Simple Summary
Cancer, a complex group of diseases marked by abnormal cell proliferation and loss of physiological functions. Furthermore, tumors in the cardiac pacemaker pocket are rare and challenging medical problems, where the location of the implanted devices employed to manage heart rhythm disorders unexpectedly becomes a site for neoplasm growth. This intersection becomes even more nuanced with the rise in cardiac pacemaker (PM) implantations, a common practice globally. This study aimed to evaluate reported cases of this condition throughout the existing literature, in addition to enhancing early detection strategies and improving the management of affected patients.
Abstract
Cancer is the abnormal proliferation of physiologically inadequate cells. Studies have identified the cardiac pacemaker pocket as a site of rare neoplasms. To evaluate the clinical outcomes, treatment, prognosis, and individualized management of tumors originating in the cardiac pacemaker pocket, a systematic review was conducted using case reports and case series available in the PubMed/Medline, Science Direct, Cochrane Central, LILACS, and Scientific Electronic Library Online (Scielo) databases. Pacemaker pocket tumors affected patients with a mean age of 72.9 years, with a higher incidence in males (76.9%, n = 10). The average time for neoplasm development was 4.4 years (54.07 months). The most prevalent model was Medtronic (38.4%, n = 5), with titanium (83.3%) being the most common metal composition. Chemotherapy was the most performed procedure among patients (38.4%), followed by radiation therapy (38.4%) and surgical tumor resection (30.7%). Six analyzed cases (46.1%) resulted in death, and four patients (30.7%) achieved a cure. Patients with pacemakers should be routinely evaluated for the occurrence of malignant tumors at the site of device implantation.
1. Introduction
Cancer is a cluster of diseases characterized by the abnormal proliferation of cells, leading to the loss of their physiological functions in terms of division, growth, and lifespan [1,2]. Oncogenesis involves intricate mechanisms influenced by genetic factors, environmental exposures, and individual lifestyle habits that contribute to the development of malignancy [3,4]. In the United States, an estimated 2 million new cancer cases are diagnosed annually, with 610,000 deaths attributed to the disease [5]. Globally, approximately 19.3 million cases are identified each year, accompanied by 10 million deaths [6].
Cardiovascular diseases (CVDs) and cancer rank as the primary causes of global mortality [7], accounting for approximately one in six deaths worldwide [8]. The correlation between anticancer therapies and alterations in left ventricular ejection function, as well as the onset of heart failure symptoms, is well documented [9]. Factors such as radiotherapy and certain medications (anthracyclines, cyclophosphamide, sunitinib) [10] have been shown to induce senescence in cardiomyocytes. Consequently, this association is justified by the exacerbation of adverse cardiac remodeling due to the secretion of pro-inflammatory molecules and matrix protease degradation, which significantly impact patient prognosis [11,12].
The cardiac pacemaker (PM), on the other hand, is an electronic device utilized to regulate heart rhythm and treat electrical conduction disorders, including bradycardia, atrioventricular blocks (AVBs), left bundle branch blocks (LBBBs), right bundle branch blocks (RBBBs), and other congenital or acquired cardiovascular diseases (CVDs) [13,14]. Over time, the use of pacemakers has progressively increased, and their implantation is associated with a reduced risk of cardiovascular complications such as heart failure, acute myocardial infarction, malignant arrhythmias, and even mortality [15,16].
Pacemaker implantation is progressively increasing. More than 1 million cardiac pacemakers are implanted per year worldwide, with 200,000 implantations being performed in the United States [17]. Although pacemaker implantation is safe, complications such as bleeding, infection, pain, and inflammation at the incision site are present in a reduced proportion of insertion procedures [18]. In rare cases, the development of malignant neoplasms around the pacemaker tissue adjacent to the pacemaker has also been described [19].
Currently, investigations regarding malignancies in the pacemaker pocket (PP) are restricted to case reports and series. Hence, this systematic review endeavored to assess, by examining reports and case series, the available evidence concerning clinical outcomes, treatment approaches, prognosis, and tailored management strategies for each case.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review followed the 27 items described in the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) protocol [20], which assist in the construction of systematic reviews and meta-analyses. This review was registered in the Prospective International Registry of Systematic Reviews (PROSPERO) of the National Institute for Health Research under number CRD42022360240.
2.2. Eligibility Criterion
The articles included in this review were only case reports and case series in patients over 18 years of age who registered malignant neoplasms with the primary site of the pacemaker pocket. Only articles with confirmation of cancer by histopathology and/or immunohistochemistry were included in this study. Only articles in English were included in this review.
Articles from literature reviews and encyclopedias, editorials, book chapters, conference abstracts, correspondence, reviews, news, and small communications were excluded. Interventional studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
Thus, we sought to answer the following question: what are the main clinical, demographic, and management-related characteristics described in cases of malignancy affecting the region covering the pacemaker implantation cavity?
2.3. Search Strategy and Data Extraction
This study used references describing cancer at the primary site in the pacemaker pocket indexed in the PubMed/Medline, SCOPUS, Web of Science, and LILACS databases. The following descriptors were used: “Cancer”, “malignancy”, “tumor”, “malignancies”, “carcinoma”, “Plasmacytoma”, “neoplasms”, “neoplasia”, “lymphoma”, “adenocarcinoma”, “leiomyosarcoma”, “histiocytoma”, “artificial pacemaker”, “Resynchronization therapy”, “CIED”, “cardiac implantable electronic devices”, “cardiac pacemaker”, “cardiac pacing artificial”, “implantable pacemaker”, “pacemaker pocket”, “pacemaker cavity”, “pacemaker implantation site”, “pacemaker sac”. For the combination of terms in the databases, we used Boolean connectors (OR, AND). For the inclusion of additional articles, a manual search was performed in the references of the selected studies and notification alerts in the databases were activated if new titles that suited the query were published. In addition, research was also carried out using abstracts, articles, and scientific presentations from virtual meetings of the American Society of Clinical Oncology [21] and the American College of Cardiology [22].
Sources found in the databases and in the references of the articles were incorporated into the reference management software (EndNote®, version X7, Thomson Reuters, Philadelphia, PA, USA). Duplicate articles were removed using both automated and manual methods. Subsequently, two reviewers (F.C.A.M. and F.R.P.) independently analyzed the titles and abstracts of the identified articles. In case of disagreements between the two reviewers, a third reviewer was responsible for the final decision (N.P.C.S.).
The following baseline characteristics were extracted: (1) age; (2) sex; (3) pacemaker model; (4) pacemaker composition metal; (5) type of cancer; (6) reported clinical symptoms; (7) comorbidities; (8) period of implementation until the development of cancer; (9) examinations, included laboratory, echocardiogram, electrocardiogram, computerized tomography, mammography, ultrasound, radiography, and histologic; (10) conduct.
2.4. Risk of Bias in Included Studies
To assess the risk of bias in the selected articles, the Critical Assessment of the Joanna Briggs Institute (JBI) [23] for case reports was used as a tool, which consists of a checklist of eight scoring items. The evaluation was carried out by two reviewers independently (L.D.M. and R.A.L.S.C.). And in case of disagreement, a third reviewer was responsible for the final opinion (M.R.F.). Additionally, to reduce the risk of bias, all studies included in this review were published in peer-reviewed journals.
Data were tabulated in Microsoft Office Excel version 2016, and patient characteristics, tumor classification, pacemaker implantation time, clinical symptoms, laboratory and imaging tests, management, and outcome were presented descriptively.
