Advances in Early Breast Cancer Risk Profiling: From Histopathology to Molecular Technologies
Abstract
:Simple Summary
Abstract
1. Introduction
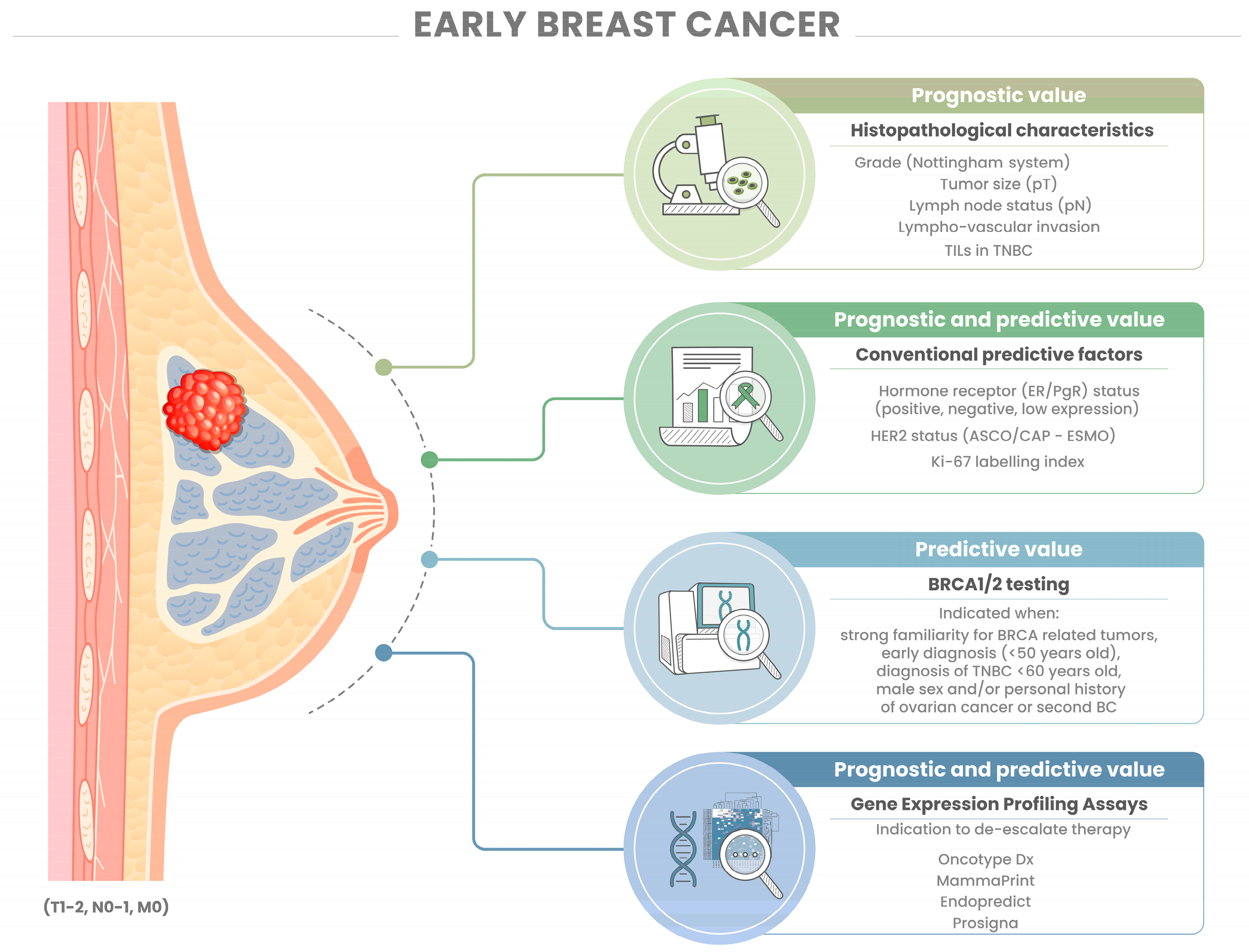
2. Dissecting the Pathology Report
2.1. Histopathology
2.1.1. Tumor Type
2.1.2. Tumor Size
2.1.3. Histological Grade
2.1.4. Lymph Node Status
2.1.5. Lymphovascular Invasion
2.2. Biomarkers
2.2.1. Hormone Receptors
2.2.2. Ki-67 Labeling Index
2.2.3. HER2
2.3. Tumor-Infiltrating Lymphocytes (TILs)
3. Molecular Subtyping and Gene Expression Profiling
3.1. Gene Expression Profiling Assays to Inform Treatment
3.2. BRCA1/2 Mutations
4. Conclusions: Challenges and Future Directions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Thomssen, C.; Balic, M.; Harbeck, N.; St. Gnant, M. Gallen/Vienna 2021: A Brief Summary of the Consensus Discussion on Customizing Therapies for Women with Early Breast Cancer. Breast Care 2021, 16, 135–143. [Google Scholar] [CrossRef]
- Schlam, I.; Tarantino, P.; Morganti, S.; Lynce, F.; Trapani, D.; Mayer, E.L.; Garrido-Castro, A.C.; Waks, A.; Tolaney, S.M. Emerging Targeted Therapies for Early Breast Cancer. Drugs 2022, 82, 1437–1451. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Thomas, A.; Parsons, H.A.; Smith, K.L. Late Recurrence Following Early Breast Cancer. J. Clin. Oncol. 2022, 40, 1400–1406. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.M.; Shao, Z.M.; Fasching, P.A.; Huang, C.S.; Jaliffe, G.G.; Tryakin, A.; Goetz, M.P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef]
- Garutti, M.; Griguolo, G.; Botticelli, A.; Buzzatti, G.; De Angelis, C.; Gerratana, L.; Molinelli, C.; Adamo, V.; Bianchini, G.; Biganzoli, L.; et al. Definition of High-Risk Early Hormone-Positive HER2-Negative Breast Cancer: A Consensus Review. Cancers 2022, 14, 1898. [Google Scholar] [CrossRef]
- Michaels, E.; Worthington, R.O.; Rusiecki, J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. N. Am. 2023, 107, 271–284. [Google Scholar] [CrossRef]
- Brett, J.O.; Mayer, E.L. New Developments in Systemic Management for High-Risk Early-Stage Hormone-Receptor-Positive, HER2-Negative Breast Cancer. Curr. Treat. Options Oncol. 2023, 24, 594–610. [Google Scholar] [CrossRef]
- Wen, H.Y.; Collins, L.C. Breast Cancer Pathology in the Era of Genomics. Hematol. Oncol. Clin. N. Am. 2023, 37, 33–50. [Google Scholar] [CrossRef]
