Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Model Development
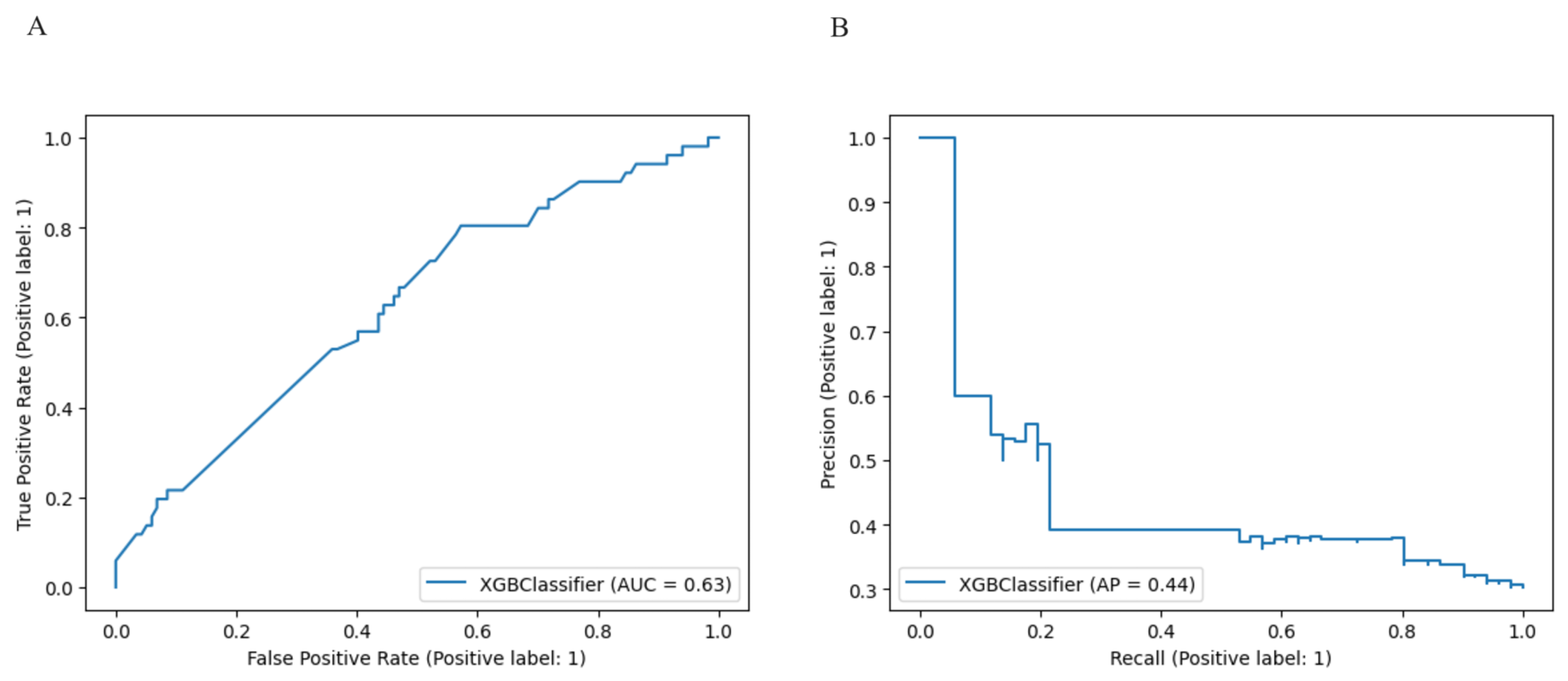
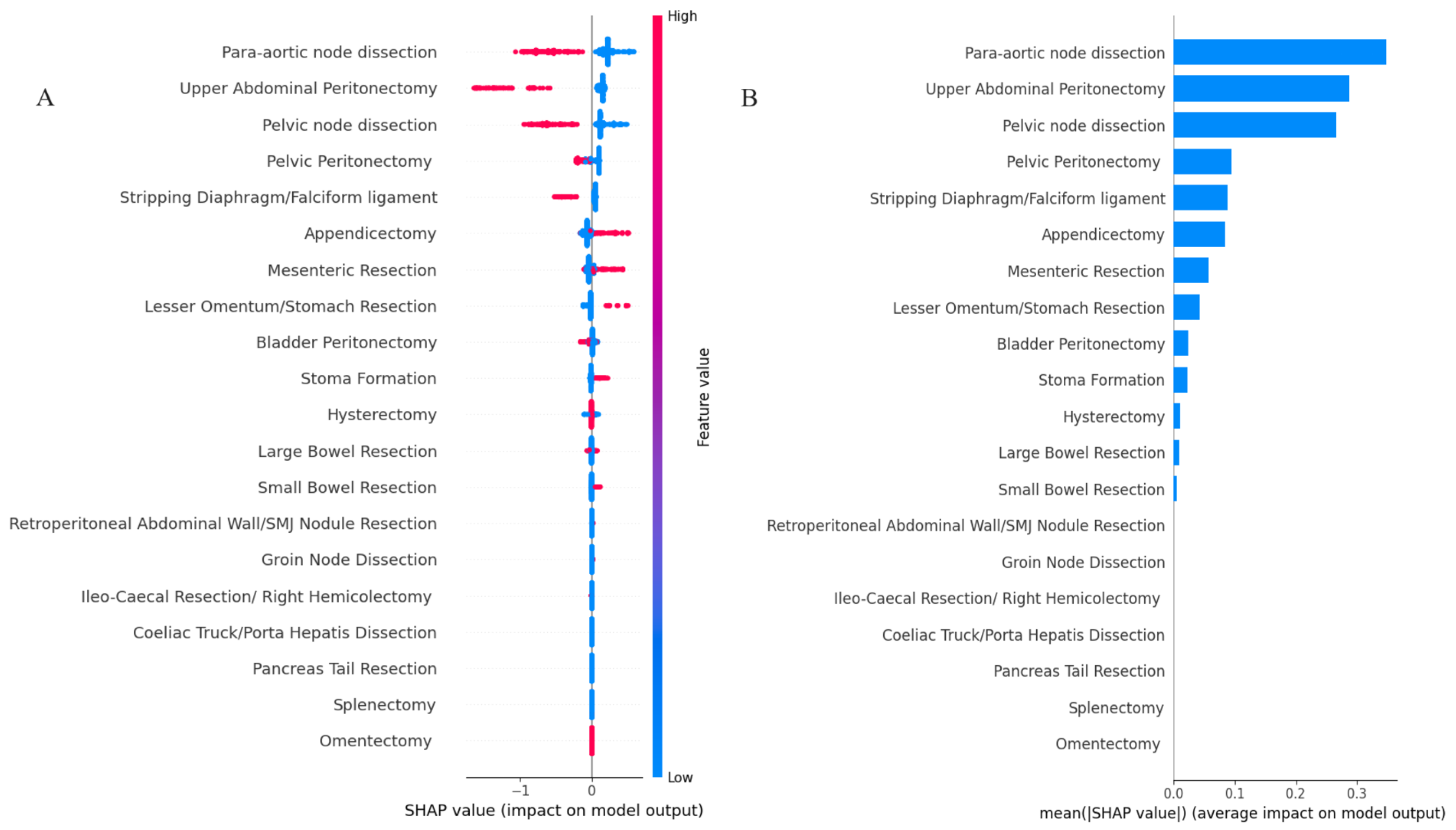
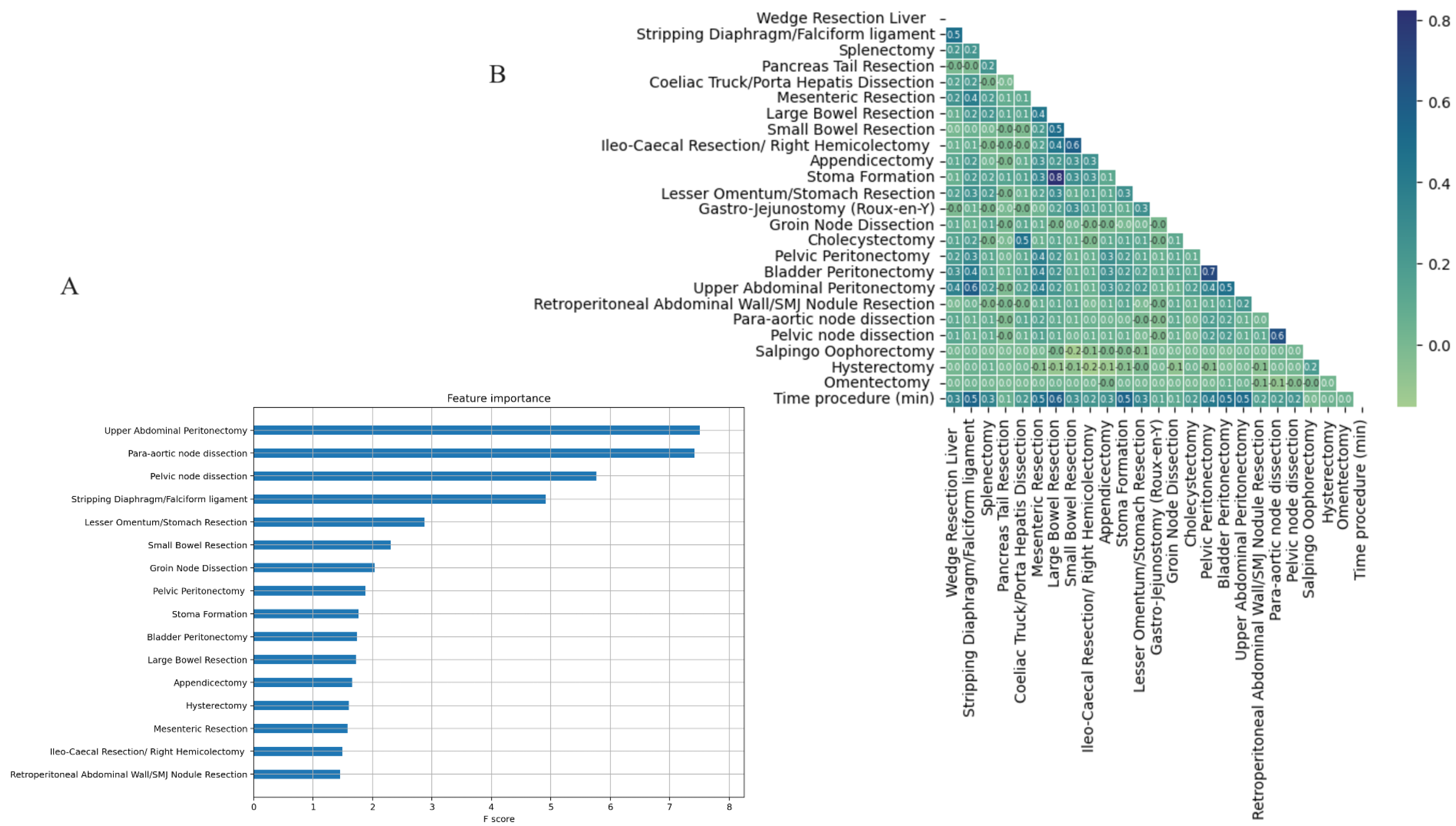
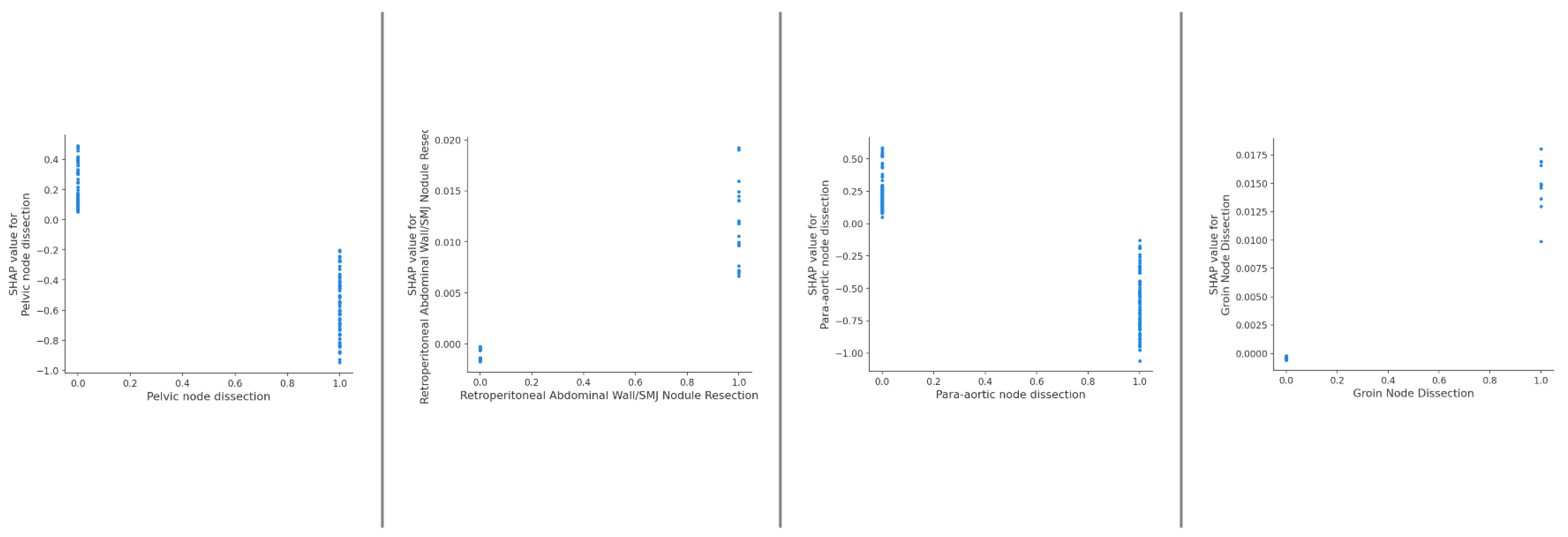
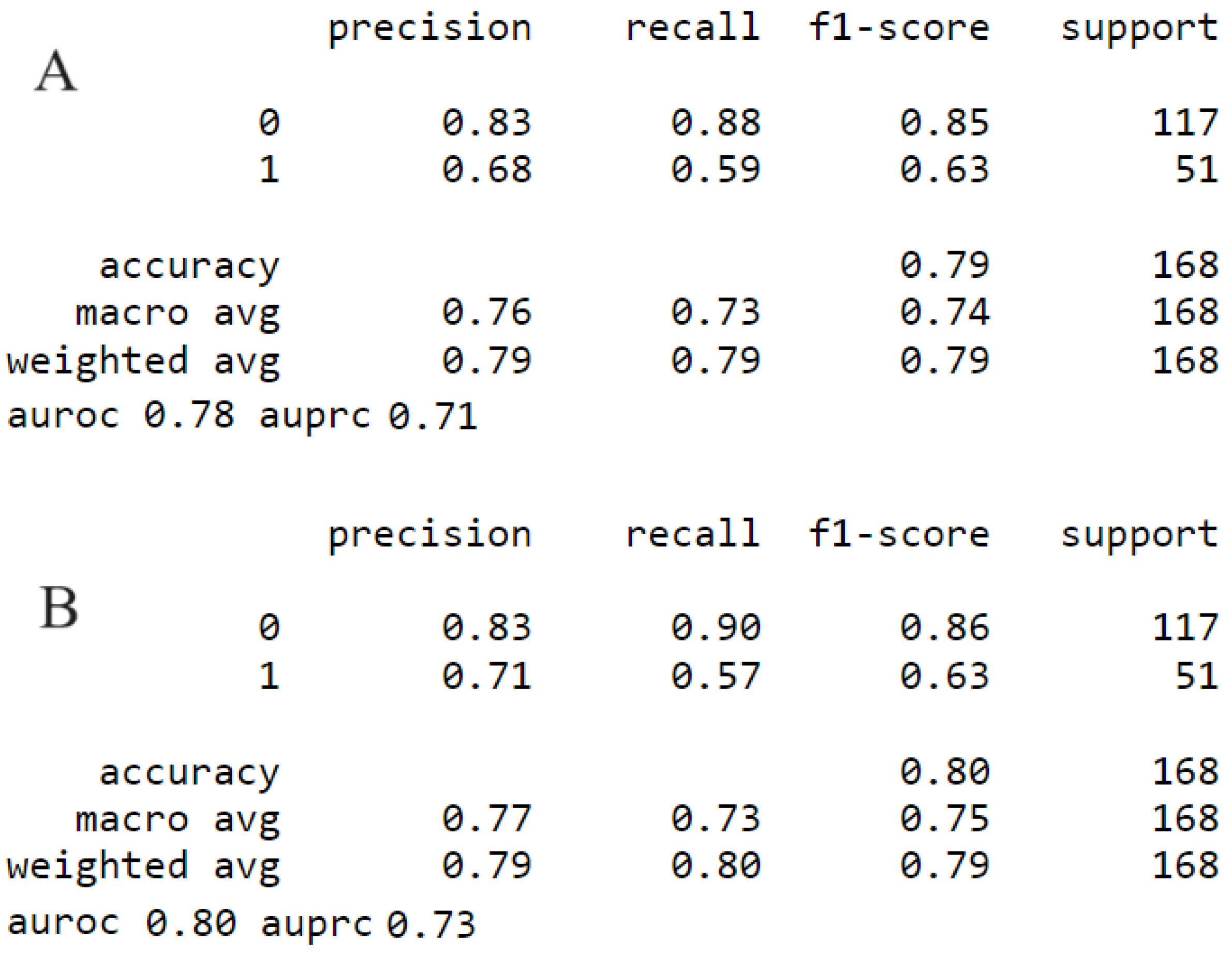
3. Results
3.1. Model Comparison
3.2. Survival Data
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| XAI | Explainable Artificial Intelligence |
| XGBoost | eXreme Gradient Boosting |
| SHAP | Shapley Additive Explanations |
| AUC-ROC | Area under Curve-Receiver Operator Curve |
| EOC | Epithelial Ovarian Cancer |
| FIGO | Federation International of Obstetrics and Gynaecology |
| ML | Machine Learning |
| MDT | Multidisciplinary team |
| ESGO | European Society Gynaecological Oncology |
| CV | Cross Validation |
| IMO | Intra-operative Mapping for Ovarian Cancer |
| PCI | Peritoneal Cancer Index |
| SCS | Surgical Complexity Score |
Appendix A
| Variable | Overall (n = 560) | Training Set (n = 448) | Testing Set (n = 112) | P-Value | No UAP (n = 481) | UAP (n = 79) | P-Value | |
|---|---|---|---|---|---|---|---|---|
| PCI | 7.37 ± 4.47 | 7.48 ± 4.51 | 6.92 ± 4.31 | 0.225 | 6.58 ± 3.87 | 12.18 ± 4.89 | <0.001 | |
| IMO | 4.92 ± 1.97 | 4.98 ± 1.99 | 4.7 ± 1.89 | 0.158 | 4.58 ± 1.81 | 7.0 ± 1.63 | <0.001 | |
| SCS | 3.8 ± 2.11 | 3.82 ± 2.06 | 3.71 ± 2.31 | 0.648 | 3.32 ± 1.56 | 6.72 ± 2.61 | <0.001 | |
| Age | 63.51 ± 11.22 | 63.23 ± 11.06 | 64.64 ± 11.82 | 0.252 | 63.91 ± 10.83 | 61.09 ± 13.15 | 0.074 | |
| EBL | 544.63 ± 623.6 | 555.15 ± 672.24 | 502.54 ± 369.17 | 0.266 | 489.51 ± 337.23 | 880.22 ± 1397.97 | 0.016 | |
| Pre Treatment CA125 | 1516.14 ± 2711.14 | 1582.85 ± 2769.98 | 1249.29 ± 2455.18 | 0.212 | 1534.8 ± 2834.62 | 1402.51 ± 1793.13 | 0.582 | |
| Pre Surgery CA125 | 410.46 ± 1175.43 | 411.43 ± 944.52 | 406.56 ± 1833.3 | 0.978 | 410.49 ± 1238.69 | 410.25 ± 679.33 | 0.998 | |
| Size Largest Bulk of Disease (cm) | 8.89 ± 5.61 | 9.13 ± 5.69 | 7.96 ± 5.23 | 0.039 | 8.57 ± 5.39 | 10.87 ± 6.51 | 0.004 | |
