Multi-Domain Screening: Identification of Patient’s Risk Profile Prior to Head-and-Neck Cancer Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
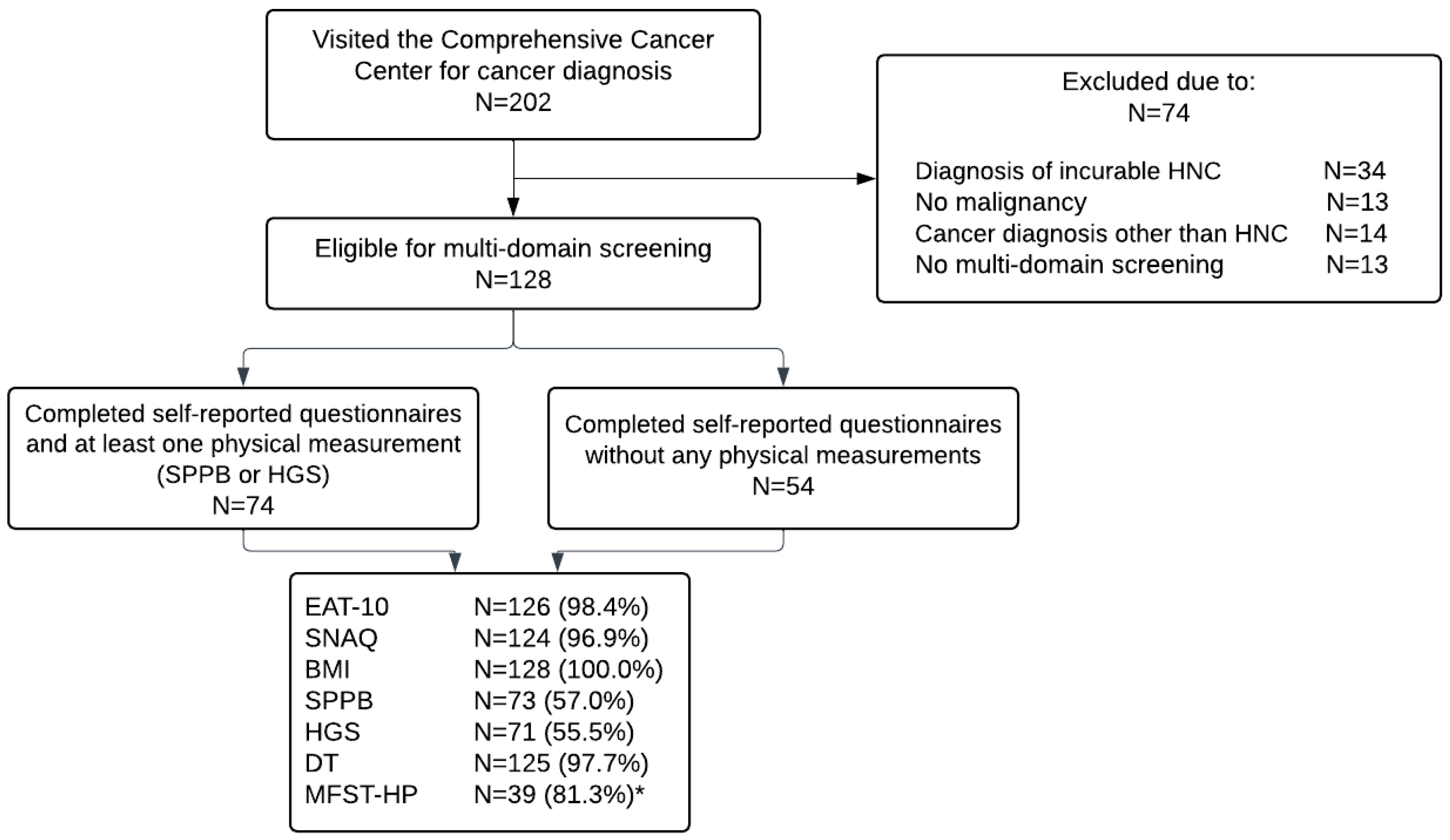
2.1. Study Design and Study Population
2.2. Demographic and Oncological Data Collection
2.3. Multi-Domain Screening
2.4. Statistical Analysis
3. Results
3.1. Screening Participation Rates
3.2. Patient Demographic Data
3.3. Prevalence of the Risk of OD, Malnutrition, Sarcopenia, and Frailty
3.4. Relationship between Age, PS, and Cancer Stage Grouping Versus Risk of OD, Malnutrition, Sarcopenia, and Frailty
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136. [Google Scholar] [CrossRef]
- DICA. Impact Report. Available online: https://dica.nl/media/2503/DICA%20Jaarrapport%202019%20-%20LR.pdf (accessed on 4 September 2023).
