The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Oncological Assessment
2.3. Data Collection
2.4. Nutritional Assessment by GNRI
2.5. Ethics Approval
2.6. Statistics
3. Results
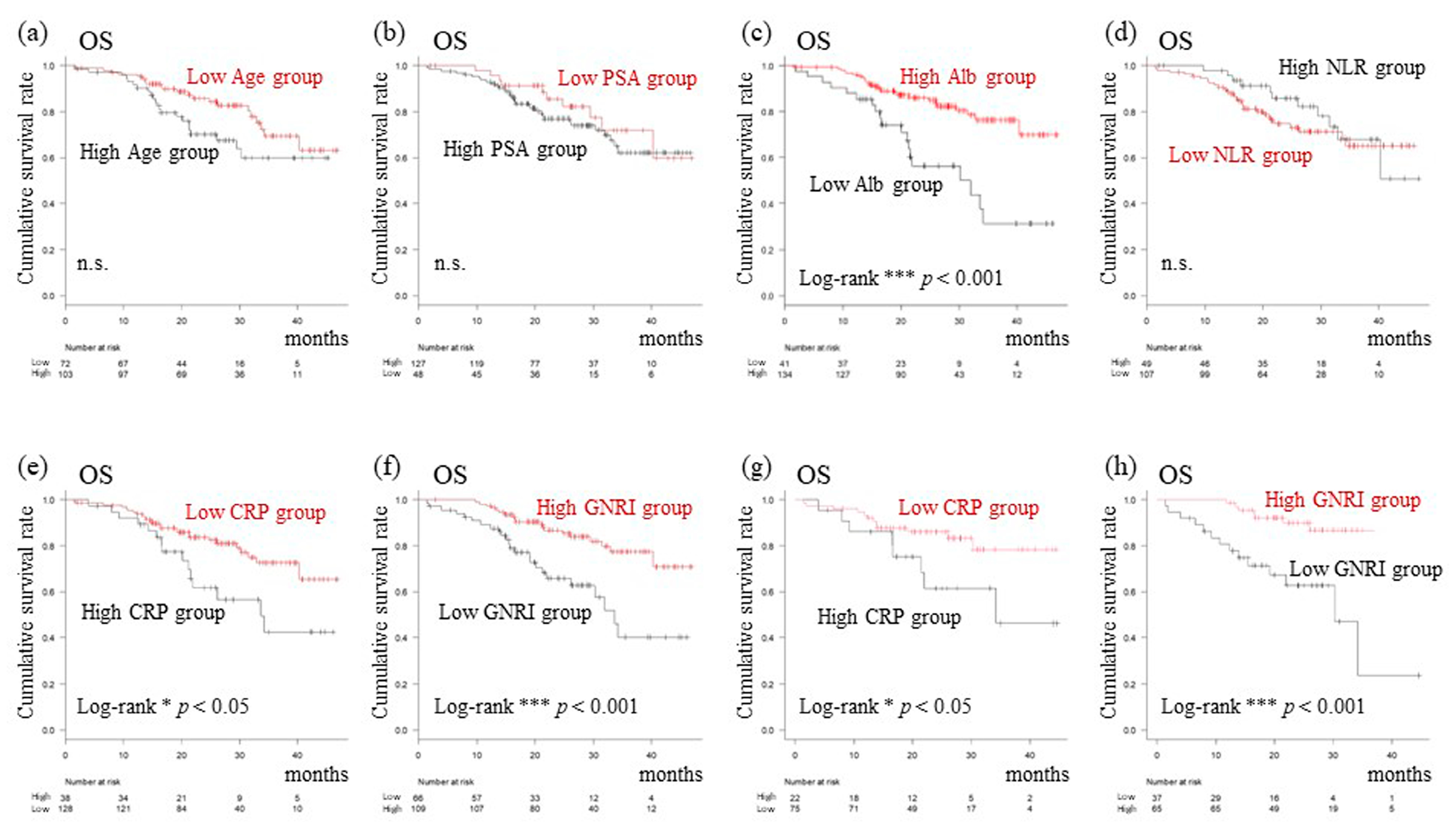
3.1. Characteristics and Outcomes of Patients by Further Follow-Up Study
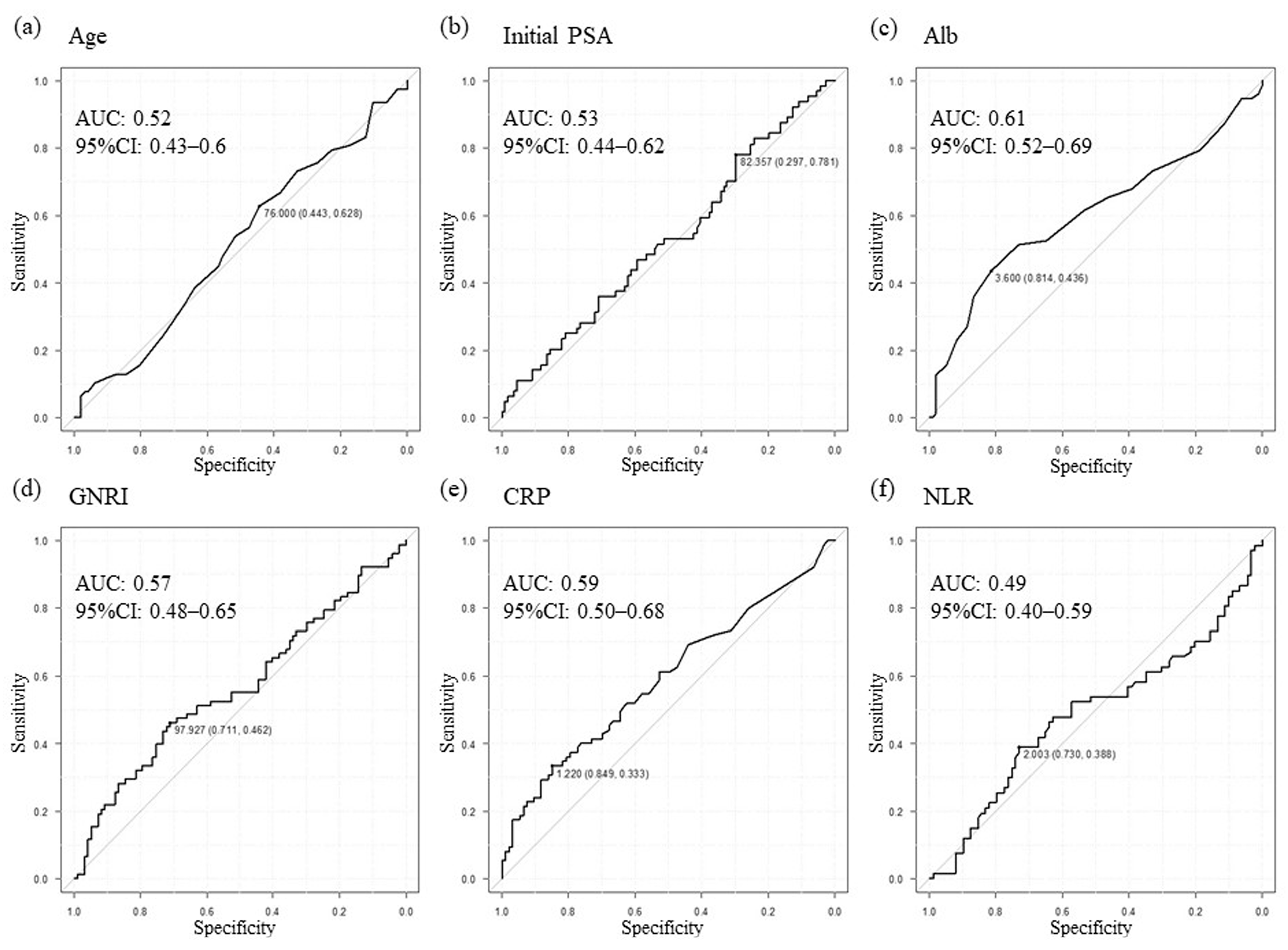
3.2. Setting New Cutoff Values and Prognostic Analysis Focusing on Serum Biomarkers
3.3. Identification of Prognostic Factors for TTCR and OS
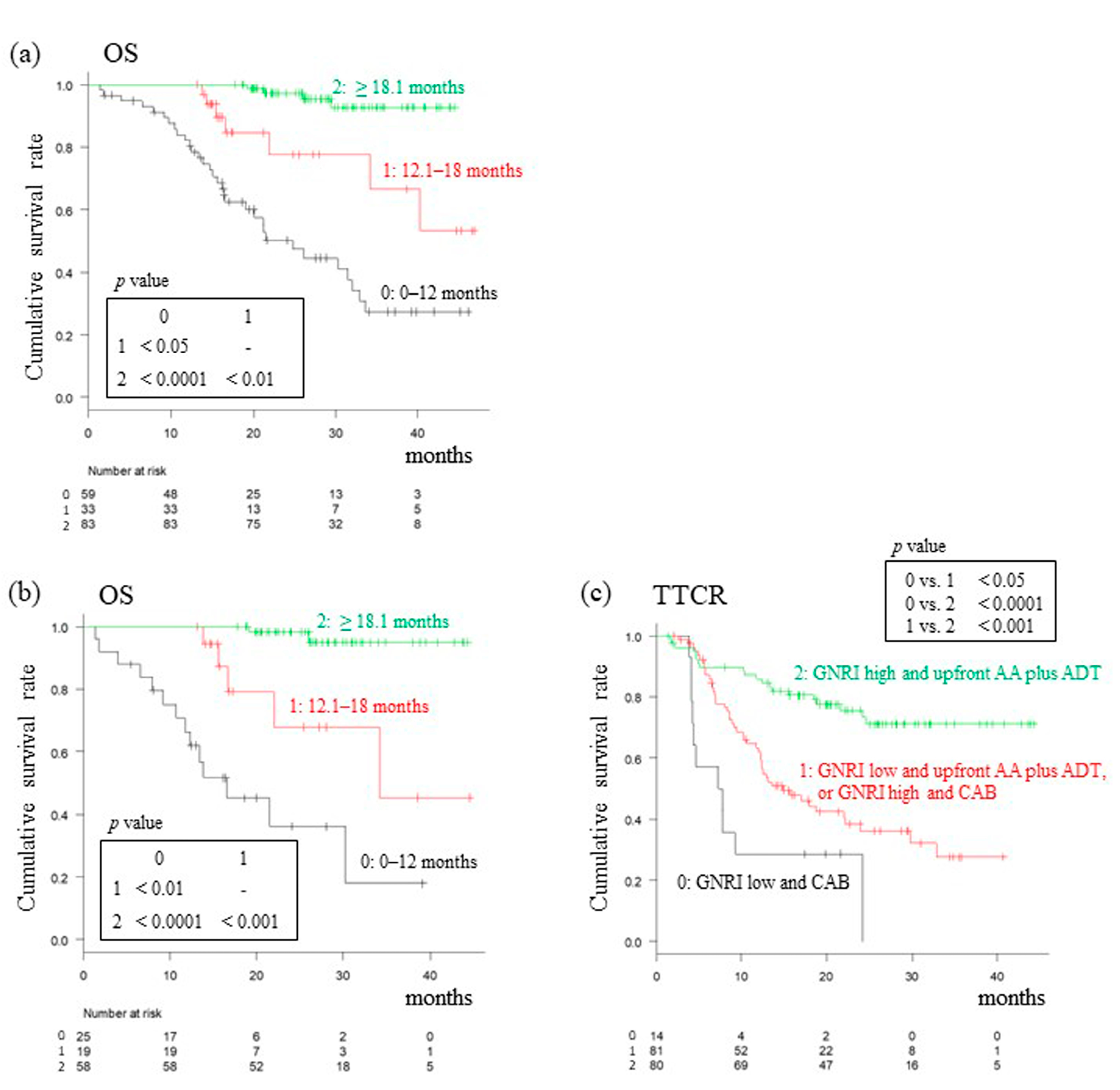
3.4. Analysis of OS Based on TTCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Protheroe, A.; Rodriguez-Antolin, A.; Facchini, G.; Suttman, H.; Matsubara, N.; Ye, Z.; Keam, B.; Damião, R.; Li, T.; et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.d.S.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Paller, C.J.; Hong, H.; De Felice, A.; Alexander, G.C.; Brawley, O. Comparison of systemic treatments for metastatic castration-sensitive prostate cancer: A systematic review and network meta-analysis. JAMA Oncol. 2021, 7, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN guideline insights: Prostate cancer, version 1.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Hinotsu, S.; Namiki, M.; Ito, K.; Broering, J.; Carroll, P.R.; Akaza, H. Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J. Clin. Oncol. 2009, 27, 4306–4313. [Google Scholar] [CrossRef]
- Naiki, T.; Takahara, K.; Ito, T.; Nakane, K.; Sugiyama, Y.; Koie, T.; Shiroki, R.; Miyake, H.; Yasui, T. Comparison of clinical outcomes between androgen deprivation therapy with up-front abiraterone and bicalutamide for Japanese patients with LATITUDE high-risk prostate cancer in a real-world retrospective analysis. Int. J. Clin. Oncol. 2022, 27, 592–601. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Kimura, T.; Mori, K.; Suzuki, H.; Sano, T.; Otsuka, T.; Iwamoto, Y.; Fukuokaya, W.; Miyajima, K.; Enei, Y.; et al. Abiraterone acetate versus nonsteroidal antiandrogen with androgen deprivation therapy for high-risk metastatic hormone-sensitive prostate cancer. Prostate 2022, 82, 3–12. [Google Scholar] [CrossRef]
