Construction of a Nomogram to Predict Overall Survival in Patients with Early-Onset Hepatocellular Carcinoma: A Retrospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Cohort Selection
2.2. Variables Management
2.3. Establishment and Validation of the Nomogram Model
2.4. Comparison of Nomogram-Based Risk Classification and the AJCC TNM Staging System
2.5. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Univariate and Multivariate Analyses
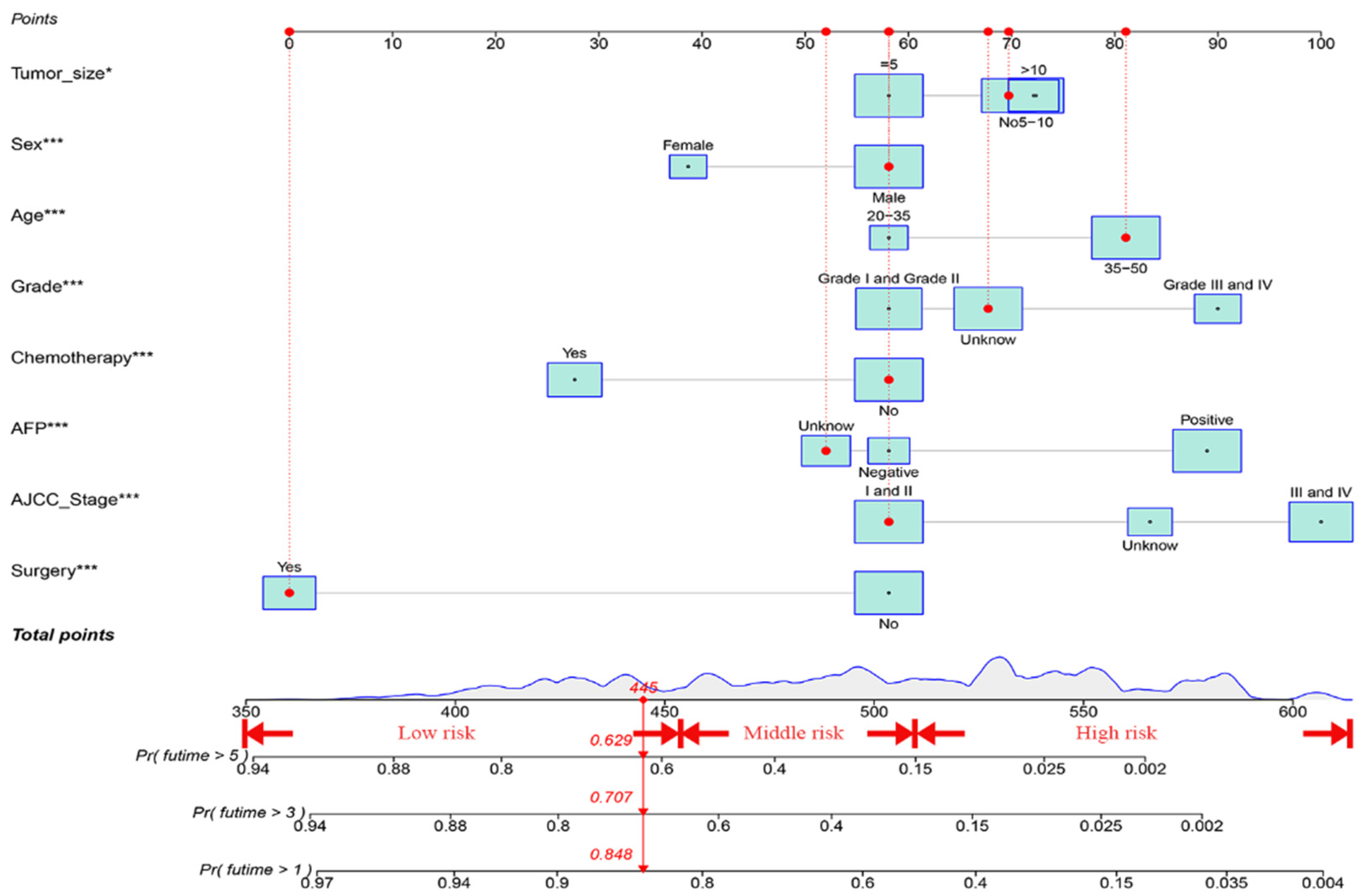
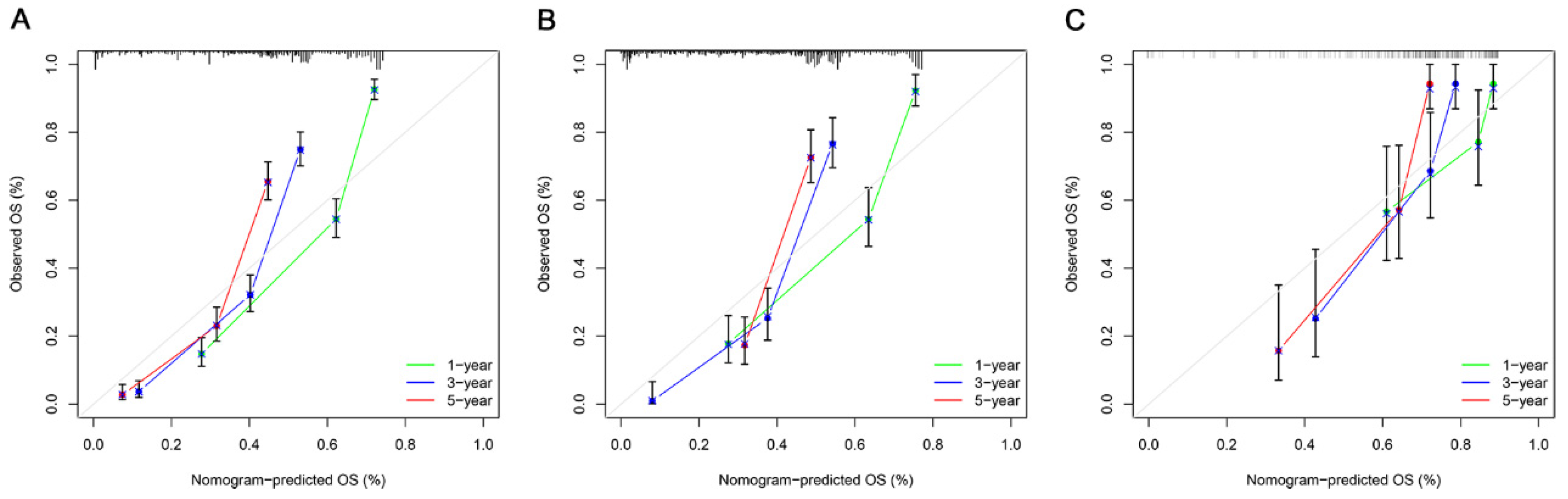
3.3. Construction and Verification of the OS Prognostic Nomogram
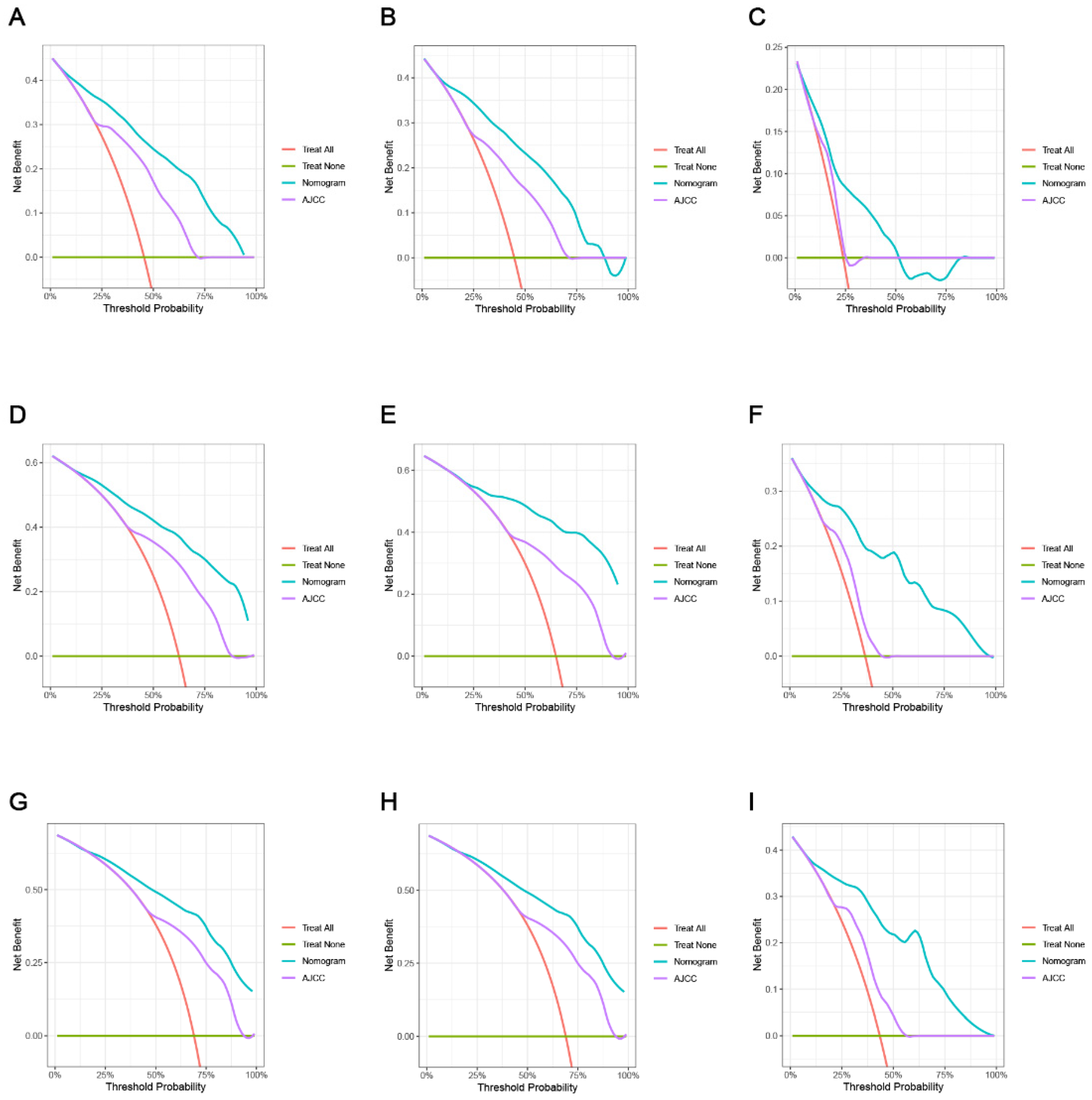
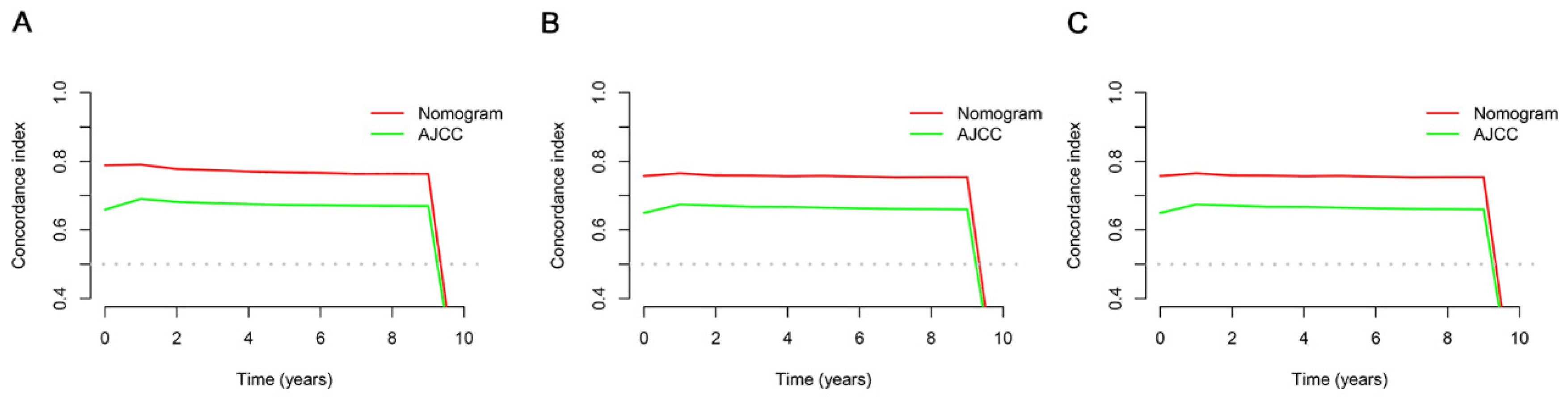
3.4. Comparing the Clinical Applicability of the New Nomogram with the AJCC TNM Staging System
3.5. Establishment of a Risk Stratification System According to the Nomogram
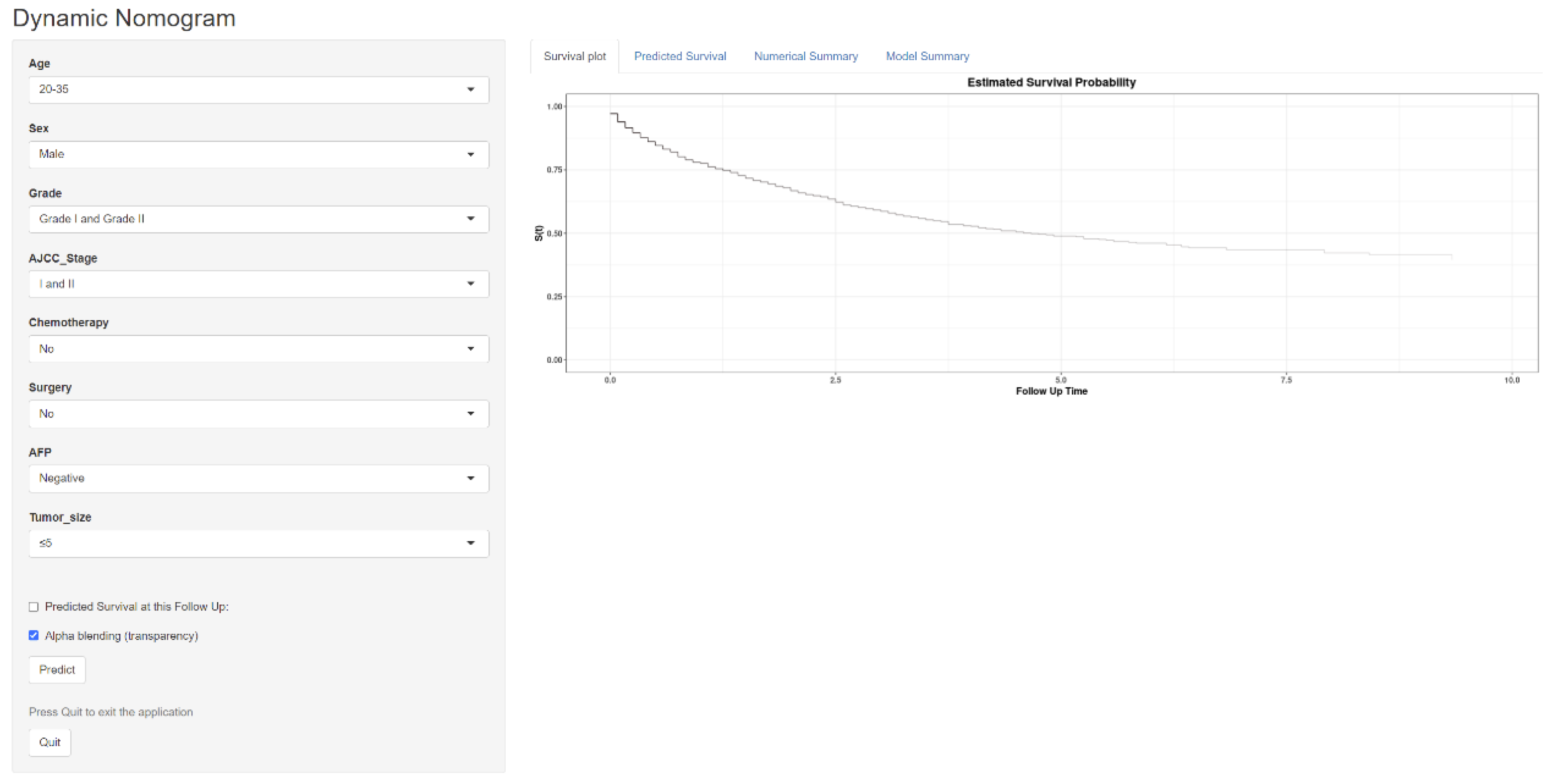
3.6. Constructing a Web-Based Survival Calculator
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public. Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Soerjomataram, I.; Lin, C.C.; Coebergh, J.W.W.; Jemal, A.U.S. Burden of Cancer by Race and Ethnicity According to Disability-Adjusted Life Years. Am. J. Prev. Med. 2016, 51, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.J.; Yabroff, K.R.; Dahman, B.; Feuer, E.J.; Mariotto, A.; Brown, M.L. Productivity costs of cancer mortality in the United States: 2000–2020. J. Natl. Cancer Inst. 2008, 100, 1763–1770. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e471. [Google Scholar] [CrossRef]