- Pisapia, P.; L’Imperio, V.; Galuppini, F.; Sajjadi, E.; Russo, A.; Cerbelli, B.; Fraggetta, F.; d’Amati, G.; Troncone, G.; Fassan, M.; et al. The evolving landscape of anatomic pathology. Crit. Rev. Oncol. Hematol. 2022, 178, 103776. [Google Scholar] [CrossRef]
- Fusco, N.; Malapelle, U.; Criscitiello, C. Editorial: Diagnosis and Treatment of Breast Cancer in 2022: The Rise of Novel Molecular Biomarkers. Front. Mol. Biosci. 2022, 9, 1117323. [Google Scholar] [CrossRef]
- Hayes, D.F. Precision Medicine and Testing for Tumor Biomarkers—Are All Tests Born Equal? JAMA Oncol. 2018, 4, 773–774. [Google Scholar] [CrossRef]
- Angerilli, V.; Galuppini, F.; Pagni, F.; Fusco, N.; Malapelle, U.; Fassan, M. The Role of the Pathologist in the Next-Generation Era of Tumor Molecular Characterization. Diagnostics 2021, 11, 339. [Google Scholar] [CrossRef]
- Beňačka, R.; Szabóová, D.; Guľašová, Z.; Hertelyová, Z.; Radoňák, J. Classic and New Markers in Diagnostics and Classification of Breast Cancer. Cancers 2022, 14, 5444. [Google Scholar] [CrossRef]
- Curigliano, G.; Dent, R.; Llombart-Cussac, A.; Pegram, M.; Pusztai, L.; Turner, N.; Viale, G. Incorporating clinicopathological and molecular risk prediction tools to improve outcomes in early HR+/HER2- breast cancer. NPJ Breast Cancer 2023, 9, 56. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, Z.; Hu, D.; Luo, H.; Yin, G.; Feng, Y.; Min, Y. Cancer-Specific Survival Outcome in Early-Stage Young Breast Cancer: Evidence From the SEER Database Analysis. Front. Endocrinol. 2021, 12, 811878. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J.; Chen, H.; Li, J.; Zhang, C.; Jiang, X.; Ni, C. The immunomodulatory effects of endocrine therapy in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 19. [Google Scholar] [CrossRef]
- Tarantino, P.; Curigliano, G.; Parsons, H.A.; Lin, N.U.; Krop, I.; Mittendorf, E.A.; Waks, A.; Winer, E.P.; Tolaney, S.M. Aiming at a Tailored Cure for ERBB2-Positive Metastatic Breast Cancer: A Review. JAMA Oncol. 2022, 8, 629–635. [Google Scholar] [CrossRef]
- Ivanova, M.; Porta, F.M.; D’Ercole, M.; Pescia, C.; Sajjadi, E.; Cursano, G.; De Camilli, E.; Pala, O.; Mazzarol, G.; Venetis, K.; et al. Standardized pathology report for HER2 testing in compliance with 2023 ASCO/CAP updates and 2023 ESMO consensus statements on HER2-low breast cancer. Virchows Arch. 2023. [Google Scholar] [CrossRef]
