 | 248 (10.6%) |
| CND | 1428 (60.8%) |
| N stage | |
| N0 | 1048 (44.6%) |
| Nx | 920 (39.2%) |
| N1a | 380 (16.2%) |
| ATA risk | |
| Low-risk | 1789 (76.2%) |
| Intermediate-risk | 559 (23.8%) |
| Follow-up | |
| Mean ± SD, months | 83.9 ± 33.4 |
| Median (range) | 80 (13–178) |
| LT4 | |
| No supplementation | 1084 (46.2%) |
| Supplementation | 1264 (53.8%) |
| Recurrence | |
| Recurrence | 62 (2.6%) |
| Characteristics | Age < 35 n = 487 (20.7%) | Age ≥ 35 n = 1861 (79.3%) | Total n = 2348 | p-Value |
|---|---|---|---|---|
| Age | ||||
| Median (range), years | 31 (18–35) | 46 (36–77) | 44 (18–77) | 0.001 |
| Sex | ||||
| Female | 408 (83.8%) | 1406 (75.6%) | 1814 (77.3%) | 0.001 |
| Male | 79 (16.2%) | 455 (24.4%) | 534 (22.7%) | |
| Surgical approach | ||||
| Conventional | 141 (29.0%) | 947 (50.9%) | 1088 (46.3%) | 0.001 |
| Minimally invasive | 134 (27.5%) | 657 (35.3%) | 791 (33.7%) | |
| RET | 212 (43.5%) | 257 (13.8%) | 469 (20.0%) | |
| Tumor size | ||||
| Mean ± SD, cm | 0.82 ± 0.50 | 0.74 ± 0.40 | 0.75 ± 0.42 | 0.002 |
| Median (range), cm | 0.7 (0.1–4.6) | 0.7 (0.1–6.9) | 0.7 (0.1–6.9) | |
| Tumor Size | ||||
| <7.5 mm | 208 (42.7%) | 910 (48.9%) | 1118 (47.6%) | 0.015 |
| ≥7.5 mm | 279 (57.3%) | 951 (51.1%) | 1230 (52.4%) | |
| ETE | 38 (7.8%) | 210 (11.3%) | 248 (10.6%) | 0.026 |
| Multifocality | 39 (8.0%) | 257 (13.8%) | 296 (12.6%) | 0.001 |
| CND | 310 (63.7%) | 1118 (60.1%) | 1428 (60.8%) | 0.150 |
| N stage | ||||
| N0 | 192 (39.4%) | 856 (46.0%) | 1048 (44.6%) | 0.001 |
| Nx | 177 (36.3%) | 743 (39.9%) | 920 (39.2%) | |
| N1a | 118 (24.2%) | 262 (14.1%) | 380 (16.2%) | |
| LT4 Supplementation | ||||
| LT4 off | 231 (47.4%) | 853 (45.8%) | 1084 (46.2%) | 0.529 |
| LT4 on | 256 (52.6%) | 1008 (54.2%) | 1264 (53.8%) | |
| Follow-up | ||||
| Mean ± SD, months | 83.8 ± 33.5 | 84.0 ± 33.4 | 83.9 ± 33.4 | 0.907 |
| Median (range) | 80 (13–173) | 79 (13–178) | 80 (13–178) | |
| Recurrence | ||||
| Recurrence | 30 (6.2%) | 32 (1.7%) | 62 (2.6%) | 0.001 |
| Covariate | n = 2348 | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age | |||||
| <35 years old | 487 | 3.69 (2.24–6.08) | 0.001 | 3.27 (1.89–5.64) | 0.001 |
| ≥35 years old | 1861 | reference | reference | ||
| Sex | |||||
| Female | 1814 | reference | reference | ||
| Male | 534 | 1.01 (0.55–1.86) | 0.978 | 1.09 (0.75–2.07) | 0.792 |
| Tumor status | |||||
| Larger than 7.5 mm | 1230 | 1.83 (1.09–3.08) | 0.023 | 1.37 (0.79–2.37) | 0.257 |
| ETE | 248 | 1.69 (0.83–3.43) | 0.150 | 1.49 (0.71–3.10) | 0.291 |
| Multifocality | 296 | 1.16 (0.55–2.43) | 0.703 | 1.28 (0.60–2.73) | 0.526 |
| Thyroiditis | 486 | 0.62 (0.31–1.25) | 0.182 | 0.62 (0.30–1.29) | 0.202 |
| Nodal status | |||||
| N0 | 1048 | reference | reference | ||
| Nx | 920 | 1.12 (0.60–2.07) | 0.728 | 1.11 (0.59–2.07) | 0.752 |
| N1a | 380 | 3.38 (1.81–6.29) | 0.001 | 2.45 (1.27–4.73) | 0.008 |
| LT4 Supplementation | |||||
| LT4 off | 1084 | reference | reference | ||
| LT4 on | 1264 | 1.16 (0.69–1.94) | 0.576 | 1.07 (0.62–1.86) | 0.798 |
| Operation types | |||||
| Conventional | 1088 | reference | reference | ||
| Minimally invasive | 791 | 1.35 (0.75–2.43) | 0.320 | 1.45 (0.79–2.66) | 0.233 |
| RET | 469 | 1.73 (0.91–3.28) | 0.095 | 1.31 (0.64–2.66) | 0.464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-S.; Na, Y.-M.; Kim, H.K. Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients. Cancers 2023, 15, 5506. https://doi.org/10.3390/cancers15235506
Cho J-S, Na Y-M, Kim HK. Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients. Cancers. 2023; 15(23):5506. https://doi.org/10.3390/cancers15235506
Chicago/Turabian StyleCho, Jin-Seong, Yong-Min Na, and Hee Kyung Kim. 2023. "Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients" Cancers 15, no. 23: 5506. https://doi.org/10.3390/cancers15235506
APA StyleCho, J.-S., Na, Y.-M., & Kim, H. K. (2023). Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients. Cancers, 15(23), 5506. https://doi.org/10.3390/cancers15235506