| Time procedure (min) | 170.39 ± 77.55 | 172.98 ± 76.53 | 160.04 ± 81.03 | 0.129 | 154.57 ± 58.7 | 266.71 ± 104.67 | <0.001 | |
| Grade (Low = 0/High = 1) | 0 | 56 (10.0) | 46 (10.27) | 10 (8.93) | 0.805 | 44 (9.15) | 12 (15.19) | 0.145 |
| 1 | 504 (90.0) | 402 (89.73) | 102 (91.07) | 0.805 | 437 (90.85) | 67 (84.81) | 0.145 | |
| FIGO | 0 | 406 (72.5) | 322 (71.88) | 84 (75.0) | 0.586 | 355 (73.8) | 51 (64.56) | 0.116 |
| 1 | 154 (27.5) | 126 (28.12) | 28 (25.0) | 0.586 | 126 (26.2) | 28 (35.44) | 0.116 | |
| PDS = 0/IDS = 1 | 0 | 172 (30.71) | 132 (29.46) | 40 (35.71) | 0.243 | 140 (29.11) | 32 (40.51) | 0.057 |
| 1 | 388 (69.29) | 316 (70.54) | 72 (64.29) | 0.243 | 341 (70.89) | 47 (59.49) | 0.057 |
References
- Du Bois, A.; Quinn, M.; Thigpen, T.; Vermorken, J.; Avall-Lundqvist, E.; Bookman, M.; Bowtell, D.; Brady, M.; Casado, A.; Cervantes, A.; et al. 2004 consensus statements on the management of ovarian cancer: Final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann. Oncol. 2005, 16, viii7–viii12. [Google Scholar] [CrossRef]
- Monk, B.J.; Coleman, R.L.; Fujiwara, K.; Wilson, M.K.; Oza, A.M.; Oaknin, A.; O’Malley, D.M.; Lorusso, D.; Westin, S.N.; Safra, T.; et al. ATHENA (GOG-3020/ENGOT-ov45): A randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA–MONO) and rucaparib in combination with nivolumab (ATHENA–COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, 1589–1594. [Google Scholar]
- Dahm-Kähler, P.; Palmqvist, C.; Staf, C.; Holmberg, E.; Johannesson, L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival-A population-based cohort study. Gynecol. Oncol. 2016, 142, 211–216. [Google Scholar] [CrossRef]
- Palmqvist, C.; Staf, C.; Mateoiu, C.; Johansson, M.; Albertsson, P.; Dahm-Kähler, P. Increased disease-free and relative survival in advanced ovarian cancer after centralized primary treatment. Gynecol. Oncol. 2020, 159, 409–417. [Google Scholar] [CrossRef]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; et al. European Society of Gynaecologic Oncology quality indicators for advanced ovarian cancer surgery. Int. J. Gynecol. Cancer 2016, 26, 1354–1363. [Google Scholar] [CrossRef]