- Jones, E.; Speyer, R.; Kertscher, B.; Denman, D.; Swan, K.; Cordier, R. Health-Related Quality of Life and Oropharyngeal Dysphagia: A Systematic Review. Dysphagia 2018, 33, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.; van Dijk, A.M.; Doornaert, P.; Kruizenga, H.M.; Langendijk, J.A.; Leemans, C.R.; Weijs, P.J.; Verdonck-de Leeuw, I.M. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr. Cancer 2013, 65, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.; Grosso, A.; Gentile, P.C.; Battista, M.; Bianciardi, F.; Di Palma, A.; Pavese, I.; Satta, F.; Tosti, M.; Palladino, A. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck J. Sci. Spec. Head Neck 2008, 30, 503–508. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Oya, R.; Inohara, H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: A meta-analysis. PLoS ONE 2021, 16, e0259288. [Google Scholar] [CrossRef] [PubMed]
- Cleere, E.F.; Davey, M.G.; O’Neill, J.P. “Age is just a number”; frailty as a marker of peri-operative risk in head and neck surgery: Systematic review and meta-analysis. Head Neck 2022, 44, 1927–1939. [Google Scholar] [CrossRef]
- Baijens, L.W.J.; Walshe, M.; Aaltonen, L.M.; Arens, C.; Cordier, R.; Cras, P.; Crevier-Buchman, L.; Curtis, C.; Golusinski, W.; Govender, R.; et al. European white paper: Oropharyngeal dysphagia in head and neck cancer. Eur. Arch. Otorhinolaryngol. 2021, 278, 577–616. [Google Scholar] [CrossRef]
- Son, Y.R.; Choi, K.H.; Kim, T.G. Dysphagia in Tongue Cancer Patients. Arm 2015, 39, 210–217. [Google Scholar] [CrossRef]
- Denaro, N.; Merlano, M.C.; Russi, E.G. Dysphagia in Head and Neck Cancer Patients: Pretreatment Evaluation, Predictive Factors, and Assessment during Radio-Chemotherapy, Recommendations. Clin. Exp. Otorhinolaryngol. 2013, 6, 117–126. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.E.; Dittus, K.; Toth, M.J.; Willis, M.S.; Guttridge, D.C.; George, J.R.; Barnes, C.A.; Gourin, C.G.; Der-Torossian, H. Cancer cachexia update in head and neck cancer: Definitions and diagnostic features. Head Neck 2015, 37, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Stapleton, N.; Bauer, J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin. Nutr. 2021, 40, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Gibb, C.; Boase, S.; Hodge, J.C.; Krishnan, S.; Foreman, A. Frailty in geriatric head and neck cancer: A contemporary review. Laryngoscope 2018, 128, E416–E424. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Orlandi, E.; Alfieri, S.; Simon, C.; Trama, A.; Licitra, L. Treatment challenges in and outside a network setting: Head and neck cancers. Eur. J. Surg. Oncol. 2019, 45, 40–45. [Google Scholar] [CrossRef]
- Netherlands Cancer Registry. Prevalence of head and neck cancer. 2022. Available online: https://iknl.nl/kankersoorten/hoofd-halskanker/registratie/prevalentie (accessed on 14 March 2023).