- Lam, C.M.; Chan, A.O.; Ho, P.; Ng, I.O.; Lo, C.M.; Liu, C.L.; Poon, R.T.; Fan, S.T. Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients—Implications for screening. Aliment. Pharmacol. Ther. 2004, 19, 771–777. [Google Scholar] [CrossRef]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Zhao, Y.N.; Zhang, T.; Chen, Z.Y.; Ma, X.L. Comprehensive radiomics nomogram for predicting survival of patients with combined hepatocellular carcinoma and cholangiocarcinoma. World J. Gastroenterol. 2021, 27, 7173–7189. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Li, J.; Tian, S.; Dong, W. Personalizing prognostic prediction in early-onset Colorectal Cancer. J. Cancer 2020, 11, 6727–6736. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.; Agha, R.; Albrecht, J.; Goel, P.; Mukherjee, I.; Pai, P.; D’Cruz, A.K.; Nixon, I.J.; Roberto, K.; Enam, S.A.; et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021, 96, 106165. [Google Scholar] [CrossRef]
- Goodrich, J.A.; Walker, D.; Lin, X.; Wang, H.; Lim, T.; McConnell, R.; Conti, D.V.; Chatzi, L.; Setiawan, V.W. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022, 4, 100550. [Google Scholar] [CrossRef]
- Yan, B.; Su, B.B.; Bai, D.S.; Qian, J.J.; Zhang, C.; Jin, S.J.; Jiang, G.Q. A practical nomogram and risk stratification system predicting the cancer-specific survival for patients with early hepatocellular carcinoma. Cancer Med. 2021, 10, 496–506. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, M.; Xiong, X.; Su, Y.; Zhao, F.; Hu, Y.; Zhang, G.; Pei, J.; Ding, Y. Clinical features and prognostic factors in patients with microvascular infiltration of hepatocellular carcinoma: Development and validation of a nomogram and risk stratification based on the SEER database. Front. Oncol. 