- Ueda, T.; Shiraishi, T.; Ito, S.; Ohashi, M.; Matsugasumi, T.; Yamada, Y.; Fujihara, A.; Hongo, F.; Okihara, K.; Ukimura, O. Abiraterone acetate versus bicalutamide in combination with gonadotropin releasing hormone antagonist therapy for high risk metastatic hormone sensitive prostate cancer. Sci. Rep. 2021, 11, 10094. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Fujita, K.; Nishimoto, M.; Yamamoto, Y.; Kuwahara, K.; Nagai, Y.; Minami, T.; Hatanaka, Y.; Nozawa, M.; Morimoto, Y.; et al. Comparison of abiraterone and combined androgen blockade therapy for high-risk metastatic hormone-sensitive prostate cancer: A propensity score-matched analysis. Front. Oncol. 2021, 11, 769068. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Kimura, T.; Hatakeyama, S.; Hata, K.; Yanagisawa, T.; Maita, S.; Chiba, S.; Sato, H.; Kashima, S.; Koizumi, A.; et al. Real-world survival outcomes of adding docetaxel or abiraterone in patients with high-volume metastatic castration-sensitive prostate cancer: Historically controlled, propensity score matched comparison with androgen deprivation therapy. World J. Urol. 2022, 40, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hatakeyama, S.; Noro, D.; Togashi, K.; Yoneyama, T.; Ohyama, C. Effect of upfront combination therapy on the overall survival of patients with metastatic castration-sensitive prostate cancer: A multicenter retrospective study. Int. J. Urol. 2022, 29, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.; Williams, R.; Garmo, H.; Holmberg, L.; Stattin, P.