- Slaets, J.P. Vulnerability in the elderly: Frailty. Med. Clin. 2006, 90, 593–601. [Google Scholar] [CrossRef]
- Paleri, V.; Wight, R.G.; Silver, C.E.; Haigentz Jr, M.; Takes, R.P.; Bradley, P.J.; Rinaldo, A.; Sanabria, A.; Bień, S.; Ferlito, A. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol. 2010, 46, 712–719. [Google Scholar] [CrossRef]
- Kulbersh, B.D.; Rosenthal, E.L.; McGrew, B.M.; Duncan, R.D.; McColloch, N.L.; Carroll, W.R.; Magnuson, J.S. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope 2006, 116, 883–886. [Google Scholar] [CrossRef]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support. Care Cancer 2010, 18, 837–845. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force. Guide to Clinical Preventive Services; Williams & Wilkins: Philadelphia, PA, USA, 1996. [Google Scholar]
- Han, S.H.; Cho, D.; Mohammad, R.; Jung, Y.H.; Ahn, S.H.; Cha, W.; Jeong, W.J. Use of the comprehensive geriatric assessment for the prediction of postoperative complications in elderly patients with head and neck cancer. Head Neck 2022, 44, 672–680. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Poelman, A.; Sidorenkov, G.; Festen, S.; de Bock, G.H.; Langendijk, J.A.; van der Laan, B.F.; Steenbakkers, R.J.; Halmos, G.B. The association of frailty and outcomes of geriatric assessment with acute radiation-induced toxicity in patients with head and neck cancer. Oral Oncol. 2022, 130, 105933. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.T.R.D. Comprehensive Geriatric Assessment. Available online: http://www.uptodate.com (accessed on 4 July 2023).
- Baitar, A.; Van Fraeyenhove, F.; Vandebroek, A.; De Droogh, E.; Galdermans, D.; Mebis, J.; Schrijvers, D. Evaluation of the Groningen Frailty Indicator and the G8 questionnaire as screening tools for frailty in older patients with cancer. J. Geriatr. Oncol. 2013, 4, 32–38. [Google Scholar] [CrossRef]
- Szumacher, E.; Sattar, S.; Neve, M.; Do, K.; Ayala, A.P.; Gray, M.; Lee, J.; Alibhai, S.; Puts, M. Use of Comprehensive Geriatric Assessment and Geriatric Screening for Older Adults in the Radiation Oncology Setting: A Systematic Review. Clin. Oncol. 2018, 30, 578–588. [Google Scholar] [CrossRef]
- Starmer, H.; Gourin, C.; Lua, L.L.; Burkhead, L. Pretreatment swallowing assessment in head and neck cancer patients. Laryngoscope 2011, 121, 1208–1211. [Google Scholar] [CrossRef]
- Righini, C.A.; Timi, N.; Junet, P.; Bertolo, A.; Reyt, E.; Atallah, I. Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 8–14. [Google Scholar] [CrossRef]
- Galli, A.; Colombo, M.; Prizio, C.; Carrara, G.; Lira Luce, F.; Paesano, P.L.; Della Vecchia, G.; Giordano, L.; Bondi, S.; Tulli, M. Skeletal muscle depletion and major postoperative complications in locally-advanced head and neck cancer: A comparison between ultrasound of rectus femoris muscle and neck cross-sectional imaging. Cancers 2022, 14, 347. [Google Scholar] [CrossRef]
- Cates, D.J.; Evangelista, L.M.; Belafsky, P.C. Effect of Pretreatment Dysphagia on Postchemoradiation Swallowing Function in Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2022, 166, 506–510. [Google Scholar] [CrossRef]
- Dewansingh, P.; Bras, L.; Ter Beek, L.; Krijnen, W.P.; Roodenburg, J.L.; van der Schans, C.P.; Halmos, G.B.; Jager-Wittenaar, H. Malnutrition risk and frailty in head and neck cancer patients: Coexistent but distinct conditions. Eur. Arch. Oto-Rhino-Laryngol. 2022, 280, 1893–1902. [Google Scholar] [CrossRef]
- Silva, J.C.; Silveira, A.; Sacau, A.; Monteiro, E.; Sequeira, T. Head and neck cancer early identification of malnutrition high risk patients and quality of life optimization. Int. J. Otolaryngol. Head Neck Surg. 2019, 8, 204. [Google Scholar] [CrossRef]
- Orell-Kotikangas, H.; Österlund, P.; Saarilahti, K.; Ravasco, P.; Schwab, U.; Mäkitie, A.A. NRS-2002 for pre-treatment nutritional risk screening and nutritional status assessment in head and neck cancer patients. Support. Care Cancer 2015, 23, 1495–1502. [Google Scholar] [CrossRef]
- Glare, P.; Sinclair, C.; Downing, M.; Stone, P.; Maltoni, M.; Vigano, A. Predicting survival in patients with advanced disease. Eur. J. Cancer 2008, 44, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A.; Hshieh, T.; Condron, N.; Wadleigh, M.; Abel, G.A.; Driver, J.A. Relationship between physician and patient assessment of performance status and survival in a large cohort of patients with haematologic malignancies. Br. J. Cancer 2016, 115, 858–861. [Google Scholar] [CrossRef]
- Derks, W.; de Leeuw, R.J.; Hordijk, G.J. Elderly patients with head and neck cancer: The influence of comorbidity on choice of therapy, complication rate, and survival. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 92–96. [Google Scholar] [CrossRef]
- Bourhis Sr, J.; Le Maıtre, A.; Pignon, J.; Ang, K.; Bernier, J.; Overgaard, J.; Tobias, J.; Saunders, M.; Adelstein, D.; O’Sullivan, B. Impact of age on treatment effect in locally advanced head and neck cancer (HNC): Two individual patient data meta-analyses. J. Clin. Oncol. 2006, 24, 5501. [Google Scholar] [CrossRef]
- Onderzoek, C.C.M. Niet-WMO-Onderzoek. Available online: https://www.ccmo.nl/onderzoekers/aanvullende-informatie-over-bepaalde-soorten-onderzoek/niet-wmo-onderzoek (accessed on 23 March 2023).