- Préfontaine, M.; Gelfand, A.T.; Donovan, J.T.; Powell, J.L. Reproducibility of tumor measurements in ovarian cancer: A study of interobserver variability. Gynecol. Oncol. 1994, 55, 87–90. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Concin, N.; Planchamp, F.; Morice, P.; Vergote, I.; du Bois, A.; Querleu, D. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. Int. J. Gynecol. Cancer 2020, 30, 436–440. [Google Scholar] [CrossRef]
- Kawakami, E.; Tabata, J.; Yanaihara, N.; Ishikawa, T.; Koseki, K.; Iida, Y.; Saito, M.; Komazaki, H.; Shapiro, J.S.; Goto, C.; et al. Application of artificial intelligence for preoperative diagnostic and prognostic prediction in epithelial ovarian cancer based on blood biomarkers. Clin. Cancer Res. 2019, 25, 3006–3015. [Google Scholar] [CrossRef]
- Laios, A.; Gryparis, A.; DeJong, D.; Hutson, R.; Theophilou, G.; Leach, C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J. Ovarian Res. 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Thangavelu, A.; Tarabanis, C.; Nugent, D.; De Jong, D. Explainable artificial intelligence for prediction of complete surgical cytoreduction in advanced-stage epithelial ovarian cancer. J. Pers. Med. 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Nugent, D.; De Jong, D. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers 2023, 15, 966. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Perit. Carcinomatosis Princ. Manag. 1996, 82, 359–374. [Google Scholar]
- Sehouli, J.; Könsgen, D.; Mustea, A.; Oskay-Özcelik, G.; Katsares, I.; Weidemann, H.; Lichtenegger, W. “IMO”-Intraoperatives Mapping des Ovarialkarzinoms. Zentralblatt für Gynäkologie 2003, 125, 129–135. [Google Scholar]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Dottino, J.A.; He, W.; Sun, C.C.; Zhao, H.; Fu, S.; Rauh-Hain, J.A.; Suidan, R.S.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. National trends in bowel and upper abdominal procedures in ovarian cancer surgery. Int. J. Gynecol. Cancer 2020, 30, 1195–1202. [Google Scholar] [CrossRef]