- Russnes, H.G.; Lingjaerde, O.C.; Borresen-Dale, A.L.; Caldas, C. Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol. 2017, 187, 2152–2162. [Google Scholar] [CrossRef]
- Eliyatkın, N.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, G.; Ghidini, M.; Botticelli, A.; Tomasello, G.; Ghidini, A.; Grossi, F.; Fusco, N.; Cabiddu, M.; Savio, T.; Petrelli, F. Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options. Cancer Manag. Res. 2020, 12, 675–686. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, T.P.; Kerin, M.J. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer 2017, 9, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Venetis, K.; Cursano, G.; Pescia, C.; D’Ercole, M.; Porta, F.M.; Blanco, M.C.; Frascarelli, C.; Ivanova, M.; Guerini Rocco, E.; Fusco, N. Liquid biopsy: Cell-free DNA based analysis in breast cancer. J. Liq. Biopsy 2023, 1, 100002. [Google Scholar] [CrossRef]
- Ben-Dror, J.; Shalamov, M.; Sonnenblick, A. The History of Early Breast Cancer Treatment. Genes 2022, 13, 960. [Google Scholar] [CrossRef]
- Kerin, E.P.; Davey, M.G.; McLaughlin, R.P.; Sweeney, K.J.; Barry, M.K.; Malone, C.M.; Elwahab, S.A.; Lowery, A.J.; Kerin, M.J. Comparison of the Nottingham Prognostic Index and OncotypeDX© recurrence score in predicting outcome in estrogen receptor positive breast cancer. Breast 2022, 66, 227–235. [Google Scholar] [CrossRef]
- Hajage, D.; de Rycke, Y.; Bollet, M.; Savignoni, A.; Caly, M.; Pierga, J.Y.; Horlings, H.M.; Van de Vijver, M.J.; Vincent-Salomon, A.; Sigal-Zafrani, B.; et al. External validation of Adjuvant! Online breast cancer prognosis tool. Prioritising recommendations for improvement. PLoS ONE 2011, 6, e27446. [Google Scholar] [CrossRef]
- Jacobson, A.; Macfarlane, C.L.; Pozniak, E. Feasibility of Integrating a Mobile Decision-Support App into a Multicomponent CME Initiative: Developing Clinician Competence at the Point of Care. J. Eur. CME 2020, 9, 1834762. [Google Scholar] [CrossRef]
- Lee, A.H.; Ellis, I.O. The Nottingham prognostic index for invasive carcinoma of the breast. Pathol. Oncol. Res. 2008, 14, 113–115. [Google Scholar] [CrossRef]
- Lambertini, M.; Pinto, A.C.; Ameye, L.; Jongen, L.; Del Mastro, L.; Puglisi, F.; Poggio, F.; Bonotto, M.; Floris, G.; Van Asten, K.; et al. The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br. J. Cancer 2016, 115, 1471–1478. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Breast Tumours; International Agency for Research on Cancer: Lyon, France, 2019.