- van Overveld, L.; Takes, R.; Smeele, L. The Dutch head and neck audit: The first steps. J. Head Neck Surg. 2018, 1, 1–8. [Google Scholar]
- DICA. Resultaten uit de Dutch Head and Neck Audit (DHNA). Available online: https://dica.nl/dhna/resultaten (accessed on 23 March 2023).
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Singh, B.; Bhaya, M.; Stern, J.; Roland, J.T.; Zimbler, M.; Rosenfeld, R.M.; Har-El, G.; Lucente, F.E. Validation of the Charlson comorbidity index in patients with head and neck cancer: A multi-institutional study. Laryngoscope 1997, 107, 1469–1475. [Google Scholar] [CrossRef]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979. [Google Scholar]
- Heijnen, B.J.; Speyer, R.; Bülow, M.; Kuijpers, L.M. ‘What About Swallowing?’ Diagnostic Performance of Daily Clinical Practice Compared with the Eating Assessment Tool-10. Dysphagia 2016, 31, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef]
- Kruizenga, H.; Beijer, S.; Huisman-de Waal, J.-S.C.; Klos, M.; Rmeijnse-Meester, W.; Thijs, A.; Tieland, M.; Vasse, E.; Witteman, B. Richtlijn Ondervoeding Herkenning, Diagnosestelling en Behandeling van Ondervoeding bij Volwassenen; Stuurgroep Ondervoeding: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, E.; De Vreede, P.; Schoene, D.; Rydwik, E.; Mueller, V.; Frändin, K.; Hopman-Rock, M. Performance-based physical function in older community-dwelling persons: A systematic review of instruments. Age Ageing 2012, 41, 712–721. [Google Scholar] [CrossRef]
- Short Physical Performance Battery (SPPB). U.S. Department of Health & Human Services, National Institute on Ageing. Available online: https://effectieveouderenzorg.nl/toolkit/functioneel/mobiliteitsstoornis/diagnostiek/ (accessed on 14 March 2023).
- Strandkvist, V.; Larsson, A.; Pauelsen, M.; Nyberg, L.; Vikman, I.; Lindberg, A.; Gustafsson, T.; Röijezon, U. Hand grip strength is strongly associated with lower limb strength but only weakly with postural control in community-dwelling older adults. Arch. Gerontol. Geriatr. 2021, 94, 104345. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.; Visser, W.; Kruizenga, H.; Reijven, N. Meetprotocol Handknijpkracht mbv Hand Dynamometer; Nutritional Assessment Platform; 2016. Available online: http://zakboekdietetiek.nl/wp-content/uploads/2016/04/Standard-Operating-Procedure-Handknijpkdracht-NAP.pdf (accessed on 14 March 2023).