- Slack, D.; Hilgard, S.; Jia, E.; Singh, S.; Lakkaraju, H. Fooling lime and shap: Adversarial attacks on post hoc explanation methods. In Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society, New York, NY, USA, 7–9 February 2020; pp. 180–186. [Google Scholar]
- NICE. Overview: Maximal Cytoreductive Surgery for Advanced Ovarian Cancer: Guidance; NICE: London, UK, 2023. [Google Scholar]
- Stepanyan, A.; Malakyan, Z.; Alaverdyan, A.; Davtyan, H.; Hovhannisyan, T. Right upper quadrant peritonectomy. Answering frequently asked questions. Int. J. Gynecol. Cancer 2021, 31, 1305–1306. [Google Scholar] [CrossRef]
- Zivanovic, O.; Eisenhauer, E.L.; Zhou, Q.; Iasonos, A.; Sabbatini, P.; Sonoda, Y.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2008, 108, 287–292. [Google Scholar] [CrossRef]
- Kehoe, S.M.; Eisenhauer, E.L.; Chi, D.S. Upper abdominal surgical procedures: Liver mobilization and diaphragm peritonectomy/resection, splenectomy, and distal pancreatectomy. Gynecol. Oncol. 2008, 111, S51–S55. [Google Scholar] [CrossRef]
- Bogani, G.; Ditto, A.; Martinelli, F.; Lorusso, D.; Chiappa, V.; Donfrancesco, C.; Di Donato, V.; Indini, A.; Aletti, G.; Raspagliesi, F. Surgical techniques for diaphragmatic resection during cytoreduction in advanced or recurrent ovarian carcinoma: A systematic Review and meta-analysis. Int. J. Gynecol. Cancer 2016, 26, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29. [Google Scholar] [CrossRef] [PubMed]
- Lago, V.; Domingo, S.; Matute, L.; Padilla-Iserte, P.; Gurrea, M. Radical en Bloc Peritonectomy in Advanced Ovarian Cancer. Ecancer 2018, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Taskiran, C. The principles of safe and efficacious upper abdominal surgery. Gynecol. Pelvic. Med. 2021, 4. [Google Scholar] [CrossRef]