3. Results
3.1. Selection of Studies
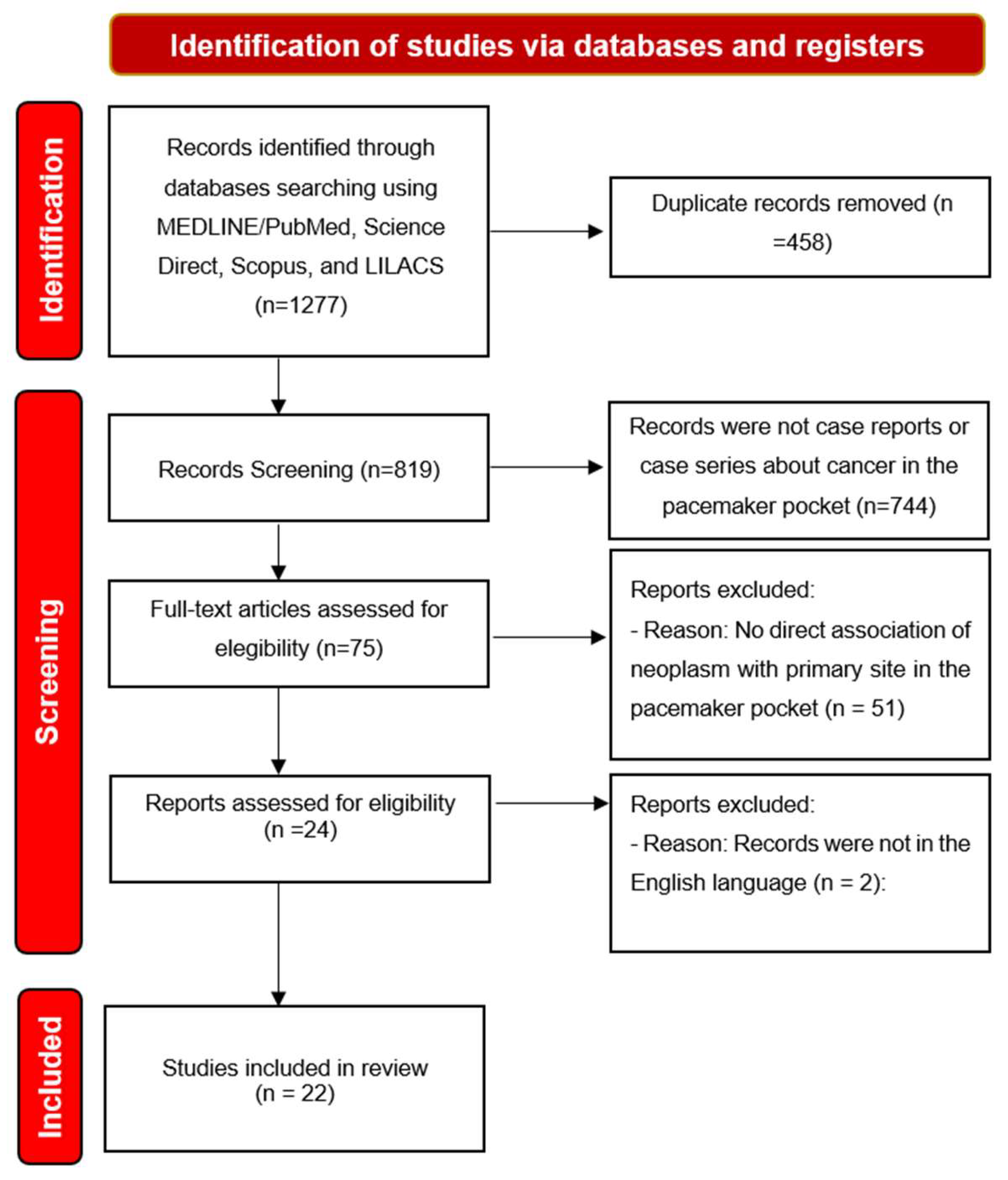
We identified 1277 titles of which, after removing duplicates, 819 titles remained for analysis. By applying the eligibility criteria, we selected 22 articles to compose the literature review. As seen on the Figure 1.
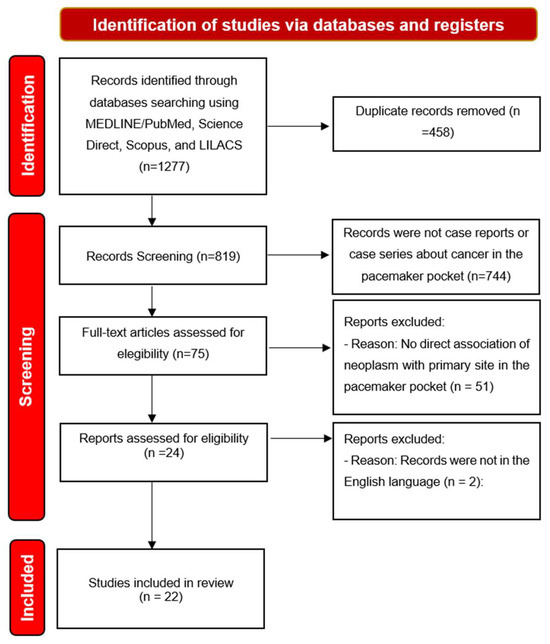
Figure 1.
Diagram of the research selection flow adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
3.2. Study Features
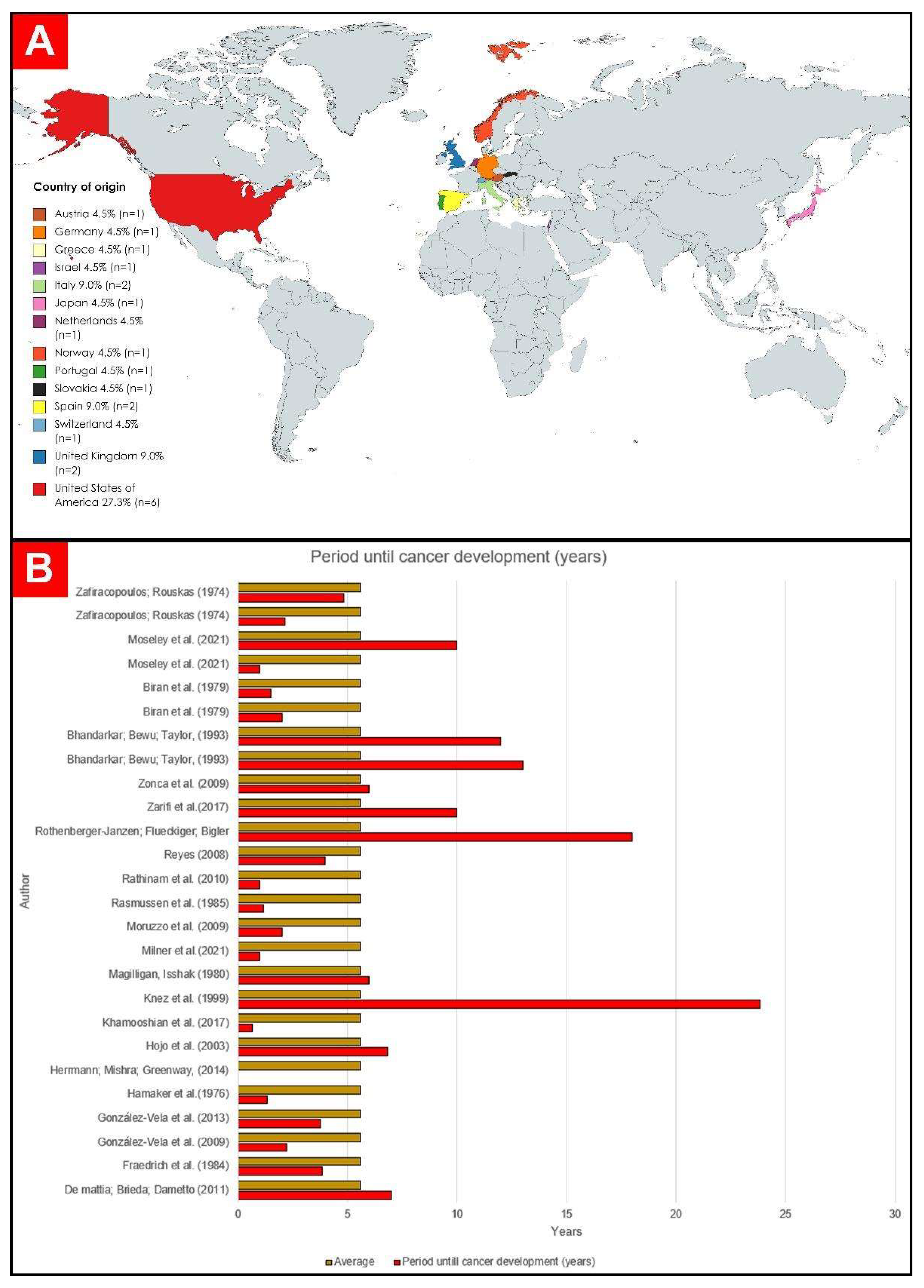
Of the 22 selected studies, 6 were carried out in the United States of America (USA): Hamaker et al. [24]; Herrmann, Mishra, and Greenway [25]; Magilligan and Isshak [26]; Moseley et al. [19]; Reyes [27]; and Zarifi et al. [28]. Fourteen were carried out in Europe: 1 in Portugal [29], 2 in Spain [30,31], 2 in the United Kingdom [32,33], 1 in Norway [34], 2 in Italy [35,36], 1 in Germany [37], 1 in Greece [38], 1 in Austria [39], 1 in Slovakia [40], 1 in the Netherlands [41], and 1 in Switzerland [42]. On the Asian continent, two studies were carried out in Japan [43] and one in Israel [44]. The graphic representation of the origin of the case reports can be seen in Figure 2.