- Spruit, M.A.; Sillen, M.J.; Groenen, M.T.; Wouters, E.F.; Franssen, F.M. New normative values for handgrip strength: Results from the UK Biobank. J. Am. Med. Dir. Assoc. 2013, 14, 775.e5–775.e11. [Google Scholar] [CrossRef] [PubMed]
- Warnier, R.M.J.; van Rossum, E.; van Kuijk, S.M.J.; Mulder, W.J.; Schols, J.; Kempen, G. The Maastricht Frailty Screening Tool for Hospitalised Patients (MFST-HP) to Identify Non-Frail Patients. Int. J. Clin. Pract. 2017, 71, e13003. [Google Scholar] [CrossRef]
- Warnier, R.M.; van Rossum, E.; van Kuijk, S.M.; Magdelijns, F.; Schols, J.M.; Kempen, G.I. Frailty screening in hospitalised older adults: How does the brief Dutch National Safety Management Program perform compared to a more extensive approach? J. Clin. Nurs. 2020, 29, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Tuinman, M.A.; Gazendam-Donofrio, S.M.; Hoekstra-Weebers, J.E. Screening and referral for psychosocial distress in oncologic practice: Use of the Distress Thermometer. Cancer 2008, 113, 870–878. [Google Scholar] [CrossRef]
- Chang, P.-H.; Yeh, K.-Y.; Huang, J.-S.; Lai, C.-H.; Wu, T.-H.; Lan, Y.-J.; Tsai, J.C.-S.; Chen, E.Y.-C.; Yang, S.-W.; Wang, C.-H. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 1909–1915. [Google Scholar] [CrossRef]
- Chalker, C.; Voutsinas, J.M.; Wu, Q.V.; Santana-Davila, R.; Hwang, V.; Baik, C.S.; Lee, S.; Barber, B.; Futran, N.D.; Houlton, J.J.; et al. Performance status (PS) as a predictor of poor response to immune checkpoint inhibitors (ICI) in recurrent/metastatic head and neck cancer (RMHNSCC) patients. Cancer Med. 2022, 11, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Obeid, N.M.; Azuh, O.; Reddy, S.; Webb, S.; Reickert, C.; Velanovich, V.; Horst, H.M.; Rubinfeld, I. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: Exploring frailty and aggressive laparoscopic approaches. J. Trauma Acute Care Surg. 2012, 72, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Steverink, N. Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). Gerontologist 2001, 41, 236. [Google Scholar]
- Soubeyran, P.; Bellera, C.; Gregoire, F.; Blanc, J.; Ceccaldi, J.; Blanc-Bisson, C.; Mertens, C.; Mathoulin-Pélissier, S.; Fonck, M.; Rainfray, M. Validation of a screening test for elderly patients in oncology. J. Clin. Oncol. 2008, 26, 20568. [Google Scholar] [CrossRef]
- Kenny, C.; Regan, J.; Balding, L.; Higgins, S.; O’Leary, N.; Kelleher, F.; McDermott, R.; Armstrong, J.; Mihai, A.; Tiernan, E.; et al. Dysphagia Prevalence and Predictors in Cancers Outside the Head, Neck, and Upper Gastrointestinal Tract. J. Pain Symptom Manag. 2019, 58, 949–958.e942. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; van der Laan, B.F.; van Oort, R.P.; Roodenburg, J.L. Malnutrition and quality of life in patients treated for oral or oropharyngeal cancer. Head Neck 2011, 33, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.C.H.; Pilz, W.; Hoeben, A.; Hoebers, F.J.P.; Schols, A.; Baijens, L.W.J. Oropharyngeal dysphagia and cachexia: Intertwined in head and neck cancer. Head Neck 2022, 45, 783–797. [Google Scholar] [CrossRef]