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; Robinsson, D.; et al. Serum inflammatory markers in relation to prostate cancer severity and death in the Swedish AMORIS study. Int. J. Cancer 2018, 142, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Luo, X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: A meta-analysis. Cancer Cell Int. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Isobe, T.; Naiki, T.; Sugiyama, Y.; Naiki-Ito, A.; Nagai, T.; Etani, T.; Nozaki, S.; Iida, K.; Noda, Y.; Shimizu, N.; et al. Chronological transition in outcome of second-line treatment in patients with metastatic urothelial cancer after pembrolizumab approval: A multicenter retrospective analysis. Int. J. Clin. Oncol. 2022, 27, 165–174. [Google Scholar] [CrossRef]
- Miyake, H.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A. Prognostic significance of time to castration resistance in patients with metastatic castration-sensitive prostate cancer. Anticancer Res. 2019, 39, 1391–1396. [Google Scholar] [CrossRef]
- Xie, H.; Ruan, G.; Zhang, H.; Zhang, Q.; Ge, Y.; Song, M.; Zhang, X.; Lin, S.; Liu, X.; Liu, Y.; et al. Association of modified geriatric nutrition risk index and handgrip strength with survival in cancer: A multi-center cohort study. Front. Nutr. 2022, 9, 850138. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, Y.; Gao, Z. Pretreatment c-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: A meta-analysis. Medicine 2020, 99, e20595. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.; Ji, R.; Tan, H.; Tian, S.; Yin, J.; Xu, J.; Chen, X.; Liu, W.; Cui, H. Preoperative sarcopenia combined with prognostic nutritional index predicts long-term prognosis of radical gastrectomy with advanced gastric cancer: A comprehensive analysis of two-center study. BMC Cancer 2023, 23, 751. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Preisser, F.; Hoeh, B.; Schroeder, M.; Würnschimmel, C.; Steuber, T.; Heinzer, H.; Banek, S.; Ahrens, M.; Becker, A.; et al. Impact of time to castration resistance on survival in metastatic hormone sensitive prostate cancer patients in the era of combination therapies. Front. Oncol. 2021, 11, 659135. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Gentilucci, A.; Salciccia, S.; Pierella, F.; Del Bianco, F.; Gentile, V.; Silvestri, I.; Cattarino, S. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: A critical review. J. Inflamm. 2016, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Shayegan, B.; Morgan, S.C.; Hamilton, R.J.; Cagiannos, I.; Basappa, N.S.; Ferrario, C.; Gotto, G.T.; Fernandes, R.; Roy, S.; et al. Prognostic association between common laboratory tests and overall survival in elderly men with de novo metastatic castration sensitive prostate cancer: A population-based study in Canada. Cancers 2021, 13, 2844. [Google Scholar] [CrossRef] [PubMed]
- McArdle, P.A.; Mir, K.; Almushatat, A.S.K.; Wallace, A.M.; Underwood, M.A.; McMillan, D. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol. Int. 2006, 77, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lan, J.; Xiong, J.; Yang, H.; Xu, X.; Tang, C.; Huang, G.; Ying, Q.; Mu, J.; Hu, Q. Efficiency of C-reactive protein in prognosis evaluation of prostate cancer: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 4432–4439. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.Y.; An, L.; Sun, D.W. Geriatric Nutritional Risk Index predicts adverse outcomes in human malignancy: A meta-analysis. Dis. Markers 2019, 2019, 4796598. [Google Scholar] [CrossRef]
- Lee, G.W.; Go, S.I.; Kim, D.W.; Kim, H.; Kim, J.; An, H.J.; Jang, J.S.; Kim, B.; Hahn, S.; Heo, D.S. Geriatric Nutritional Risk Index as a prognostic marker in patients with extensive-stage disease small cell lung cancer: Results from a randomized controlled trial. Thorac. Cancer 2020, 11, 62–71. [Google Scholar] [CrossRef]