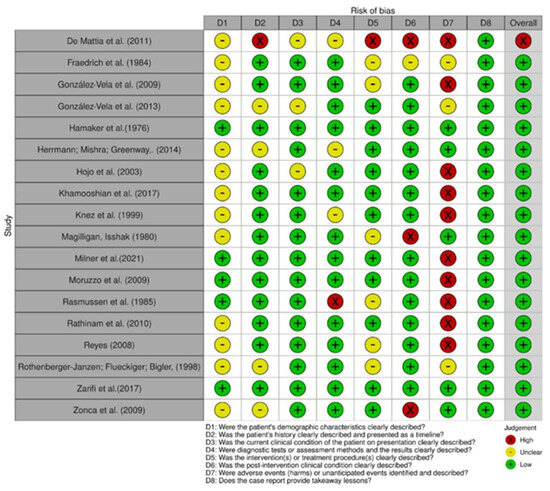
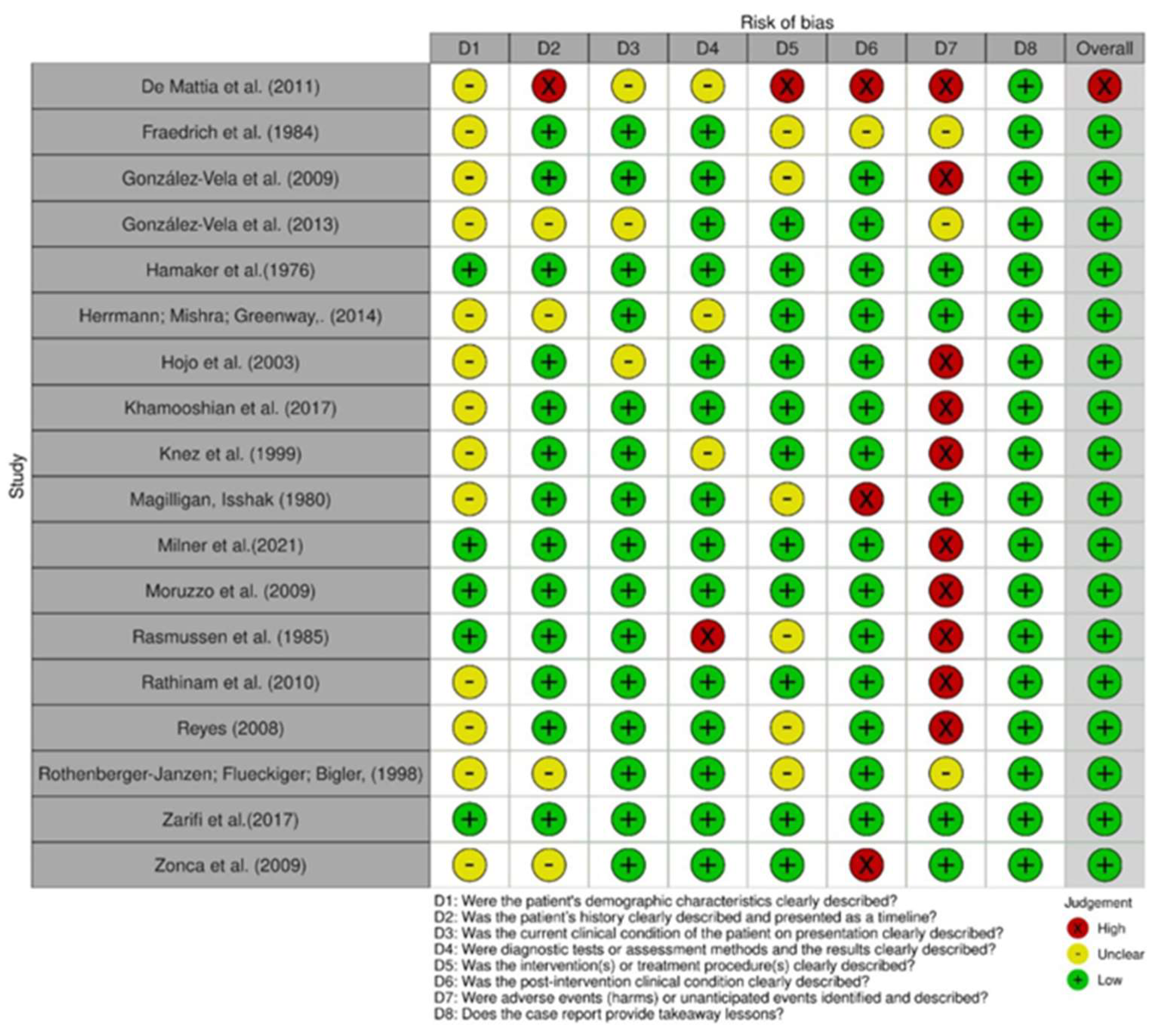
Figure 2.
Risk of bias among case reports [24,25,26,27,28,29,30,31,32,34,35,36,37,39,40,41,42,43].