- Fusco, N.; Sajjadi, E.; Venetis, K.; Ivanova, M.; Andaloro, S.; Guerini-Rocco, E.; Montagna, E.; Caldarella, P.; Veronesi, P.; Colleoni, M.; et al. Low-risk triple-negative breast cancers: Clinico-pathological and molecular features. Crit. Rev. Oncol. Hematol. 2022, 172, 103643. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Quinn, C.M.; Foschini, M.P.; Bianchi, S.; Callagy, G.; Chmielik, E.; Decker, T.; Fend, F.; Kovács, A.; van Diest, P.J.; et al. Triple-Negative Breast Cancer Histological Subtypes with a Favourable Prognosis. Cancers 2021, 13, 5694. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Niu, Y. Triple negative breast cancer: Special histological types and emerging therapeutic methods. Cancer Biol. Med. 2020, 17, 293–306. [Google Scholar] [CrossRef]
- Hamza, A.; Sakhi, R.; Alrajjal, A.; Ibrar, W.; Miller, S.; Salehi, S.; Edens, J.; Ockner, D. Tumor Size in Breast Carcinoma: Gross Measurement Is Important! Int. J. Surg. Pathol. 2018, 26, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.; Ashikaga, T.; Oppenheimer, R.G.; Weaver, D.L. Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Mod. Pathol. 2004, 17, 905–910. [Google Scholar] [CrossRef]
- Choi, B.; Jegatheeswaran, L.; Nakhoul, M.; Haria, P.; Srivastava, R.; Karki, S.; Lupi, M.; Patel, V.; Chakravorty, A.; Babu, E. Axillary staging in ductal carcinoma in situ with microinvasion: A meta-analysis. Surg. Oncol. 2021, 37, 101557. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Eijkelboom, A.H.; de Munck, L.; de Vries, M.; Francken, A.B.; Hendriks, M.P.; Strobbe, L.; Witteveen, A.; van Maaren, M.C.; Siesling, S. Routine and interval detection of locoregional breast cancer recurrences and risk of subsequent distant metastasis. Breast Cancer Res. Treat. 2022, 197, 123–135. [Google Scholar] [CrossRef]
- Caldonazzi, N.; Rizzo, P.C.; Eccher, A.; Girolami, I.; Fanelli, G.N.; Naccarato, A.G.; Bonizzi, G.; Fusco, N.; d’Amati, G.; Scarpa, A.; et al. Value of Artificial Intelligence in Evaluating Lymph Node Metastases. Cancers 2023, 15, 2491. [Google Scholar] [CrossRef]
- Ma, X.; Yang, X.; Yang, W.; Shui, R. Prognostic value of extranodal extension in axillary lymph node-positive breast cancer. Sci. Rep. 2021, 11, 9534. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Corti, C.; Lopez, G.; Michelotti, A.; Despini, L.; Gambini, D.; Lorenzini, D.; Guerini-Rocco, E.; Maggi, S.; Noale, M.; et al. Lymphovascular invasion and extranodal tumour extension are risk indicators of breast cancer related lymphoedema: An observational retrospective study with long-term follow-up. BMC Cancer 2018, 18, 935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Yi, S.; Gong, M.; Lu, C.; Cai, Y.; Tang, X.; Zou, L. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.W.; Gelber, R.; Goldhirsch, A.; Hartmann, W.H.; Hollaway, L.; Russell, I.; Rudenstam, C.M. Prognostic significance of peritumoral vessel invasion in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Hum. Pathol. 1985, 16, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Torous, V.F.; Simpson, R.W.; Balani, J.P.; Baras, A.S.; Berman, M.A.; Birdsong, G.G.; Giannico, G.A.; Paner, G.P.; Pettus, J.R.; Sessions, Z. College of American pathologists cancer protocols: From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. JCO Clin. Cancer Inform. 2021, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Cohen, M.; Classe, J.M.; Reyal, F.; Mazouni, C.; Chopin, N.; Martinez, A.; Daraï, E.; Coutant, C.; Colombo, P.E.; et al. Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study. ESMO Open. 2021, 6, 100316. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Aebi, S.; et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Kuhn, E.; Gambini, D.; Despini, L.; Asnaghi, D.; Runza, L.; Ferrero, S. Updates on Lymphovascular Invasion in Breast Cancer. Biomedicines 2023, 11, 968. [Google Scholar] [CrossRef]