- Hu, S.P.; Chen, L.; Lin, C.Y.; Lin, W.H.; Fang, F.Q.; Tu, M.Y. The prognostic value of preoperative geriatric nutritional risk index in patients with pancreatic ductal adenocarcinoma. Cancer Manag. Res. 2020, 12, 385–395. [Google Scholar] [CrossRef]
- Lidoriki, I.; Schizas, D.; Frountzas, M.; Machairas, N.; Prodromidou, A.; Kapelouzou, A.; Karavokyros, I.; Pikoulis, E.; Kales, S.N.; Liakakos, T. GNRI as a prognostic factor for outcomes in cancer patients: A systematic review of the literature. Nutr. Cancer 2021, 73, 391–403. [Google Scholar] [CrossRef]
- Okamoto, T.; Hatakeyama, S.; Narita, S.; Takahashi, M.; Sakurai, T.; Kawamura, S.; Hoshi, S.; Ishida, M.; Kawaguchi, T.; Ishidoya, S.; et al. Impact of nutritional status on the prognosis of patients with metastatic hormone-naive prostate cancer: A multicenter retrospective cohort study in Japan. World J. Urol. 2019, 37, 1827–1835. [Google Scholar] [CrossRef]
| Characteristics | Low GNRI Group (n = 66) | High GNRI Group (n = 109) | p Value | |
|---|---|---|---|---|
| Median age (range), years | 77 (55–91) | 72 (48–89) | <0.05 * | |
| Median initial serum PSA levels (range), ng/mL | 465.7 (4.7–16720) | 189.6 (0.76–13433) | <0.01 ** | |
| Gleason score at initial diagnosis, n (%) | 4 + 3 | 1 (1.5) | 1 (0.9) | 0.05 |
| 4 + 4 | 26 (39.4) | 41 (37.6) | ||
| 4 + 5 | 15 (22.7) | 42 (38.5) | ||
| 5 + 4 | 13 (19.7) | 19 (17.4) | ||
| 5 + 5 | 11 (16.7) | 6 (5.5) | ||
| Clinical T stage | 2a | 0 (0) | 4 (3.7) | 0.35 |
| 2b | 3 (4.5) | 5 (4.6) | ||
| 2c | 8 (12.1) | 8 (7.3) | ||
| 3a | 12 (18.2) | 28 (25.7) | ||
| 3b | 19 (28.8) | 35 (32.1) | ||
| 4 | 24 (36.4) | 29 (26.6) | ||
| Clinical N stage | N0 | 32 (48.5) | 55 (50.5) | 0.88 |
| N1 | 34 (51.5) | 54 (49.5) | ||
| Visceral metastasis, n (%) | no | 45 (68.2) | 72 (66.1) | 0.87 |