- Spirtos, N.M.; Gross, G.M.; Freddo, J.L.; Ballon, S.C. Cytoreductive surgery in advanced epithelial cancer of the ovary: The impact of aortic and pelvic lymphadenectomy. Gynecol. Oncol. 1995, 56, 345–352. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- Straubhar, A.M.; Filippova, O.T.; Cowan, R.A.; Lakhman, Y.; Sarasohn, D.M.; Nikolovski, I.; Torrisi, J.M.; Ma, W.; Abu-Rustum, N.R.; Gardner, G.J.; et al. A multimodality triage algorithm to improve cytoreductive outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer: A Memorial Sloan Kettering Cancer Center team ovary initiative. Gynecol. Oncol. 2020, 158, 608–613. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, R.; Yin, S.; You, C.; Liu, D.; Cheng, X.; Tang, J.; Zang, R. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: An observational study. BMC Cancer 2015, 15, 1–12. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Spruance, S.L.; Reid, J.E.; Grace, M.; Samore, M. Hazard ratio in clinical trials. Antimicrob. Agents Chemother. 2004, 48, 2787–2792. [Google Scholar] [CrossRef]
- Laios, A.; De Jong, D.; Kalampokis, E. Beauty is in the explainable artificial intelligence (XAI) of the “agnostic” beholder. Transl. Cancer Res. 2023, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, CD005343. [Google Scholar] [PubMed]
- Chang, S.J.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J. Gynecol. Oncol. 2015, 26, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Pannu, H.K.; Bristow, R.E.; Montz, F.J.; Fishman, E.K. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 2003, 23, 687–701. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Shah, M.Y.; Shaikh, S.; Prabhu, A.; Mishra, S.; et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur. J. Surg. Oncol. 2020, 46, 582–589. [Google Scholar] [CrossRef] [PubMed]


| Variable | Overall (n = 560) | Training Set (n = 448) | Testing Set (n = 112) | P-Value | No UAP (n = 481) | UAP (n = 79) | P-Value | |
|---|---|---|---|---|---|---|---|---|