- Fusco, N.; Rizzo, A.; Costarelli, L.; Santinelli, A.; Cerbelli, B.; Scatena, C.; Macri, E.; Pietribiasi, F.; d’Amati, G.; Sapino, A.; et al. Pathological examination of breast cancer samples before and after neoadjuvant therapy: Recommendations from the Italian Group for the Study of Breast Pathology—Italian Society of Pathology (GIPaM-SIAPeC). Pathologica 2022, 114, 104–110. [Google Scholar] [CrossRef]
- Viale, G.; Fusco, N. Pathology after neoadjuvant treatment—How to assess residual disease. Breast 2021, 62, S25–S28. [Google Scholar] [CrossRef]
- Cucciniello, L.; Gerratana, L.; Del Mastro, L.; Puglisi, F. Tailoring adjuvant endocrine therapy in early breast cancer: When, how, and how long? Cancer Treat. Rev. 2022, 110, 102445. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; Hussainzada, A.; Berger, S.; Fietze, E.; Linke, J.; Siedentopf, F.; Schoenegg, W. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast 2016, 29, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Tse, G.M. Immunohistochemical Surrogates for Molecular Classification of Breast Carcinoma: A 2015 Update. Arch. Pathol. Lab. Med. 2016, 140, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris, R.; Davis, A. Optimal adjuvant endocrine therapy for early breast cancer. Womens Health 2010, 6, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Lopez, G.; Costanza, J.; Colleoni, M.; Fontana, L.; Ferrero, S.; Miozzo, M.; Fusco, N. Molecular Insights into the Classification of Luminal Breast Cancers: The Genomic Heterogeneity of Progesterone-Negative Tumors. Int. J. Mol. Sci. 2019, 20, 510. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. S2), S26–S35. [Google Scholar] [CrossRef]
- Fusco, N.; Ragazzi, M.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Morganti, S.; Santandrea, G.; Fanelli, G.N.; Despini, L.; Invernizzi, M.; et al. Assessment of estrogen receptor low positive status in breast cancer: Implications for pathologists and oncologists. Histol. Histopathol. 2021, 36, 1235–1245. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Wu, Y.; Zheng, H.; Gou, Q. Adjuvant endocrine therapy in patients with estrogen receptor-low positive breast cancer: A prospective cohort study. Breast 2022, 66, 89–96. [Google Scholar] [CrossRef]
- Reinert, T.; Cascelli, F.; de Resende, C.A.A.; Gonçalves, A.C.; Godo, V.S.P.; Barrios, C.H. Clinical implication of low estrogen receptor (ER-low) expression in breast cancer. Front. Endocrinol. 2022, 13, 1015388. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Cai, Y.W.; Wu, S.Y.; Shui, R.H.; Shao, Z.M. Estrogen receptor-low breast cancer: Biology chaos and treatment paradox. Cancer Commun. 2021, 41, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Park, M.H.; Choi, J.E.; Kang, S.H.; Bae, Y.K. Characteristics and Prognosis of Estrogen Receptor Low-Positive Breast Cancer. J. Breast Cancer 2022, 25, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. JNCI J. Natl. Cancer Inst. 2020, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Li, F.; Ren, P.; Wu, H.; Zhang, C.; Gu, K. Expression changes of ER, PR, HER2, and Ki-67 in primary and metastatic breast cancer and its clinical significance. Front. Oncol. 2023, 13, 1053125. [Google Scholar] [CrossRef]
- Najjar, S.; Allison, K.H. Updates on breast biomarkers. Virchows Arch. 2022, 480, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef]
- Kreipe, H.; Harbeck, N.; Christgen, M. Clinical validity and clinical utility of Ki67 in early breast cancer. Ther. Adv. Med. Oncol. 2022, 14, 17588359221122725. [Google Scholar] [CrossRef]
- Escala Cornejo, R.A.; Muñoz García, M.; Olivares Hernández, A.; Sancho de Salas, M.; Gómez muñoz, M.A.; Claros Ampuero, J.; Figuero Pérez, L.; Escalera Martín, E.; Barrios Collado, B.; Martín García, G.; et al. 215P Identifying the best Ki67 cut-off for determining luminal breast cancer subtypes using immunohistochemical analysis and PAM50 genomic classification. Ann. Oncol. 2020, 31, S327. [Google Scholar] [CrossRef]