| yes | 21 (31.8) | 37 (33.9) | ||
| ECOG–PS, n (%) | 0 | 36 (54.5) | 91 (83.5) | <0.001 *** |
| 1 | 20 (30.3) | 10 (9.2) | ||
| 2 | 7 (10.6) | 8 (7.3) | ||
| 3 | 3 (4.5) | 0 (0) | ||
| Existence of symptoms, n (%) | 44 (66.7) | 58 (53.2) | 0.09 | |
| Median BMI (range), kg/m2 | 20.4 (14.2–25.6) | 23.7 (17.3–34.1) | NA | |
| The use of bone modifying agents, n (%) | 43 (65.2) | 59 (54.1) | 0.16 | |
| Median serum albumin level (range), g/dL | 3.5 (1.5–4.3) | 4.2 (2.8–6.6) | NA | |
| Median serum ALP level (range), U/L | 626 (136–11620) | 333 (81–8300) | <0.01 ** | |
| Agents, combined with ADT | Bicalutamide | 29 (43.9) | 44 (40.4) | 0.75 |
| Abiraterone | 37 (56.1) | 65 (59.6) | ||
| PSA decline > 50%, n (%) | 56 (84.8) | 93 (85.3) | 1 | |
| Median period to PSA nadir (range), days | 143 (27–770) | 330 (21–1215) | <0.001 *** | |
| CRPC development, n (%) | 36 (54.5) | 42 (38.5) | <0.05 * | |
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Ages, <76 vs. ≥76 years | 1.14 | 0.72–1.80 | 0.58 | 1.36 | 0.79–2.32 | 0.27 |
| Initial PSA levels, <82.4 vs. ≥82.4 ng/mL | 0.62 | 0.36–1.06 | 0.08 | 0.92 | 0.49–1.72 | 0.80- |
| ECOG–PS, 1, 2, 3 vs. 0 | 1.53 | 0.94–2.48 | 0.08 | 1.06 | 0.59–1.91 | 0.84 |
| Existence of liver metastasis, yes vs. no | 1.66 | 0.41–6.78 | 0.48 | 3.18 | 0.72–14.1 | 0.13 |
| Existence of symptoms, yes vs. no | 1.87 | 1.16–3.03 | < 0.05 * | 1.61 | 0.90–2.88 | 0.11 |
| Agent at the start of treatment, AA vs. Bica | 0.38 | 0.24–0.60 | <0.0001 **** | 0.39 | 0.23–0.66 | <0.001 *** |
| NLR, >2.003 vs. ≤2.003 | 0.79 | 0.48–1.30 | 0.36 | 0.46 | 0.25–0.84 | <0.05 * |
| Serum CRP levels, <1.22 vs. ≥1.22 mg/dL | 0.45 | 0.28–0.74 | <0.01 ** | 0.65 | 0.37–1.12 | 0.12 |
| GNRI, ≥98 vs. <98 | 0.44 | 0.28–0.69 | <0.001 *** | 0.38 | 0.21–0.67 | <0.001 *** |
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Ages, <76 vs. ≥76 years | 0.57 | 0.31–1.04 | 0.07 | 0.67 | 0.34–1.30 | 0.23 |