2022, 12, 987603. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Hoofnagle, J.H. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer 1989, 64, 2117–2120. [Google Scholar] [CrossRef]
- Yamada, R.; Hiramatsu, N.; Oze, T.; Morishita, N.; Harada, N.; Yakushijin, T.; Iio, S.; Doi, Y.; Yamada, A.; Kaneko, A.; et al. Impact of alpha-fetoprotein on hepatocellular carcinoma development during entecavir treatment of chronic hepatitis B virus infection. J. Gastroenterol. 2015, 50, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, M.; He, Z.; Zhang, L.; Li, H.; Lin, J.; He, Y.; Dai, S.; Hu, W.; Liu, W. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int. J. Biol. Sci. 2019, 15, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Wang, J.J.; Luu, M.; Noureddin, M.; Nissen, N.N.; Patel, T.C.; Roberts, L.R.; Singal, A.G.; Gores, G.J.; Yang, J.D. Comparison of Clinical Features and Outcomes Between Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma in the United States. Hepatology 2021, 74, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Chen, T.; Liu, X.; Zhang, B.; Yue, S.; Cao, J.; Zhang, G. A Web-Based Prediction Model for Cancer-Specific Survival of Elderly Patients With Early Hepatocellular Carcinoma: A Study Based on SEER Database. Front. Public. Health 2021, 9, 789026. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cai, J.; Chen, H.; Yao, J.; Xiao, C.; Li, R.; Xiao, J.; Zhang, J.; Sui, X.; Lu, T.; et al. Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: A retrospective analysis of the SEER database. Front. Oncol. 2023, 13, 1098686. [Google Scholar] [CrossRef]