- Williams, G.R.; Chen, Y.; Kenzik, K.M.; McDonald, A.; Shachar, S.S.; Klepin, H.D.; Kritchevsky, S.; Bhatia, S. Assessment of Sarcopenia Measures, Survival, and Disability in Older Adults Before and After Diagnosis With Cancer. JAMA Netw. Open 2020, 3, e204783. [Google Scholar] [CrossRef]
- Tzeng, P.-L.; Lin, C.-Y.; Lai, T.-F.; Huang, W.-C.; Pien, E.; Hsueh, M.-C.; Lin, K.-P.; Park, J.-H.; Liao, Y. Daily lifestyle behaviors and risks of sarcopenia among older adults. Arch. Public Health 2020, 78, 113. [Google Scholar] [CrossRef]
- Frowen, J.; Hughes, R.; Skeat, J. The prevalence of patient-reported dysphagia and oral complications in cancer patients. Support. Care Cancer 2020, 28, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Perracini, M.R.; Mello, M.; de Oliveira Máximo, R.; Bilton, T.L.; Ferriolli, E.; Lustosa, L.P.; da Silva Alexandre, T. Diagnostic Accuracy of the Short Physical Performance Battery for Detecting Frailty in Older People. Phys. Ther. 2020, 100, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients (N = 128) |
|---|---|
| Age, mean (SD) (N = 128) | 67 (9.8) |
| Age ≤ 70 years, N (%) | 81 (63.3) |
| Age > 70 years, N (%) | 47 (36.7) |
| Sex, N (%) | 128 (100.0) |
| Male | 84 (65.6) |
| Female | 44 (34.4) |
| Tobacco consumption, N (%) | 128 (100.0) |
| Never | 20 (15.6) |
| Former | 57 (44.5) |
| Current | 51 (39.8) |
| Number of pack years of smoking, median (IQR) (N = 128) | 28 (5–45) |
| Alcohol consumption, N (%) | 127 (100.0) |
| Never or irregular drinker (<one alcoholic drink per day) | 69 (54.3) |
| Former drinker (at least one alcoholic drink per day in the past) | 10 (7.9) |
| Current regular drinker (at least one alcoholic drink per day) | 48 (37.8) |
| Number of alcoholic drinks per day, median (IQR) (N = 127) | 1 (0–3) |
| Marital status, N (%) | 126 (100.0) |
| Single | 23 (18.3) |
| Married | 81 (64.3) |
| Widower | 14 (11.1) |
| Having a partner without being married | 8 (6.3) |
| Occupation, N (%) | 91 (100.0) |
| Employed | 35 (38.5) |
| Unemployed | 15 (16.5) |
| Retired | 38 (41.8) |
| Voluntary work | 3 (3.3) |
| Tumor site, N (%) | 128 (100.0) |
| Oral cavity | 46 (35.9) |
| (Para)nasal cavity | 8 (6.3) |
| Pharynx | 39 (30.5) |
| Larynx | 28 (21.9) |
| Salivary gland | 4 (3.1) |
| Lymph node metastasis of unknown origin | 3 (2.3) |
| T classification, N (%) | 128 (100.0) |
| T0–1 | 56 (43.8) |
| T2 | 27 (21.1) |
| T3 | 22 (17.2) |
| T4 | 23 (18.0) |
| N classification, N (%) | 128 (100.0) |
| N0 | 82 (64.1) |
| N1 | 19 (14.8) |
| N2 | 21 (16.4) |
| N3 | 6 (4.7) |
| M classification, N (%) | 128 (100.0) |
| M0 | 127 (99.2) |
| M1 a | 1 (0.8) |
| Cancer stage grouping, N (%) | 128 (100.0) |
| 0–1 | 55 (43.0) |
| 2 | 19 (14.8) |
| 3 | 19 (14.8) |
| 4 | 35 (27.3) |
| Tumor histopathology, N (%) | 128 (100.0) |
| Squamous cell carcinoma | 115 (89.8) |
| Verrucous carcinoma | 4 (3.1) |
| Other histopathology | 9 (7.0) |