- Lombardi, A.; Lazzeroni, R.; Bersigotti, L.; Vitale, V.; Amanti, C. The Proper Ki-67 Cut-Off in Hormone Responsive Breast Cancer: A Monoinstitutional Analysis with Long-Term Follow-Up. Breast Cancer 2021, 13, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Freedman, R.A.; Somerfield, M.R. Abemaciclib With Endocrine Therapy in the Treatment of High-Risk Early Breast Cancer: ASCO Optimal Adjuvant Chemotherapy and Targeted Therapy Guideline Rapid Recommendation Update. J. Clin. Oncol. 2021, 40, 307–309. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, A.; Friedl, T.W.P.; Hering, E.; Widschwendter, P.; de Gregorio, N.; Bekes, I.; Janni, W.; Dayan, D.; Huober, J.B. Ki67 as Proliferative Marker in Patients with Early Breast Cancer and Its Association with Clinicopathological Factors. Oncology 2021, 99, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, D.; Yu, Y.; Li, J.; Liu, Y. Artificial intelligence-assisted interpretation of Ki-67 expression and repeatability in breast cancer. Diagn. Pathol. 2022, 17, 20. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Pathmanathan, N.; Provan, P.J.; Mahajan, H.; Hall, G.; Byth, K.; Bilous, A.M.; Balleine, R.L. Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing. Breast 2012, 21, 724–729. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Swain, S.M. Pertuzumab: Evolving therapeutic strategies in the management of HER2-overexpressing breast cancer. Expert. Opin. Biol. Ther. 2013, 13, 779–790. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO–College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef]
- Tsang, J.Y.; Tse, G.M. Update on triple-negative breast cancers - highlighting subtyping update and treatment implication. Histopathology 2023, 82, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, E.; Boscolo Bielo, L.; Curigliano, G.; Tarantino, P. The HER2-low revolution in breast oncology: Steps forward and emerging challenges. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152842. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.-Y.; Viret, F.; Levy, C.; Salabert, L.; Du, F.L. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82, PD8-02. [Google Scholar] [CrossRef]
- Pruneri, G.; Vingiani, A.; Denkert, C. Tumor infiltrating lymphocytes in early breast cancer. Breast 2018, 37, 207–214. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Kim, R.S.; Song, N.; Gavin, P.G.; Salgado, R.; Bandos, H.; Kos, Z.; Floris, G.; Eynden, G.G.G.M.V.d.; Badve, S.; Demaria, S.; et al. Stromal Tumor-infiltrating Lymphocytes in NRG Oncology/NSABP B-31 Adjuvant Trial for Early-Stage HER2-Positive Breast Cancer. J. Natl. Cancer Inst. 2019, 111, 867–871. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhang, P.; Xue, S.; Chen, Y.; Sun, L.; Yang, R. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: A meta-analysis. Breast 2022, 66, 97–109. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Zhang, Y.; Liu, J.; Chen, X.; Shen, K. The Value of Tumor Infiltrating Lymphocytes (TILs) for Predicting Response to Neoadjuvant Chemotherapy in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e115103. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Loi, S.; Salgado, R.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 2022, 8, 3. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Ivanova, M.; Noale, M.; Blundo, C.; Di Loreto, E.; Scarfone, G.; Ferrero, S.; Maggi, S.; Veronesi, P.; et al. Immune microenvironment dynamics in breast cancer during pregnancy: Impact of gestational age on tumor-infiltrating lymphocytes and prognosis. Front. Oncol. 2023, 13, 1116569. [Google Scholar] [CrossRef]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.B.; et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- Karn, T.; Denkert, C.; Weber, K.E.; Holtrich, U.; Hanusch, C.; Sinn, B.V.; Higgs, B.W.; Jank, P.; Sinn, H.P.; Huober, J.; et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. 2020, 31, 1216–1222. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Noale, M.; Azim, H.A., Jr.; Blundo, C.; Bonizzi, G.; Di Loreto, E.; Scarfone, G.; Ferrero, S.; Maggi, S.; et al. Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles. Cells 2022, 11, 2286. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; St. Harbeck, N. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Gnant, M.; St. Harbeck, N. Gallen/Vienna 2023: Optimization of Treatment for Patients with Primary Breast Cancer—A Brief Summary of the Consensus Discussion. Breast Care 2023, 18, 213–222. [Google Scholar] [CrossRef]