- Xie, Q.; Yang, Y.; Qu, B.; Xiao, P.; Tang, F.; Shen, H. Comparison of surgical resection and radiofrequency ablation for stages I and II elderly hepatocellular carcinoma patients (≥65 years): A SEER population-based propensity score matching’s study. Front. Oncol. 2022, 12, 903231. [Google Scholar] [CrossRef]
- VoPham, T.; Bertrand, K.A.; Fisher, J.A.; Ward, M.H.; Laden, F.; Jones, R.R. Emissions of dioxins and dioxin-like compounds and incidence of hepatocellular carcinoma in the United States. Environ. Res. 2022, 204, 112386. [Google Scholar] [CrossRef]
- Zhan, G.; Peng, H.; Zhou, L.; Jin, L.; Xie, X.; He, Y.; Wang, X.; Du, Z.; Cao, P. A web-based nomogram model for predicting the overall survival of hepatocellular carcinoma patients with external beam radiation therapy: A population study based on SEER database and a Chinese cohort. Front. Endocrinol. 2023, 14, 1070396. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kong, C.; Zhao, Z.; Chen, W.; Lv, X.; Shu, G.; Ye, M.; Song, J.; Ying, X.; Weng, Q.; Weng, W.; et al. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur. Radiol. 2021, 31, 7500–7511. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Yan, Y.; Zhang, J.; Wang, J.; Wang, R.; Ling, X.; Liu, Y.; Lau, W.Y.; Jiang, S.; Liu, J.; et al. The impact of liver resection on survival outcomes of hepatocellular carcinoma patients with extrahepatic metastases: A propensity score matching study. Cancer Med. 2018, 7, 4475–4484. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hoffmann, K.; Schemmer, P. Treatment of hepatocellular carcinoma: A systematic review. Liver Cancer 2012, 1, 144–158. [Google Scholar] [CrossRef]