| HPV/p16 positive, N (%) (N = 128) | 16 (12.5) |
| EBV positive, N (%) (N = 128) | 2 (1.6) |
| CCI grade, N (%) | 128 (100.0) |
| 0 No comorbidity | 52 (40.6) |
| 1 Low-level comorbidity | 51 (39.8) |
| 2 Moderate-level comorbidity | 16 (12.5) |
| 3 Advanced-level comorbidity | 9 (7.0) |
| PS, N (%) | 128 (100.0) |
| 0 Asymptomatic | 90 (70.3) |
| 1 Symptomatic, fully ambulatory | 24 (18.8) |
| 2 Symptomatic, in bed <50% of the day | 12 (9.4) |
| 3 Symptomatic, in bed >50% of the day but not bedridden | 2 (1.6) |
| 4 Completely disabled, bedridden | 0 (0.0) |
| Multi-Domain Screening | Patients (N = 128) |
|---|---|
| Domain OD | |
| EAT-10, N (%) | 126 (100.0) |
| EAT-10 median (IQR) | 0 (0–3) |
| <3, N (%) | 93 (73.8) |
| ≥3, N (%) | 33 (26.2) |
| Domain malnutrition | |
| SNAQ, N (%) | 124 (100.0) |
| SNAQ median (IQR) | 0 (0–1) |
| <2, N (%) | 99 (79.8) |
| ≥2, N (%) | 25 (20.2) |
| BMI, N (%) | 128 (100.0) |
| BMI mean (SD) | 25.8 (5.0) |
| ≥20 kg/m2 if age < 70 y and ≥22 kg/m2 if age ≥ 70 y, N (%) | 108 (84.4) |
| <20 kg/m2 if age < 70 y and <22 kg/m2 if age ≥ 70 y, N (%) | 20 (15.6) |
| Domain sarcopenia | |
| SPPB, N (%) | 73 (100.0) |
| SPPB median (IQR) | 9 (8–10) |
| >9, N (%) | 22 (30.1) |
| 4–9, N (%) | 48 (65.8) |
| <4, N (%) | 3 (4.1) |
| HGS a, N (%) | 71 (100.0) |
| ≥10th percentile, N (%) | 62 (87.3) |
| <10th percentile, N (%) | 9 (12.7) |
| Domain frailty | |
| DT, N (%) | 125 (100.0) |
| DT median (IQR) | 4 (1–7) |
| <5, N (%) | 67 (53.6) |
| ≥5, N (%) | 58 (46.4) |
| MFST-HP b, N (%) | 128 (100.0) |
| MFST-HP median (IQR) | 2 (1–3) |
| <6, N (%) | 35 (89.7) |
| ≥6, N (%) | 4 (10.3) |
| Multi-Domain Screening | Age ≤ 70 Years (N = 81) | Age > 70 Years (N = 47) | p-Value | PS 0–1 (N = 114) | PS 2–3 (N = 14) | p-Value | CSG 0–2 (N = 74) | CSG 3–4 (N = 54) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Domain OD | |||||||||
| EAT-10 (N = 126) | |||||||||
| EAT-10 median (IQR) | 0 (0–2) | 0 (0–5) | 0.400 a | 0 (0–2) | 0 (0–8) | 0.664 a | 0 (0–1) | 1 (0–12) | 0.001 a |
| ≥3, N (%) | 19 (23.8) | 14 (30.4) | 0.528 b | 28 (25.0) | 5 (35.7) | 0.519 c | 8 (11.1) | 25 (46.3) | <0.001 b |
| Domain malnutrition | |||||||||
| SNAQ (N = 124) | |||||||||
| SNAQ median (IQR) | 0 (0–0) | 0 (0–2) | 0.032 a | 0 (0–1) | 0 (0–1) | 0.507 a | 0 (0–0) | 0 (0–2) | 0.014 a |
| ≥2, N (%) | 12 (15.4) | 13 (28.3) | 0.106 b | 22 (20.0) | 3 (21.4) | 1.000 c | 11 (15.1) | 14 (27.5) | 0.113 b |
| BMI (N = 126) | |||||||||
| BMI mean (SD) | 26.1 (5.3) | 25.5 (4.5) | 0.518 d | 25.7 (4.8) | 26.9 (6.6) | 0.427 d | 26.0 (5.3) | 25.5 (4.6) | 0.469 d |
| <20 kg/m2 if age < 70 y and <22 kg/m2 if age ≥ 70 y, N (%) | 10 (12.3) | 10 (21.3) | 0.211 b | 18 (15.8) | 2 (14.3) | 1.000 c | 10 (13.5) | 10 (18.5) | 0.677 c |
| Domain sarcopenia | |||||||||
| SPPB (N = 73) | |||||||||
| SPPB median (IQR) | 9 (9–10) | 8 (5–9) | 0.001 a | 9 (8–10) | 6 (5–8) | <0.001 a | 9 (9–10) | 9 (7–9) | 0.031 a |
| ≤9, N (%) | 31 (62.0) | 20 (87.0) | 0.053 b | 41 (65.1) | 10 (100.0) | 0.027 c | 25 (61.0) | 26 (81.3) | 0.075 b |