3.3. Risk of Bias in Studies
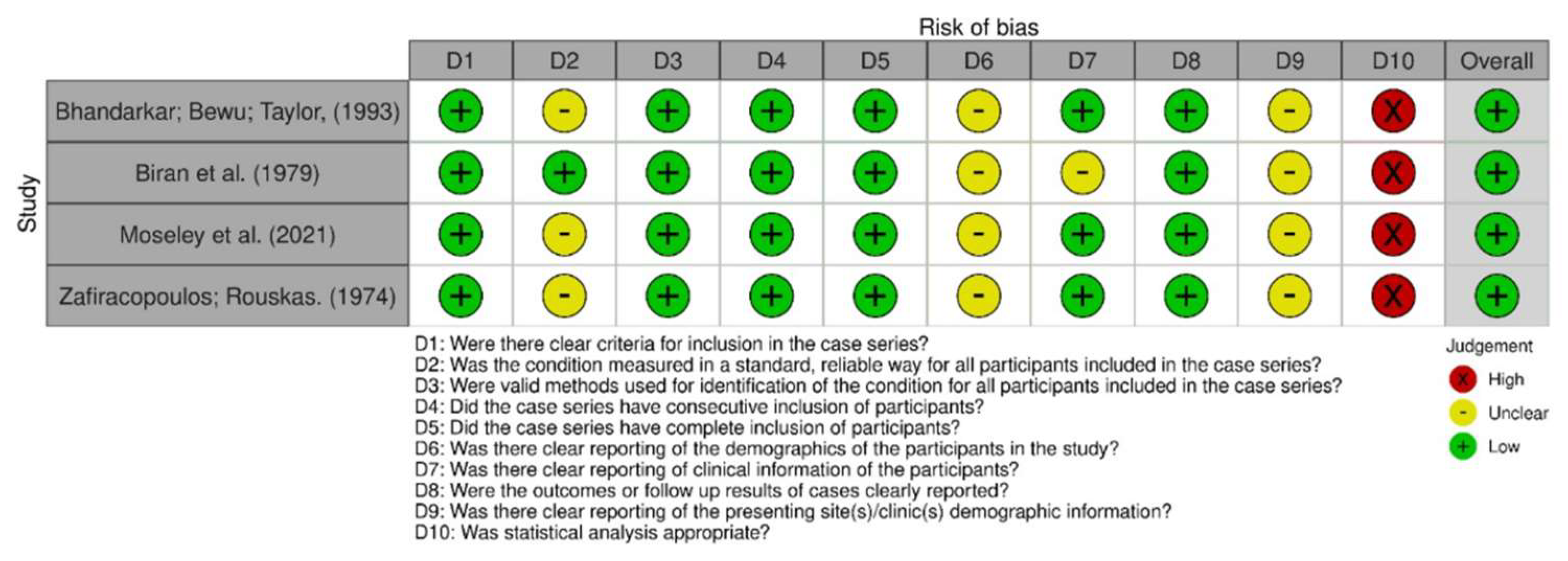
In comparing the case reports, 22 articles were determined to be at low risk of bias [24,25,26,27,28,29,30,31,32,33,34,35,37,38,39,40,41,42,43,44]. Only one study was identified as having a high risk of bias [36], as shown in Figure 2 and Figure 3. Respective graphics representations are in Figures S1 and S2.
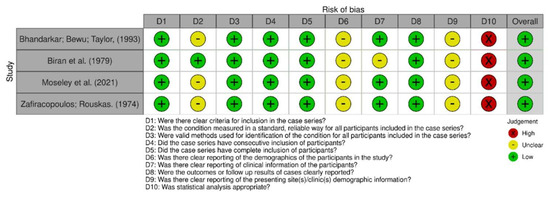
Figure 3.
Risk of bias among case series [19,33,38,44].
3.4. Results of Individual Studies
Eighteen (n = 18) case reports and four (n = 4) case series were included in the systematic review [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Milner et al. [29] reported an ulcerative and expansive plasmacytic lymphoma in the pacemaker pocket (PP) of a 78-year-old man from Portugal. De Mattia, Brieda, and Dametto [36] presented a case of an 87-year-old female patient with an invasive ductal carcinoma developing in the pacemaker pocket, which, along with Moruzzo et al.’s study [35], represented non-Hodgkin’s lymphomas identified consecutively in the adjacent region of the pocket; these were the two reported Italian cases. In the UK, cases were reported by Bhandarkar, Bewu, and Taylor and Rathinam et al. [32,33], describing two adenocarcinomas and one inflammatory myofibroblastic tumor, respectively. The study from Spain conducted by González-Vela et al. in 2009 [32] described a cutaneous leiomyosarcoma in the subpectoral pouch of a 74-year-old man, and another case report published in 2013 [31] described an atypical fibroxanthoma in the PP of an 89-year-old man with four years of implantation. Rasmussen et al. [34] reported a case of papillary adenocarcinoma that developed one year after implantation in a 75-year-old man in Norway. Hamaker et al. [24] reported a 48-year-old male patient with a plasmacytoma in the PP region 16 months after implantation. The study conducted in Germany by Fraedrich et al. [37] described a malignant fibrous histiocytoma in the PP of an 82-year-old male patient three years after implantation. Hojo et al. [43] described, in their Japanese study, a case of a 29-year-old man with stage II diffuse large B-cell lymphoma that developed six years after implantation. The Slovak study by Zonca et al. [40] reported a case of invasive ductal carcinoma in the PP of a 78-year-old woman with ulcerations in the affected region. The Dutch case report by Khamooshian et al. [41] described a pleomorphic sarcoma in the PP of a 43-year-old man eight months into the third device replacement.
In the United States, six cases have been reported. In Hamaker et al. [24], a man was described who developed plasmocytoma, diagnosed 1 year and 4 months after implantation of the generator. Reyes [27] reported in his study a case of clear cell hidradenocarcinoma affecting the pacemaker region of an 88-year-old woman four years later. In the work by Herrmann, Mishra, and Greenway [25], the case of a nodular basal cell carcinoma with features of an erythematous plaque on the left pectoral under the generator was cited. Magilligan and Ishak [26] described in their study a case of an 89-year-old woman who developed a breast adenocarcinoma in the region located in the PP. Zarifi et al. [28] described a case of plasmablastic lymphoma affecting the PP of a 100-year-old male patient after a period of 10 years from implantation until the onset of symptoms, as observed in Table 1 below.

Table 1.
Identification of articles included in the study.
3.5. Demographic Characteristics
Years of publication were listed between 1974 and 2021 (Table 1). The country with the most publications was the United States [19,24,25,26,27,28] (n = 6), followed by Spain (n = 2) [30,31], the UK [32,35] (n = 2), Italy (n = 2) [35,37], Austria (n = 1) [39], Germany (n = 1) [37], Slovakia (n = 1) [40], Greece (n = 1) [38], Israel (n = 1) [44], Japan (n = 1) [43], Norway (n = 1) [34], the Netherlands (n = 1) [41], Portugal (n = 1) [29], and Switzerland (n = 1) [42]. The mean age range of patients ranged from 29 to 100 years old. In total, 26 patients were analyzed, 14 men (53.8%) [19,24,25,28,29,30,31,32,35,37,39,41,43] and 12 women [19,24,26,33,36,38,40,42,44] (46.1%).
3.6. Malignancies
When analyzing the malignancies, there was a predominance of adenocarcinoma in 29.62% (n = 8) [26,33,34,38,42,44], lymphoma in 22.22% (n = 6) [19,28,29,35,43], and carcinoma in 22.22% (n = 6) [19,25,36,39,40,44]. Adenocarcinomas, were classified as the papillary type (n = 1) [34], breast (n = 1) [26], clear cell hidradenocarcinoma (n = 1) [27], intraductal with extracellular mucus (n = 1) [42], unspecified adenocarcinoma (n = 3), [33,44], and ecchymosal adenocarcinoma (n = 2) [38]. The lymphomas were all of the non-Hodgkin type: lymphoplasmacytic lymphoma (n = 1) [29], stage I–E non-Hodgkin lymphoma (n = 1) [35], stage II diffuse large B-cell lymphoma (n = 1) [43], plasmablastic lymphoma (n = 1) [28], large B-cell lymphoma (n = 1) [19], and B-cell lymphoma (n = 1) [19].
As for carcinomas, they were subdivided into moderately differentiated squamous cell carcinoma (n = 1) [19], invasive ductal carcinoma (n = 3) [36,39,40], nodular basal cell carcinoma (n = 1) [25], and intraductal carcinoma (n = 1) [44]. Other tumors affecting the pacemaker pouch described were: atypical fibroxanthoma [31] (n = 1), plasmacytoma (n = 1) [24], malignant fibrous histioma (n = 1) [37], clear cell hidradenocarcinoma (n = 1) [27], undifferentiated pleomorphic sarcoma (n = 1) [41], cutaneous leiomyosarcoma (n = 1) [30], and inflammatory myofibroblastic tumor (n = 1) [32].
3.7. Pacemaker Features
Of the 26 patients, 8 (30.7%) registered the insertion of the Medtronic model (Table 2), with varying subtypes: KVDD 901 [31], 5841 [24], Xytron [37], 5950 [26], Adapt ADDR01 [28], Capsure SP 4024 [39], and 5942 [44]. The most frequent composition metal was titanium in 30.7% (n = 8) [24,26,28,30,31,43,44], followed by mercury zinc with one patient (3.8%), who used the Unipolar Cordis Stanicor model [34] according to the information obtained on the constitution of pacemakers.