- Liang, L.J.; Hu, W.J.; Yin, X.Y.; Zhou, Q.; Peng, B.G.; Li, D.M.; Lu, M.D. Adjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomy. World J. Surg. 2008, 32, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef]
- Yuan, G.; Song, Y.; Li, Q.; Hu, X.; Zang, M.; Dai, W.; Cheng, X.; Huang, W.; Yu, W.; Chen, M.; et al. Development and Validation of a Contrast-Enhanced CT-Based Radiomics Nomogram for Prediction of Therapeutic Efficacy of Anti-PD-1 Antibodies in Advanced HCC Patients. Front. Immunol. 2020, 11, 613946. [Google Scholar] [CrossRef]
- Yang, Z.; Zi, Q.; Xu, K.; Wang, C.; Chi, Q. Development of a macrophages-related 4-gene signature and nomogram for the overall survival prediction of hepatocellular carcinoma based on WGCNA and LASSO algorithm. Int. Immunopharmacol. 2021, 90, 107238. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, Q.; Li, Q.; Zhong, H.; Wang, Y.; Yang, H.; Li, H.; Wang, X.; Li, K.; et al. Multi-institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front. Immunol. 2022, 13, 1007176. [Google Scholar] [CrossRef]




| Characteristics | Training Cohort | Validation Cohort 1 | p Value |
|---|---|---|---|
| n | 899 | 388 | |
| Age, n (%) | 0.662 | ||
| ≥35 | 685 (53.2%) | 300 (23.3%) | |
| <35 | 214 (16.6%) | 88 (6.8%) | |
| Sex, n (%) | 0.418 | ||
| Male | 693 (53.8%) | 291 (22.6%) | |
| Female | 206 (16%) | 97 (7.5%) | |
| Race, n (%) | 0.859 | ||
| White | 536 (41.6%) | 225 (17.5%) | |
| Other | 246 (19.1%) | 111 (8.6%) | |
| Black | 117 (9.1%) | 52 (4%) | |