| HGS (N = 71) | |||||||||
| <10th percentile, N (%) e | 5 (10.4) | 4 (17.4) | 0.458 c | 6 (9.8) | 3 (30.0) | 0.108 c | 5 (11.9) | 4 (13.8) | 1.000 c |
| Domain frailty | |||||||||
| DT (N = 125) | |||||||||
| DT median (IQR) | 4 (1–7) | 3 (1–7) | 0.751 a | 3 (1–7) | 7 (2–8) | 0.076 a | 2 (0–7) | 6 (2–7) | 0.003 a |
| ≥5, N (%) | 38 (46.9) | 20 (45.5) | 1.000 b | 49 (44.1) | 9 (64.3) | 0.169 b | 27 (38.0) | 31 (57.4) | 0.046 b |
| MFST–HP (N = 39) | |||||||||
| MFST-HP median (IQR) | 2 (2–2) | 2 (1–3) | 0.892 a | 2 (1–3) | 4 (2–9) | 0.015 a | 2 (1–4) | 2 (1–3) | 0.728 a |
| ≥6, N (%) | 0 (0.0) | 4 (10.5) | 1.000 c | 2 (6.1) | 2 (33.3) | 0.104 c | 3 (14.3) | 1 (5.6) | 0.609 c |
| Variables | Age > 70 Years Versus Age ≤ 70 Years (after Correction for CSG) | Age > 70 Years Versus Age ≤ 70 Years (after Correction for CSG and Tobacco and Alcohol Consumption) | CSG 3–4 Versus CSG Grouping 1–2 (after Correction for Age) | CSG 3–4 Versus CSG 1–2 (after Correction for Age and Tobacco and Alcohol Consumption) | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Domain OD | ||||||||
| EAT-10 (≥3) (N = 33) | 1.3 (0.5–3.2) | 0.531 | 1.3 (0.5–3.3) | 0.546 | 6.5 (2.6–16.2) | <0.001 | 6.9 (2.7–17.4) | <0.001 |
| Domain malnutrition | ||||||||
| SNAQ (≥2) (N = 25) | 2.3 (0.9–5.7) | 0.078 | 2.3 (0.9–5.8) | 0.075 | 1.9 (0.8–4.7) | 0.173 | 1.9 (0.8–4.7) | 0.179 |
| BMI (<20 kg/m2 if age < 70 y and <22 kg/m2 if age ≥ 70 y) (N = 20) | 2.1 (0.8–5.6) | 0.141 | 2.1 (0.8–5.8) | 0.142 | 1.2 (0.5–3.3) | 0.672 | 1.3 (0.5–3.5) | 0.651 |
| Domain sarcopenia | ||||||||
| SPPB (≤9) (N = 51) | 3.5 (0.9–13.6) | 0.075 | 3.5 (0.9–14.1) | 0.075 | 2.3 (0.7–7.0) | 0.149 | 2.2 (0.7–6.8) | 0.180 |
| Domain frailty | ||||||||
| DT (≥5) (N = 58) | 0.9 (0.4–1.9) | 0.752 | 0.9 (0.4–1.9) | 0.718 | 2.2 (1.04–4.5) | 0.039 | 2.2 (1.02–4.6) | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieland, M.W.M.; Pilz, W.; Winkens, B.; Hoeben, A.; Willemsen, A.C.H.; Kremer, B.; Baijens, L.W.J. Multi-Domain Screening: Identification of Patient’s Risk Profile Prior to Head-and-Neck Cancer Treatment. Cancers 2023, 15, 5254. https://doi.org/10.3390/cancers15215254
Wieland MWM, Pilz W, Winkens B, Hoeben A, Willemsen ACH, Kremer B, Baijens LWJ. Multi-Domain Screening: Identification of Patient’s Risk Profile Prior to Head-and-Neck Cancer Treatment. Cancers. 2023; 15(21):5254. https://doi.org/10.3390/cancers15215254
Chicago/Turabian StyleWieland, Monse W. M., Walmari Pilz, Bjorn Winkens, Ann Hoeben, Anna C. H. Willemsen, Bernd Kremer, and Laura W. J. Baijens. 2023. "Multi-Domain Screening: Identification of Patient’s Risk Profile Prior to Head-and-Neck Cancer Treatment" Cancers 15, no. 21: 5254. https://doi.org/10.3390/cancers15215254
APA StyleWieland, M. W. M., Pilz, W., Winkens, B., Hoeben, A., Willemsen, A. C. H., Kremer, B., & Baijens, L. W. J. (2023). Multi-Domain Screening: Identification of Patient’s Risk Profile Prior to Head-and-Neck Cancer Treatment. Cancers, 15(21), 5254. https://doi.org/10.3390/cancers15215254