Table 2.
Characteristics of patients and pacemakers.
3.8. Reported Clinical Symptoms
In general, the most frequent clinical manifestation observed was local expansion over or close to the pacemaker pocket (Table 2); 5 patients (19.23%) were registered with local expansion [19,24,28,29,37] and 11 (42.30%) had a palpable mass [26,27,30,31,33,34,35,38,41,42,43,44]. In addition, other symptoms were recorded, such as ulceration in five patients (19.23%) [29,30,31,34,40], necrosis in two (7.7%) [24,43], fever in three (11.53%) [24,32,35], secretion in one (3.8%) [34], infection in one (3.8%) [34], and the presence of erythema in one (3.8%) [19].
3.9. Comorbidities
Thirteen patients (50.0%) had a history of AVB [19,24,29,31,34,37,38,41,43,44] and five of systemic arterial hypertension (SAH) (19.2%) [19,28,29,35]. There were five cases of Stokes-Adams syndrome (19.2%) [34,37,38,43], four cases of type 2 diabetes mellitus (DM2) (15.4%) [19,28,29], three cases of atrial fibrillation (AF) (11.5%) [25,29,34], and two cases with a previous cancer (7.7%) [29,44]. These comorbidities are listed in Table 2.
3.10. Time Elapsed from Implantation to Development of Cancer
The average time for the evolution of neoplasia was 6.57 years (78.92 months). A total of 12 patients developed cancer between 0 and 48 months after pacemaker implantation [19,24,27,30,31,34,35,37,38,41,44], 5 patients developed some neoplasm between 49 and 96 months [26,36,38,40,43], and 8 had a period longer than 97 months [19,28,29,32,33,39,42]. The graphical representation of these data can be seen below in Figure 4.
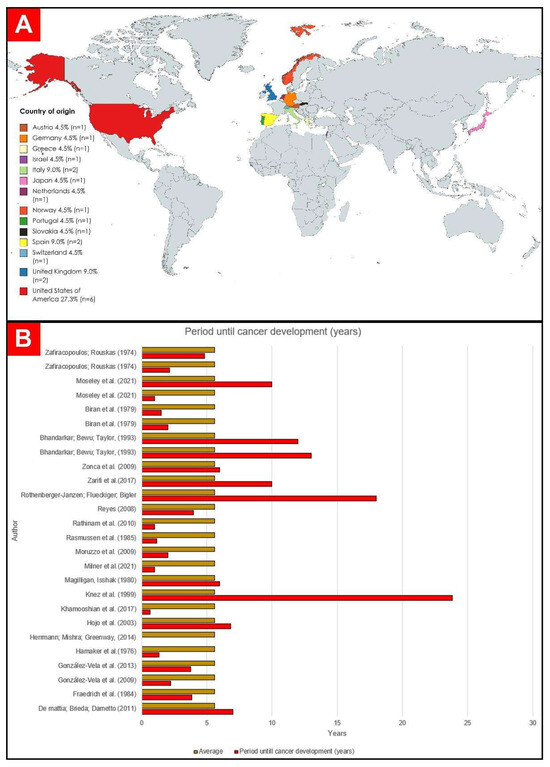
Figure 4.
(A) Countries of origin of the selected publications. (B) Graphic representation of time to development of cancer in years [19,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
3.11. Laboratory Tests
There was no predominant alteration among the evaluated cases, as seen in Table S1. However, the occurrence of reduced hemoglobin [24,29,32], positive QuantiFERON-TB Gold [29]; elevated LDH and lambda IgA [35], increased soluble interleukin 2 (IL-2) receptor [43], elevated CRP [32], increased ESR [28,32], and leukocytosis and changes in biochemical markers of cardiovascular damage [28] should be noted, in addition to leukopenia associated with thrombocytopenia [24].
3.12. Cardiovascular Assessment
3.12.1. Echocardiogram
Three patients (11.54%) [29,32,41] underwent echocardiography (ECHO). The results were: one ECHO case indicating mildly reduced ejection fraction [29], Three ECHO case with reduced ejection fraction [41], and one case showing no evidence of endocarditis [32]
3.12.2. Electrocardiogram
Five patients (19.23%) [24,25,26,43,44] recorded an ECG after admission. In all of them, the presence of AVB was observed.
3.13. Imaging Exams
In total, 6 cases (23%) underwent CT (Table S1). Among them, one case had a mass measuring 65 × 24 mm associated with left axillary lymphadenopathy [29]; one case had a mass on the pacemaker battery measuring 6 cm in diameter [35], also with localized lymphadenopathy; and one case had a round mass measuring 8–9 cm in the left clavicular region superficial to the left pectoral muscle on the upper surface of the pacemaker [32]. Furthermore, two suspected metastases were ruled out [41,42] and one was identified as highly suggestive. [38]
Regarding mammography, it was performed in three cases, presenting the following results: spiculated round mass caudal to the pacemaker pocket [36], absence of abnormalities or suspicious changes in both breasts [27], and findings difficult to interpret [40]. Four cases (15.3%) underwent USG, revealing a solid-looking lesion in two cases [27,41] and liquid in one case [28]
A total of seven case reports (26.9%) presented in their descriptions the results of chest X-rays performed [24,26,29,33,37,42], of which two reported no visible changes [26,29], one reported the presence of soft tissue mass around the pulse generator [24], one reported a shadow in the posterobasal segment of the lung with a suspicious indication of bronchial carcinoma with enlargement [37], one reported no metastasis [42], one reported a clinically palpable mass [33], and one reported nodular bilateral round shadows typical of metastatic lesions in the lungs [38].
3.14. Histopathological
Histopathological analysis (Table S1), with the aid of immunohistochemistry, revealed the identification of proteins among the lymphomas (n = 6) [19,28,29,43], which acted as markers, the majority (n = 3) being positive for CD138 [28,29,35] and for Ki-67 [28,29] (n = 2), in addition to other markers, such as CD4 [29], CD30, and CD43 [35]. Expression of CD10, CD99, CD68 (focal) and smooth muscle actin (focal), S100 protein, melan-A, desmin, CD34, p63, CD31, and human herpesvirus latent nuclear antigen 8 were markers also identified in atypical fibroxanthoma [31], together with the presence of hyperchromatic cells described both in atypical fibroxanthoma [31] and in undifferentiated pleomorphic sarcoma [41].
In addition to lymphomas, two other groups were identified in a large portion of the samples; namely, carcinomas (n = 6) [19,25,36,39,40,44] of the intraductal (n = 2) [39,44], nodular basal [25], ductal (n = 3) [36,39,40], and squamous cell types [19] and adenocarcinomas, which accounted for 30.7% of the total (n = 8) [26,33,34,38,42], with samples of being not specified (n = 4) [28,33,45] and of the schirrous (n = 2) [38], intraductal [42], and papillary types [34].
Diagnostic patterns of cutaneous leiomyosarcoma [30], inflammatory myofibroblastic tumors [32], and malignant fibrous histiocytoma [37] were also identified by the study in single samples.
Another tumor that stood out after consultation was a clear cell hidradenocarcinoma mimicking the immunohistochemistry of a metastatic lobular carcinoma by presenting a positive estrogen receptor, a progesterone receptor, mammaglobin, and CK7 cytokeratin [27]. Finally, the anatomopathological analysis can be highlighted, which identified invasion in different tissues in 19.2% (n = 5) of the samples [24,29,34], with one (1) in the bone marrow [29], one (1) in the right scapular region [24], and three (3) in the axillary lymph node region [19,34,38].
3.15. Management
Surgical resection of the tumor in the pacemaker pocket was the most performed procedure among study patients (34.61%, n = 9) (Table S1) [25,26,30,31,32,37,39,41,42]. Then came the use of chemotherapy (30.76%, n = 8) [33,34,35,38,40,42,44] and radiotherapy (30.76%, n = 8) [24,32,34,38,40,41,43,44].