| Grade, n (%) | 0.966 | ||
| Well/Moderate | 366 (28.4%) | 158 (12.3%) | |
| Poor/Undifferentiated | 158 (12.3%) | 66 (5.1%) | |
| Unknown | 375 (29.1%) | 164 (12.7%) | |
| AJCC_Stage, n (%) | 0.699 | ||
| Unknown | 166 (12.9%) | 64 (5%) | |
| stage I–II | 393 (30.5%) | 174 (13.5%) | |
| stage III–IV | 340 (26.4%) | 150 (11.7%) | |
| Stage_T, n (%) | 0.218 | ||
| Unknown | 179 (13.9%) | 65 (5.1%) | |
| T1/T2 | 474 (36.8%) | 201 (15.6%) | |
| T3/T4 | 246 (19.1%) | 122 (9.5%) | |
| Stage_N, n (%) | 0.163 | ||
| Unknown | 180 (14%) | 63 (4.9%) | |
| N0 | 648 (50.3%) | 286 (22.2%) | |
| N1 | 71 (5.5%) | 39 (3%) | |
| Stage_M, n (%) | 0.633 | ||
| Unknown | 73 (5.7%) | 29 (2.3%) | |
| M0 | 656 (51%) | 293 (22.8%) | |
| M1 | 170 (13.2%) | 66 (5.1%) | |
| Dissected lymph nodes, n (%) | 0.176 | ||
| 0 | 820 (63.7%) | 344 (26.7%) | |
| 1–3 | 69 (5.4%) | 35 (2.7%) | |
| ≥4 | 10 (0.8%) | 9 (0.7%) | |
| Surgery, n (%) | 0.765 | ||
| No | 564 (43.8%) | 240 (18.6%) | |
| Yes | 335 (26%) | 148 (11.5%) | |
| Chemotherapy, n (%) | 0.248 | ||
| No | 551 (42.8%) | 251 (19.5%) | |
| Yes | 348 (27%) | 137 (10.6%) | |
| AFP, n (%) | 0.101 | ||
| Negative | 178 (13.8%) | 90 (7%) | |
| Positive | 477 (37.1%) | 213 (16.6%) | |
| Unknown | 244 (19%) | 85 (6.6%) | |
| Tumor_size, n (%) | 0.358 | ||
| ≤5 cm | 317 (24.6%) | 135 (10.5%) | |
| 5–10 cm | 209 (16.2%) | 94 (7.3%) | |
| >10 cm | 173 (13.4%) | 87 (6.8%) | |
| Unknown | 200 (15.5%) | 72 (5.6%) | |
| Tumor_Number, n (%) | 0.133 | ||
| 1 | 876 (68.1%) | 372 (28.9%) | |
| 2 | 23 (1.8%) | 16 (1.2%) |
| Variable | Univariate | p | Multivariate | p | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age | ||||||