- Le, H.; Gupta, R.; Hou, L.; Abousamra, S.; Fassler, D.; Torre-Healy, L.; Moffitt, R.A.; Kurc, T.; Samaras, D.; Batiste, R.; et al. Utilizing Automated Breast Cancer Detection to Identify Spatial Distributions of Tumor-Infiltrating Lymphocytes in Invasive Breast Cancer. Am. J. Pathol. 2020, 190, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.M.; Sajjadi, E.; Venetis, K.; Frascarelli, C.; Cursano, G.; Guerini-Rocco, E.; Fusco, N.; Ivanova, M. Immune Biomarkers in Triple-Negative Breast Cancer: Improving the Predictivity of Current Testing Methods. J. Pers. Med. 2023, 13, 1176. [Google Scholar] [CrossRef]
- Oliveira, L.J.C.; Amorim, L.C.; Megid, T.B.C.; de Resende, C.A.A.; Mano, M.S. Gene expression signatures in early breast cancer: Better together with clinicopathological features. Crit. Rev. Oncol. Hematol. 2022, 175, 103708. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Ibrahim, M.; El Nakib, C.; Hajjar, R.; Assi, H.I. Genomic Assays in Node Positive Breast Cancer Patients: A Review. Front. Oncol. 2021, 10, 609100. [Google Scholar] [CrossRef]
- Dias, K.; Dvorkin-Gheva, A.; Hallett, R.M.; Wu, Y.; Hassell, J.; Pond, G.R.; Levine, M.; Whelan, T.; Bane, A.L. Claudin-Low Breast Cancer; Clinical & Pathological Characteristics. PLoS ONE 2017, 12, e0168669. [Google Scholar] [CrossRef]
- Fougner, C.; Bergholtz, H.; Norum, J.H.; Sørlie, T. Re-definition of claudin-low as a breast cancer phenotype. Nat. Commun. 2020, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Che, J.; Hamy, A.S.; Porcher, R.; Barritault, M.; Bouhidel, F.; Habuellelah, H.; Leman-Detours, S.; de Roquancourt, A.; Cahen-Doidy, L.; Bourstyn, E.; et al. Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res. 2013, 15, R37. [Google Scholar] [CrossRef]
- Wan, A.; Zhang, G.; Ma, D.; Zhang, Y.; Qi, X. An overview of the research progress of BRCA gene mutations in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188907. [Google Scholar] [CrossRef]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021, 16, 255–282. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
- Eikesdal, H.P.; Yndestad, S.; Blix, E.S.; Lundgren, S.; Vagstad, G.; Espelid, H.; Gilje, B.; Janssen, E.A.; Geisler, J.; Aas, T.; et al. Neoadjuvant olaparib monotherapy in primary triple negative breast cancer. Ann. Oncol. 2019, 30, v60. [Google Scholar] [CrossRef]
- Spring, L.M.; Han, H.; Liu, M.C.; Hamilton, E.; Irie, H.; Santa-Maria, C.A.; Reeves, J.; Pan, P.; Shan, M.; Tang, Y.; et al. Neoadjuvant study of niraparib in patients with HER2-negative, BRCA-mutated, resectable breast cancer. Nat. Cancer 2022, 3, 927–931. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Pinto, L.; Almeida-Santos, T. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: A systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- t’Kint de Roodenbeke, M.D.; Pondé, N.; Buisseret, L.; Piccart, M. Management of early breast cancer in patients bearing germline BRCA mutations. Semin. Oncol. 2020, 47, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Fogle, R.L.; Gulfidan, G.; Stanley, A.E.; Walter, V.; Hollenbeak, C.S.; Arga, K.Y.; Sinha, R. Potential Early Markers for Breast Cancer: A Proteomic Approach Comparing Saliva and Serum Samples in a Pilot Study. Int. J. Mol. Sci. 2023, 24, 4164. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Khan, M.I.; Kumar, A.; Gupta, A.K.; Rani, K.; Ramesh Parashar, T.; Jayaram, J.; Ranjan Mishra, P.; Srivastava, A.; Mathur, S.; et al. Proteomics of Sentinel Lymph Nodes in Early Breast Cancer for Identification of Thymidylate Synthase as a Potential Biomarker to Flag Metastasis: A Preliminary Study. Cancer Manag. Res. 2020, 12, 4841–4854. [Google Scholar] [CrossRef]