The use of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone was described by Rasmussen et al. [34], while Zonca et al. [40]; Bhandarkar, Bewu, and Taylor [33]; and Rothenberger-Janzen, Flueckiger, and Bigler [42] described the use of antiestrogen therapy with tamoxifen. Biran et al. [44] performed chemotherapy with cytoxan, methotrexate and 5-fluoracil. The studies by Moruzzo et al. [35] and Zafirocopoulos and Rouskas [38] also reported the use of chemotherapy but did not present the drugs used.
Radiotherapy was described in 34.61% of cases (n = 9) [24,27,32,34,38,40,41,43,44]. Of the nine studies with radiotherapy, only three provided details on how the procedure was performed. The description presented by Hamaker et al. [24] in their study included the use of cobalt therapy with 2000 rads at the pacemaker site, Hojo et al. [43] reported the use of 30 Gy at the tumor site in the pacemaker pocket of a patient with AC, and Khamooshian [41] described the use of 60 Gy of adjuvant radiotherapy in the treatment. However, Reyes [27] presented a patient who refused the recommended irradiation.
Other procedures employed were drainage of hematoma [29], antibiotic therapy for suspected infection at the site of the pacemaker bag after hospital admission [19,28,29], simple mastectomy [38], and unilateral mastectomy with unilateral lymphadenectomy [34,44].
3.16. Clinical Outcomes
Regarding the clinical outcomes, seven patients died [24,27,34,35,37,38,40] and nine were cured after surgical intervention, chemotherapy, or radiotherapy [25,26,29,30,31,32,39,42,43]. One patient was described as still undergoing radiotherapy [41], one patient was in palliative care [28], and one patient [19] was undergoing outpatient follow-up with no therapeutic or palliative proposal yet. Four patients did not have the outcomes recorded in their respective studies [19,33,36,44]. These data can be seen in Table S1.
4. Discussion
Every year, approximately 1 million pacemakers are implanted worldwide [46], with a total of 19 million in the USA over the period from 1993 to 2009. Nevertheless, only 15 cases of malignancies are described in the literature, which substantiates the infrequent occurrence of malignancies within the pacemaker pocket [28]. Publications reporting and describing the appearance of tumors with a primary site in the pocket of these devices are still rare in the current medical literature.
The process of formation of malignant neoplasms around the cardiac pacemaker is still not well understood. One hypothesis would be that titanium is involved in tumor formation in this region [28,45]. The wearing away and corrosion of titanium in the human body, associated with the release of metal ions in the reaction, can cause toxicity in the body due to the potential pro-inflammatory effects mediated by interleucins, tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), and β-glucuronidase (GLU), and oxidative effects on the tissue surrounding the metal may cause apoptosis, genomic instabilities, and production of titanium-specific T-lymphocytes [45,47,48,49,50]. In addition, titanium particles in their oxidized form (TiO2) are capable of inducing DNA damage, genetic mutations, DNA deletions, and formation of micronuclei indicative of chromosomal aberrations in different cell lines [45,51,52].
Another possibility is that there may be genetic factors involved in the neoplasia formation process within the pacemaker pocket. The occurrence of cancer at the site of silicone breast implantation has been linked to mutations in the JAK1/STAT3 signaling pathway and the TP53 gene, which are involved in the modulation and prevention of clonal expansion of tumor cells [53,54,55]. Overexpression of the MYC gene and mutations in BRCA1/2 have also been associated with a higher likelihood of lymphomagenesis in patients following silicone implants [53,56,57]. Mutations in the p53 gene have also been described in the development of oral cancer after metal implants [58,59]. Currently, there is no evidence to support the presence of any specific common genetic component for the development of cancer in the pacemaker pocket among the patients described in the study.
Two other hypotheses regarding the formation of tumors in the pacemaker pocket would be chronic inflammation due to prolonged pacemaker presence and as a result of electrical stimulation [24,28]. The inflammation and the electric stimulation caused by a pacemaker are linked to chronic mechanical irritation, electrochemical disbalance, and trauma, all of which contribute to the development of cancer by inducing prolonged immune activation, cellular damage, and cancer cell migration resulting from electrical activity (galvanotaxis/electrotaxis) [60,61,62].
In the current study, titanium was the main metal described in the composition of pacemakers (30.7%, n = 8) [24,26,28,30,31,43,44]. Moreover, Pinchasov et al. [63] also reported in their study a series of cases of oral cancer of the squamous cell type in patients with dental implants made of titanium. For Onega et al. [64], in their meta-analysis with patients undergoing total arthroplasty who developed cancer at the implant site, there was no identification of an increased risk of cancer with the use of metal prostheses.
A study carried out with the population of Denmark observed a greater chance of developing bladder cancer and multiple myeloma in patients with a cardiac pacemaker, suggesting that the use of the device or the shared risk factors between cancer and cardiovascular diseases would be involved in the development of these tumors [65].
The most frequent comorbidities in the publications were arterial hypertension (20.0%) [28,29,35], DM2 (15.3%) [28,29], and cardiomyopathy (15.3%) [29]. These cardiovascular diseases have several well-established risk factors in common with cancer, such as smoking, alcoholism, physical inactivity, unhealthy diet, dyslipidemia, hypertension, age, obesity, and diabetes. These factors could explain the occurrence of the two pathologies concomitantly [11,66,67,68].
The presence of lymphomas was reported in 23.07% of the observed studies (n = 6) [19,28,29,35,43]. A similar result was described by Kricheldorff et al. [69], who also reported the predominance of lymphoma-type cancer—more specifically, anaplastic large cell lymphoma (BIA-ALCL)—in women undergoing silicone breast implantation. BIA-ALCL is a type of non-Hodgkin’s lymphoma that also originates from a silicone implant in the pectoral region of women [70]. Its genesis involves factors related to immunological interaction between tissue and prosthesis, bacterial growth in the implanted site, and genetic factors [70,71].
The most common clinical manifestation was local expansion over or near the pacemaker pocket, which was reported in terms of either local expansion (n = 5) [19,24,28,29,37] or cutaneous nodules (n = 11) [26,27,30,31,33,34,35,38,41,42,43,44], in addition to infection (n = 1) [34], ulceration (n = 5) [29,30,31,34,40], necrosis (n = 2) [24,43], fever (n = 3) [24,32,35], discharge (n = 1) [34], and erythema [19] (n = 1). The constant presence of a foreign body, the pacemaker, can lead to chronic inflammation, tissue damage, and production of reactive oxygen species, which can increase the risk of tumor development and the proliferation of microorganisms [72,73].
As for tumor development time, 12 patients developed cancer within 4 years of pacemaker implantation [19,24,27,30,31,34,35,37,38,41,44], 5 patients were diagnosed in a period greater than 4 years and less than 10 years [26,36,38,40,43], and 8 developed cancer over a period of more than 10 years [19,28,29,32,33,39,42]. In comparison, the International Agency for Research on Cancer (IARC) [74] described the growth of sarcomas and lymphomas at sites of orthopedic metal implants as occurring over variable periods, with cases reported between a few months and 30 years after implantation but with an average time of diagnosis that was also less than 10 years.
Chest radiography (26.9%) was the most used imaging test due to its ability to analyze the insertion of the cardiac device and identify tumors or structures adjacent to the implant [75,76], and it was also used in some patients to assess metastases [38,42], an action that is recurrent in underdeveloped countries [77]. Of the patients who underwent computed tomography (26.9%) (n = 7), in four of them the exact location of the mass and its relationship with adjacent structures (pacemaker bag and muscle bundles) were verified, in addition to definition of the presence or absence of lymphadenopathy in the axillary and mediastinal chains [29,32,35,41,78,79,80]. Finally, the third most used imaging test was USG (19.2%) due to its usefulness in observing soft tissues and differentiating the described tumors from isolated abscesses [81] and because it serves as a quick and cost-effective way to locate a lesion in relation to the pacemaker pocket [82], as seen in the studies by De Mattia [36], Khamooshian et al. [41], Zonca et al. [40], and Reyes [27].