| Wedge Resection Liver | 0 | 537 (95.89) | 428 (95.54) | 109 (97.32) | 0.558 | 475 (98.75) | 62 (78.48) | <0.001 |
| 1 | 23 (4.11) | 20 (4.46) | 3 (2.68) | 0.558 | 6 (1.25) | 17 (21.52) | <0.001 | |
| Stripping Diaphragm/Falciform ligament | 0 | 484 (86.43) | 385 (85.94) | 99 (88.39) | 0.6 | 455 (94.59) | 29 (36.71) | <0.001 |
| 1 | 76 (13.57) | 63 (14.06) | 13 (11.61) | 0.6 | 26 (5.41) | 50 (63.29) | <0.001 | |
| Splenectomy | 0 | 543 (96.96) | 435 (97.1) | 108 (96.43) | 0.951 | 474 (98.54) | 69 (87.34) | <0.001 |
| 1 | 17 (3.04) | 13 (2.9) | 4 (3.57) | 0.951 | 7 (1.46) | 10 (12.66) | <0.001 | |
| Pancreas Tail Resection | 0 | 559 (99.82) | 447 (99.78) | 112 (100.0) | 1.0 | 480 (99.79) | 79 (100.0) | 1 |
| 1 | 1 (0.18) | 1 (0.22) | 0 (0.0) | 1.0 | 1 (0.21) | 0 (0.0) | 1 | |
| Coeliac Truck/Porta Hepatis Dissection | 0 | 554 (98.93) | 443 (98.88) | 111 (99.11) | 1.0 | 479 (99.58) | 75 (94.94) | 0.002 |
| 1 | 6 (1.07) | 5 (1.12) | 1 (0.89) | 1.0 | 2 (0.42) | 4 (5.06) | 0.002 | |
| Mesenteric Resection | 0 | 427 (76.25) | 340 (75.89) | 87 (77.68) | 0.785 | 399 (82.95) | 28 (35.44) | <0.001 |
| 1 | 133 (23.75) | 108 (24.11) | 25 (22.32) | 0.785 | 82 (17.05) | 51 (64.56) | <0.001 | |
| Large Bowel Resection | 0 | 496 (88.57) | 399 (89.06) | 97 (86.61) | 0.572 | 440 (91.48) | 56 (70.89) | <0.001 |
| 1 | 64 (11.43) | 49 (10.94) | 15 (13.39) | 0.572 | 41 (8.52) | 23 (29.11) | <0.001 | |
| Small Bowel Resection | 0 | 537 (95.89) | 430 (95.98) | 107 (95.54) | 1.0 | 464 (96.47) | 73 (92.41) | 0.168 |
| 1 | 23 (4.11) | 18 (4.02) | 5 (4.46) | 1.0 | 17 (3.53) | 6 (7.59) | 0.168 | |
| Ileo-Caecal Resection/Right Hemicolectomy | 0 | 539 (96.25) | 432 (96.43) | 107 (95.54) | 0.868 | 465 (96.67) | 74 (93.67) | 0.326 |
| 1 | 21 (3.75) | 16 (3.57) | 5 (4.46) | 0.868 | 16 (3.33) | 5 (6.33) | 0.326 | |
| Appendicectomy | 0 | 439 (78.39) | 352 (78.57) | 87 (77.68) | 0.939 | 398 (82.74) | 41 (51.9) | <0.001 |
| 1 | 121 (21.61) | 96 (21.43) | 25 (22.32) | 0.939 | 83 (17.26) | 38 (48.1) | <0.001 | |