- Asleh, K.; Negri, G.L.; Spencer Miko, S.E.; Colborne, S.; Hughes, C.S.; Wang, X.Q.; Gao, D.; Gilks, C.B.; Chia, S.K.L.; Nielsen, T.O.; et al. Proteomic analysis of archival breast cancer clinical specimens identifies biological subtypes with distinct survival outcomes. Nat. Commun. 2022, 13, 896. [Google Scholar] [CrossRef]
- Da Cunha, P.A.; Nitusca, D.; Canto, L.M.D.; Varghese, R.S.; Ressom, H.W.; Willey, S.; Marian, C.; Haddad, B.R. Metabolomic Analysis of Plasma from Breast Cancer Patients Using Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry: An Untargeted Study. Metabolites 2022, 12, 447. [Google Scholar] [CrossRef]
- Wei, Y.; Jasbi, P.; Shi, X.; Turner, C.; Hrovat, J.; Liu, L.; Rabena, Y.; Porter, P.; Gu, H. Early Breast Cancer Detection Using Untargeted and Targeted Metabolomics. J. Proteome Res. 2021, 20, 3124–3133. [Google Scholar] [CrossRef]
- Eghlimi, R.; Shi, X.; Hrovat, J.; Xi, B.; Gu, H. Triple Negative Breast Cancer Detection Using LC-MS/MS Lipidomic Profiling. J. Proteome Res. 2020, 19, 2367–2378. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Guryleva, M.V.; Stepanova, E.S.; Makarenkova, L.M.; Ptitsyna, E.V.; Goriainov, S.V.; Nikolskaya, A.I.; Astakhova, A.A.; Klimenko, A.S.; Bezborodova, O.A.; et al. Multi-Omics Approach Points to the Importance of Oxylipins Metabolism in Early-Stage Breast Cancer. Cancers 2022, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.-J.; Crispin-Ortuzar, M.; Chin, S.-F.; Provenzano, E.; Bardwell, H.A.; Ma, W.; Cope, W.; Dariush, A.; Dawson, S.-J.; Abraham, J.E.; et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 2022, 601, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Chae, H. moBRCA-net: A breast cancer subtype classification framework based on multi-omics attention neural networks. BMC Bioinform. 2023, 24, 169. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.M.O.; Andrechek, E.R. Molecular Characterization and Landscape of Breast cancer Models from a multi-omics Perspective. J. Mammary Gland. Biol. Neoplasia 2023, 28, 12. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Hinoue, T.; Wheeler, G.L.; Kelly, B.J.; Garrido-Castro, A.C.; Pascual, T.; De Cubas, A.A.; Xia, Y.; Felsheim, B.M.; McClure, M.B.; et al. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat. Cancer 2023, 4, 128–147. [Google Scholar] [CrossRef]
- Sajjadi, E.; Frascarelli, C.; Venetis, K.; Bonizzi, G.; Ivanova, M.; Vago, G.; Guerini-Rocco, E.; Fusco, N. Computational pathology to improve biomarker testing in breast cancer: How close are we? Eur. J. Cancer Prev. 2023, 32, 460–467. [Google Scholar] [CrossRef]
| N | Premenopausal or ≤50 Years Old | Postmenopausal or >50 Years Old |
|---|---|---|
| pN0 | Oncotype Dx (***) | Oncotype Dx (***) MammaPrint (**) EndoPredict (*) Prosigna (*) BCI (*) |
| pN1a-c | Not recommended | Oncotype Dx (***) MammaPrint (**) EndoPredict (*) BCI (*) |
| pN2 | Not recommended | Not recommended |
| Genomic Signatures | Method | LoE | GoR |
|---|---|---|---|
| Oncotype Dx | qRT-PCR | I | A |
| MammaPrint | DNA microarray | I | A |
| Prosigna | nCounter | I | B |
| EndoPredict | qRT-PCR | I | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescia, C.; Guerini-Rocco, E.; Viale, G.; Fusco, N. Advances in Early Breast Cancer Risk Profiling: From Histopathology to Molecular Technologies. Cancers 2023, 15, 5430. https://doi.org/10.3390/cancers15225430
Pescia C, Guerini-Rocco E, Viale G, Fusco N. Advances in Early Breast Cancer Risk Profiling: From Histopathology to Molecular Technologies. Cancers. 2023; 15(22):5430. https://doi.org/10.3390/cancers15225430
Chicago/Turabian StylePescia, Carlo, Elena Guerini-Rocco, Giuseppe Viale, and Nicola Fusco. 2023. "Advances in Early Breast Cancer Risk Profiling: From Histopathology to Molecular Technologies" Cancers 15, no. 22: 5430. https://doi.org/10.3390/cancers15225430
APA StylePescia, C., Guerini-Rocco, E., Viale, G., & Fusco, N. (2023). Advances in Early Breast Cancer Risk Profiling: From Histopathology to Molecular Technologies. Cancers, 15(22), 5430. https://doi.org/10.3390/cancers15225430