In this systematic review, the approaches described were heterogeneous in terms of treatment, with significant differences between them. The treatment was carried out according to the specific indications of each histological type of tumor, the resources and evidence available at the time of the studies’ respective publications and the clinical condition of the patients and the indications for surgery [29,32,35,41,78,79,80], chemotherapy [33,34,35,38,40,42,44], and radiotherapy [24,32,34,38,40,41,43,44].
Primary pacemaker pocket tumors are rare. The relationship between pacemaker components and the appearance of malignancies is not yet well understood, but occurrence is probably due to coincidence [28,83]. Currently, there is insufficient evidence to establish a clear link between the occurrence of pacemaker pocket tumors and a specific factor, such as genetic characteristics, pacemaker composition, or immunological processes. Due to the rarity of these tumors, there is a lack of consensus on the approach to diagnosis and treatment. More studies are needed to improve our understanding of the biology and treatment of these rare tumors.
5. Conclusions
Patients who have been implanted with a pacemaker should be routinely clinically evaluated for the occurrence of malignant tumors at the implantation site of these devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15215206/s1, Figure S1: Graphical representation regarding Figure 2; Figure S2: Graphical representation regarding Figure 3; Table S1: Tests used, conduct and clinical outcomes.
Author Contributions
Conceptualization: F.C.A.d.M. and F.R.P.; methodology: F.C.A.d.M. and R.A.L.S.C.; resources: L.D.M. and E.S.d.R.P.; data curation: L.D.M. and R.A.L.S.C.; writing—original draft preparation: F.R.P., E.S.d.R.P., R.A.L.S.C. and L.D.M.; writing—review and editing, L.D.M.; visualization: R.A.L.S.C.; supervision: L.D.M., F.R.P., M.R.F. and N.P.C.d.S.; project administration: F.C.A.d.M.; funding acquisition: F.C.A.d.M., D.d.S.M.d.S., D.F. and R.M.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
Coordenação de Aperfeiçoamento de Pessoal e Nível Superior (CAPES); Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA); and Universidade Federal do Pará (UFPA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We acknowledge Universidade Federal do Pará (UFPA) and the Oncology Research Center (nPO/UFPA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- CancerProgressReport.org [Internet]. Available online: http://www.CancerProgressReport.org/ (accessed on 6 June 2023).
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2020, 8, 655–661. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Kamal, N.; Ilowefah, M.A.; Hilles, A.R.; Anua, N.A.; Awin, T.; Alshwyeh, H.A.; Aldosary, S.K.; Jambocus, N.G.S.; Alosaimi, A.A.; Rahman, A.; et al. Genesis and Mechanism of Some Cancer Types and an Overview on the Role of Diet and Nutrition in Cancer Prevention. Molecules 2022, 27, 1794. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Dzaye, O.; Bødtker, H.; Reiter-Brennan, C.; Blaha, M.J.; Mortensen, M.B. Danish National Trends in Cardiovascular Disease and Cancer Drug Expenditure in Relation to Trends in Cardiovascular Disease and Cancer Deaths. Am. J. Med. 2020, 133, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer. (accessed on 7 June 2023).
- Lenneman, C.G.; Sawyer, D.B. Cardio-Oncology. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef]
- Roule, V.; Verdier, L.; Blanchart, K.; Ardouin, P.; Lemaitre, A.; Bignon, M.; Sabatier, R.; Alexandre, J.; Beygui, F. Revisão sistemática e meta-análise do impacto prognóstico do câncer entre pacientes com síndrome coronariana aguda e/ou intervenção coronária percutânea. BMC Cardiovasc. Disord. 2020, 20, 38. [Google Scholar] [CrossRef]
- Dongchen, X.; Tongyi, L.; Xueping, M.; Jingjing, S.; Quanhong, L. Risk of mortality and other adverse outcomes from myocardial infarction in cancer survivors: A meta-analysis. Int. J. Clin. Oncol. 2023, 28, 41–51. [Google Scholar] [CrossRef]
- Truong, L.-L.; Scott, L.; Pal, R.S.; Jalink, M.; Gunasekara, S.; Wijeratne, D.T. Cancer and cardiovascular disease: Can understanding the mechanisms of cardiovascular injury guide us to optimise care in cancer survivors? Ecancermedicalscience 2022, 16, 1430. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 932–987. [Google Scholar] [CrossRef] [PubMed]
- Dalia, T.; Amr, B.S. Pacemaker Indications. [Updated 2022 August 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507823/ (accessed on 7 June 2023).
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. Corrigendum to: 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC): With the special contribution of the European Heart Rhythm Association (EHRA). EP Europace 2022, 24, 71–164. [Google Scholar] [CrossRef]
- Wood, M.A.; Ellenbogen, K.A. Cardiac pacemakers from the patient’s perspective. Circulation 2002, 105, 2136–2138. [Google Scholar] [CrossRef]
- Bhatia, N.; El-Chami, M. Leadless pacemakers: A contemporary review. J. Geriatr. Cardiol. 2018, 15, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Camacho, M.R.; Marín-León, I.; Molina-Doñoro, J.M.; González-López, J.R. Safety of Permanent Pacemaker Implantation: A Prospective Study. J. Clin. Med. 2019, 8, 35. [Google Scholar] [CrossRef]
- Moseley, T.; Birgersdotter-Green, U.; Feld, G.; Pollema, T. Malignancies masquerading as device pocket infections. Heart Case Rep. 2021, 7, 694–697. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Asco Hub—American Society of Clinical Oncology. (n.d.). Available online: https://www.asco.org/. (accessed on 10 June 2023).
- Home. American College of Cardiology. Available online: https://www.acc.org/. (accessed on 10 June 2023).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBL: Balanod, France, 2020; Available online: https://synthesismanual.jbi.global (accessed on 2 July 2023).