| <35 | Reference | |||||
| ≥35 | 1.45 | 1.19–1.77 | <0.001 | 1.68 | 1.36–2.09 | <0.001 |
| Sex | ||||||
| Female | 0.63 | 0.51–0.76 | <0.001 | 0.67 | 0.54–0.82 | <0.001 |
| Male | Reference | Reference | ||||
| AJCC Stages | ||||||
| I and II | Reference | Reference | ||||
| III and IV | 4.11 | 3.41–4.95 | <0.001 | 2.59 | 1.59–2.52 | <0.001 |
| Unknown | 2.48 | 1.97–3.11 | <0.001 | 1.81 | 0.99–1.49 | <0.001 |
| Grade | ||||||
| Well | Reference | Reference | ||||
| Bad | 2.68 | 2.15–3.34 | <0.001 | 2 | 1.59–2.52 | <0.001 |
| Unknown | 2.19 | 1.82–2.64 | <0.001 | 1.21 | 0.99–1.49 | 0.067 |
| Surgery | ||||||
| No | Reference | Reference | ||||
| Yes | 0.23 | 0.19–0.28 | <0.001 | 0.3 | 0.24–0.38 | <0.001 |
| Chemotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | 1.03 | 0.88–1.21 | 0.72 | 0.52 | 0.44–0.62 | <0.001 |
| AFP | ||||||
| Negative | Reference | Reference | ||||
| Positive | 2.71 | 2.14–3.43 | <0.001 | 1.94 | 1.51–2.48 | <0.001 |
| Unknown | 1.54 | 1.18–2.01 | <0.01 | 0.91 | 0.67–1.22 | 0.52 |
| Tumor size | ||||||
| ≤5 cm | Reference | Reference | ||||
| 5–10 cm | 2.22 | 1.79–2.76 | <0.001 | 1.36 | 1.07–1.73 | <0.05 |
| >10 cm | 2.32 | 1.84–2.92 | <0.001 | 1.33 | 1.01–1.76 | <0.05 |
| Unknown | 2.95 | 2.37–3.67 | <0.001 | 1.27 | 0.96–1.69 | 0.099 |
| Dissected lymph nodes | ||||||
| 0 | Reference | Reference | ||||
| 1–3 | 0.57 | 0.41–0.79 | <0.001 | 0.89 | 0.63–1.25 | 0.499 |
| ≥4 | 0.26 | 0.08–0.81 | <0.05 | 0.47 | 0.15–1.52 | 0.208 |
| Index | Training Cohort | p | Validation Cohort | p | ||
|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |||