| Stoma Formation | 0 | 509 (90.89) | 407 (90.85) | 102 (91.07) | 1.0 | 449 (93.35) | 60 (75.95) | <0.001 |
| 1 | 51 (9.11) | 41 (9.15) | 10 (8.93) | 1.0 | 32 (6.65) | 19 (24.05) | <0.001 | |
| Lesser Omentum/Stomach Resection | 0 | 534 (95.36) | 427 (95.31) | 107 (95.54) | 1.0 | 468 (97.3) | 66 (83.54) | <0.001 |
| 1 | 26 (4.64) | 21 (4.69) | 5 (4.46) | 1.0 | 13 (2.7) | 13 (16.46) | <0.001 | |
| Gastro-Jejunostomy (Roux-en-Y) | 0 | 558 (99.64) | 447 (99.78) | 111 (99.11) | 0.859 | 480 (99.79) | 78 (98.73) | 0.658 |
| 1 | 2 (0.36) | 1 (0.22) | 1 (0.89) | 0.859 | 1 (0.21) | 1 (1.27) | 0.658 | |
| Groin Node Dissection | 0 | 549 (98.04) | 440 (98.21) | 109 (97.32) | 0.819 | 473 (98.34) | 76 (96.2) | 0.407 |
| 1 | 11 (1.96) | 8 (1.79) | 3 (2.68) | 0.819 | 8 (1.66) | 3 (3.8) | 0.407 | |
| Cholecystectomy | 0 | 553 (98.75) | 442 (98.66) | 111 (99.11) | 1.0 | 479 (99.58) | 74 (93.67) | <0.001 |
| 1 | 7 (1.25) | 6 (1.34) | 1 (0.89) | 1.0 | 2 (0.42) | 5 (6.33) | <0.001 | |
| Pelvic Peritonectomy | 0 | 277 (49.46) | 216 (48.21) | 61 (54.46) | 0.281 | 273 (56.76) | 4 (5.06) | <0.001 |
| 1 | 283 (50.54) | 232 (51.79) | 51 (45.54) | 0.281 | 208 (43.24) | 75 (94.94) | <0.001 | |
| Bladder Peritonectomy | 0 | 358 (63.93) | 284 (63.39) | 74 (66.07) | 0.676 | 351 (72.97) | 7 (8.86) | <0.001 |
| 1 | 202 (36.07) | 164 (36.61) | 38 (33.93) | 0.676 | 130 (27.03) | 72 (91.14) | <0.001 | |
| Upper Abdominal Peritonectomy | 0 | 481 (85.89) | 383 (85.49) | 98 (87.5) | 0.693 | 481 (100.0) | 0 (0.0) | <0.001 |
| 1 | 79 (14.11) | 65 (14.51) | 14 (12.5) | 0.693 | 0 (0.0) | 79 (100.0) | <0.001 | |
| Retroperitoneal Abdominal Wall/SMJ Nodule Resection | 0 | 537 (95.89) | 432 (96.43) | 105 (93.75) | 0.312 | 470 (97.71) | 67 (84.81) | <0.001 |
| 1 | 23 (4.11) | 16 (3.57) | 7 (6.25) | 0.312 | 11 (2.29) | 12 (15.19) | <0.001 | |
| Para-aortic node dissection | 0 | 381 (68.04) | 303 (67.63) | 78 (69.64) | 0.768 | 333 (69.23) | 48 (60.76) | 0.172 |