| Initial PSA levels, <82.4 vs. ≥82.4 ng/mL | 0.74 | 0.36–1.50 | 0.40 | 1.47 | 0.65–3.31 | 0.35 |
| ECOG–PS, 1, 2, 3 vs. 0 | 2.21 | 1.20–4.05 | <0.05 * | 1.82 | 0.90–3.71 | 0.10 |
| Existence of liver metastasis, yes vs. no | 1.56 | 0.21–11.4 | 0.66 | 1.27 | 0.15–10.50 | 0.83 |
| Existence of symptoms, yes vs. no | 1.47 | 0.78–2.79 | 0.23 | 1.07 | 0.52–2.22 | 0.86 |
| Agent at the start of treatment, AA vs. Bica | 0.83 | 0.45–1.51 | 0.53 | 1.07 | 0.54–2.10 | 0.85 |
| NLR, >2.003 vs. ≤2.003 | 1.31 | 0.65–2.63 | 0.45 | 0.72 | 0.32–1.62 | 0.42 |
| Serum CRP levels, <1.22 vs. ≥1.22, mg/dL | 0.46 | 0.24–0.86 | <0.05 * | 0.53 | 0.23–1.22 | 0.14 |
| GNRI, ≥98 vs. <98 | 0.35 | 0.19–0.64 | <0.001 *** | 0.45 | 0.21–0.96 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naiki, T.; Takahara, K.; Watanabe, H.; Nakane, K.; Sugiyama, Y.; Koie, T.; Shiroki, R.; Miyake, H.; Yasui, T. The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study. Cancers 2023, 15, 5333. https://doi.org/10.3390/cancers15225333
Naiki T, Takahara K, Watanabe H, Nakane K, Sugiyama Y, Koie T, Shiroki R, Miyake H, Yasui T. The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study. Cancers. 2023; 15(22):5333. https://doi.org/10.3390/cancers15225333
Chicago/Turabian StyleNaiki, Taku, Kiyoshi Takahara, Hiromitsu Watanabe, Keita Nakane, Yosuke Sugiyama, Takuya Koie, Ryoichi Shiroki, Hideaki Miyake, and Takahiro Yasui. 2023. "The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study" Cancers 15, no. 22: 5333. https://doi.org/10.3390/cancers15225333
APA StyleNaiki, T., Takahara, K., Watanabe, H., Nakane, K., Sugiyama, Y., Koie, T., Shiroki, R., Miyake, H., & Yasui, T. (2023). The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study. Cancers, 15(22), 5333. https://doi.org/10.3390/cancers15225333