| NRI | ||||||
| 1-year OS | 0.794 | 0.636–0.929 | 0.757 | 0.326–0.927 | ||
| 3-year OS | 0.699 | 0.211–0.814 | 0.521 | 0.292–0.783 | ||
| 5-year OS | 0.204 | 0.099–0.328 | 0.223 | 0.114–0.558 | ||
| IDI | ||||||
| 1-year OS | 0.197 | 0.161–0.243 | <0.001 | 0.228 | 0.161–0.300 | <0.001 |
| 3-year OS | 0.204 | 0.156–0.251 | <0.001 | 0.306 | 0.224–0.398 | <0.001 |
| 5-year OS | 0.189 | 0.145–0.237 | <0.001 | 0.323 | 0.234–0.406 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, T.; Ma, W.; Zhang, J.; Yu, J.; Deng, W.; Dong, K.; Wang, W. Construction of a Nomogram to Predict Overall Survival in Patients with Early-Onset Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers 2023, 15, 5310. https://doi.org/10.3390/cancers15225310
Kuang T, Ma W, Zhang J, Yu J, Deng W, Dong K, Wang W. Construction of a Nomogram to Predict Overall Survival in Patients with Early-Onset Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers. 2023; 15(22):5310. https://doi.org/10.3390/cancers15225310
Chicago/Turabian StyleKuang, Tianrui, Wangbin Ma, Jiacheng Zhang, Jia Yu, Wenhong Deng, Keshuai Dong, and Weixing Wang. 2023. "Construction of a Nomogram to Predict Overall Survival in Patients with Early-Onset Hepatocellular Carcinoma: A Retrospective Cohort Study" Cancers 15, no. 22: 5310. https://doi.org/10.3390/cancers15225310
APA StyleKuang, T., Ma, W., Zhang, J., Yu, J., Deng, W., Dong, K., & Wang, W. (2023). Construction of a Nomogram to Predict Overall Survival in Patients with Early-Onset Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers, 15(22), 5310. https://doi.org/10.3390/cancers15225310