| 1 | 179 (31.96) | 145 (32.37) | 34 (30.36) | 0.768 | 148 (30.77) | 31 (39.24) | 0.172 | |
| Pelvic node dissection | 0 | 414 (73.93) | 335 (74.78) | 79 (70.54) | 0.427 | 363 (75.47) | 51 (64.56) | 0.056 |
| 1 | 146 (26.07) | 113 (25.22) | 33 (29.46) | 0.427 | 118 (24.53) | 28 (35.44) | 0.056 | |
| Salpingo Oophorectomy | 0 | 6 (1.07) | 3 (0.67) | 3 (2.68) | 0.182 | 6 (1.25) | 0 (0.0) | 0.683 |
| 1 | 554 (98.93) | 445 (99.33) | 109 (97.32) | 0.182 | 475 (98.75) | 79 (100.0) | 0.683 | |
| Hysterectomy | 0 | 56 (10.0) | 41 (9.15) | 15 (13.39) | 0.245 | 49 (10.19) | 7 (8.86) | 0.871 |
| 1 | 504 (90.0) | 407 (90.85) | 97 (86.61) | 0.245 | 432 (89.81) | 72 (91.14) | 0.871 | |
| Omentectomy | 0 | 7 (1.25) | 4 (0.89) | 3 (2.68) | 0.296 | 7 (1.46) | 0 (0.0) | 0.594 |
| 1 | 553 (98.75) | 444 (99.11) | 109 (97.32) | 0.296 | 474 (98.54) | 79 (100.0) | 0.594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laios, A.; Kalampokis, E.; Mamalis, M.E.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Nugent, D.; De Jong, D. Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence. Cancers 2023, 15, 5386. https://doi.org/10.3390/cancers15225386
Laios A, Kalampokis E, Mamalis ME, Thangavelu A, Hutson R, Broadhead T, Nugent D, De Jong D. Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence. Cancers. 2023; 15(22):5386. https://doi.org/10.3390/cancers15225386
Chicago/Turabian StyleLaios, Alexandros, Evangelos Kalampokis, Marios Evangelos Mamalis, Amudha Thangavelu, Richard Hutson, Tim Broadhead, David Nugent, and Diederick De Jong. 2023. "Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence" Cancers 15, no. 22: 5386. https://doi.org/10.3390/cancers15225386
APA StyleLaios, A., Kalampokis, E., Mamalis, M. E., Thangavelu, A., Hutson, R., Broadhead, T., Nugent, D., & De Jong, D. (2023). Exploring the Potential Role of Upper Abdominal Peritonectomy in Advanced Ovarian Cancer Cytoreductive Surgery Using Explainable Artificial Intelligence. Cancers, 15(22), 5386. https://doi.org/10.3390/cancers15225386