- Hamaker, W.R.; Lindell, M.E.; Gomez, A.C. Plasmacytoma Arising in a Pacemaker Pocket. Ann. Thorac. Surg. 1976, 21, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.L.; Mishra, V.; Greenway, H.T. Basal Cell Carcinoma Overlying a Cardiac Pacemaker Successfully Treated Using Mohs Micrographic Surgery. Dermatol. Surg. 2014, 40, 474–477. [Google Scholar] [CrossRef]
- Magilligan, D.J., Jr.; Isshak, G. Carcinoma of the Breast in a Pacemaker Pocket-Simple Recurrence or Oncotaxis? Pacing Clin. Electrophysiol. 1980, 3, 220–223. [Google Scholar] [CrossRef]
- Reyes, C.V. Clear Cell Hidradenocarcinoma Developing in Pacemaker Pocket. Pacing Clin. Electrophysiol. 2008, 31, 1513–1515. [Google Scholar] [CrossRef]
- Zarifi, C.; Deutsch, S.; Dullet, N.; Mukherjee, K.K.; Mukherjee, A.; Abubaker, F. An enlarging pacemaker pocket: A case report of a plasmablastic lymphoma arising as a primary tumor around a cardiac pacemaker and systematic literature review of various malignancies arising at the pacemaker pocket. J. Cardiol. Cases 2017, 17, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.; Gonçalves, F.; Gonçalves, L. A pacemaker pocket mass has many faces. J. Cardiol. Cases 2021, 24, 244–246. [Google Scholar] [CrossRef] [PubMed]
- González-Vela, C.M.; Val-Bernal, F.J.; Rubio, S.; Olalla, J.J.; González-López, M.A. Cutaneous leiomyosarcoma developing on a pacemaker pocket. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2009, 35, 863–867. [Google Scholar] [CrossRef] [PubMed]
- González-Vela, M.C.; Salcedo, W.; Neira, C.; González-López, M.A.; Ayala, H.; Val-Bernal, J.F. Atypical fibroxanthoma developing on a pacemaker pocket mimicking a pyogenic granuloma. Cardiovasc. Pathol. 2013, 22, 102–104. [Google Scholar] [CrossRef]
- Rathinam, S.; Kuntz, H.; Panting, J.; Kalkat, M.S. Inflammatory myofibroblastic tumour at the pacemaker site. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 443–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhandarkar, D.S.; Bewu, A.D.M.; Taylor, T.V. Carcinoma of the breast at the site of migrated pacemaker generators. Heart 1993, 69, 883–885. [Google Scholar] [CrossRef]
- Rasmussen, K.; Grimsgaard, G.; Vik-Mo, H.; Stalsberg, H. Male Breast Cancer from Pacemaker Pocket. Pacing Clin. Electrophysiol. 1985, 8, 761–763. [Google Scholar] [CrossRef]
- Moruzzo, D.; Bindi, M.; Bongiorni, M.G.; Castiglioni, M. A rare case of non-Hodgkin lymphoma in a pacemaker pocket. Leuk. Lymphoma 2009, 50, 1384–1385. [Google Scholar] [CrossRef]
- De Mattia, L.; Brieda, M.; Dametto, E. A carcinoma of the breast mimicking a pacemaker pocket infection. Europace 2011, 13, 220. [Google Scholar] [CrossRef]
- Fraedrich, G.; Kracht, J.; Scheld, H.H.; Jundt, G.; Mulch, J. Sarcoma of the Lung in a Pacemaker Pocket—Simple Coincidence or Oncotaxis? Thorac. Cardiovasc. Surg. 1984, 32, 67–69. [Google Scholar] [CrossRef]
- Zafiracopoulos, P.; Rouskas, A. Breast Cancer at Site of Implantation of Pacemaker Generator. Lancet 1974, 303, 1114. [Google Scholar] [CrossRef]
- Knez, I.; Cerwenka, H.; Moinfar, F.; Hoff, M.; Machler, H.; Anelli-Monti, M.; Radner, H.; Rigler, B. Invasive Ductal Carcinoma of the Male Breast Expanding from Pacemaker Pocket Decubitus. Pacing Clin. Electrophysiol. 1999, 22, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Zonca, P.; Herokova, J.; Cambal, M.; Jacobi, C.A. Ductal carcinoma of the breast in the pacemaker generator’s pocket. Bratisl. Lek. Listy 2009, 110, 719–722. [Google Scholar]
- Khamooshian, A.; Klinkenberg, T.J.; Maass, A.H.; Mariani, M.A. Management of device-related malignant sarcoma. Heart Case Rep. 2017, 3, 373–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rothenberger-Janzen, K.; Flueckiger, A.; Bigler, R. Carcinoma of the breast and pacemaker generators. Pacing Clin. Electrophysiol. 1998, 21, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Hojo, N.; Yakushijin, Y.; Narumi, H.; Minamoto, Y.; Sakai, I.; Takada, K.; Yasukawa, M.; Fujita, S.; Hato, T. Non-Hodgkin’s Lymphoma Developing in a Pacemaker Pocket. Int. J. Hematol. 2003, 77, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Biran, S.; Keren, A.; Farkas, T.; Stern, S. Development of carcinoma of the breast at the site of an implanted pacemaker in two patients. J. Surg. Oncol. 1979, 11, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Tjong, F.V.; Reddy, V.Y. Permanent leadless cardiac pacemaker therapy: A comprehensive review. Circulation 2017, 135, 1458–1470. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are Titania Photocatalysts and Titanium Implants Safe? Review on the Toxicity of Titanium Compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Sidun, J.; Świderska, M.; Zalewska, A. Free Radical Production, Inflammation and Apoptosis in Patients Treated with Titanium Mandibular Fixations—An Observational Study. Front. Immunol. 2019, 10, 2662. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Cadosch, D.; Gautschi, O.P.; Sprengel, K.; Filgueira, L. Influence of metal ions on human lymphocytes and the generation of titanium-specific T-lymphocytes. J. Appl. Biomater. Funct. Mater. 2011, 9, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Prestat, M.; Thierry, D. Corrosion of titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.-E.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoScience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Rondón-Lagos, M.; Rangel, N.; Camargo-Villalba, G.; Forero-Castro, M. Biological and genetic landscape of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Eur. J. Surg. Oncol. (EJSO) 2020, 47, 942–951. [Google Scholar] [CrossRef]
- Oishi, N.; Miranda, R.N.; Feldman, A.L. Genetics of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthetic Surg. J. 2019, 39 (Suppl. 1), S14–S20. [Google Scholar]
- Quesada, A.E.; Zhang, Y.; Ptashkin, R.; Ho, C.; Horwitz, S.; Benayed, R.; Dogan, A.; Arcila, M.E. Next generation sequencing of breast implant-associated anaplastic large cell lymphomas reveals a novel STAT3-JAK2 fusion among other activating genetic alterations within the JAK-STAT pathway. Breast J. 2021, 27, 314–321. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.; Ryland, G.L.; Joyce, R.; Byrne, D.J.; Khoo, C.; Lade, S.; Hertzberg, M.; Hapgood, G.; Marlton, P.; et al. Frequent activating STAT3 mutations and novel recurrent genomic abnormalities detected in breast implant-associated anaplastic large cell lymphoma. Oncotarget 2018, 9, 36126–36136. [Google Scholar] [CrossRef]
- Carbonaro, R.; Accardo, G.; Mazzocconi, L.; Pileri, S.; Derenzini, E.; Veronesi, P.; Caldarella, P.; De Lorenzi, F. BIA-ALCL in patients with genetic predisposition for breast cancer: Our experience and a review of the literature. Eur. J. Cancer Prev. 2023, 32, 370–376. [Google Scholar] [CrossRef]
- Salgado-Peralvo, A.O.; Arriba-Fuente, L.; Mateos-Moreno, M.V.; Salgado-García, A. Cancerous lesions in the vicinity of dental implants: A systematic review. J. Oral Med. Oral Surg. 2020, 26, 45. [Google Scholar] [CrossRef]
- Salgado-Peralvo, A.O.; Arriba-Fuente, L.; Mateos-Moreno, M.V.; Salgado-García, A. Is there an association between dental implants and squamous cell carcinoma? Br. Dent. J. 2016, 221, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hum, N.R.; Reid, B.; Sun, Q.; Loots, G.G.; Zhao, M. Electric Fields at Breast Cancer and Cancer Cell Collective Galvanotaxis. Sci. Rep. 2020, 10, 8712. [Google Scholar] [CrossRef]
- Fontvieille, E.; His, M.; Biessy, C.; Navionis, A.-S.; Torres-Mejía, G.; Ángeles-Llerenas, A.; Alvarado-Cabrero, I.; Sánchez, G.I.; Navarro, E.; Cortes, Y.R.; et al. Inflammatory biomarkers and risk of breast cancer among young women in Latin America: A case-control study. BMC Cancer 2022, 22, 877. [Google Scholar] [CrossRef]
- Pinchasov, G.; Haimov, H.; Druseikaite, M.; Pinchasov, D.; Astramskaite, I.; Sarikov, R.; Juodzbalys, G. Oral Cancer around Dental Implants Appearing in Patients with\without a History of Oral or Systemic Malignancy: A Systematic Review. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef]
- Onega, T.; Baron, J.; MacKenzie, T.; Palmer, J.R.; Wise, L.A.; Hatch, E.E.; Troisi, R.; Titus-Ernstoff, L.; Strohsnitter, W.; Kaufman, R.; et al. Cancer after Total Joint Arthroplasty: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Johansen, C.; Arnsbo, P.; Moller, M.; McLaughlin, J.K.; Olsen, J.H. Cancer risk among pacemaker recipients in Denmark, 1982–1996. J. Long-Term Eff. Med. Implant. 2002, 12, 263–270. [Google Scholar] [CrossRef]
- de Boer, R.A.; Meijers, W.C.; van der Meer, P.; van Veldhuisen, D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019, 21, 1515–1525. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Han, X.; Sun, J.; Li, C.; Adhikari, B.K.; Zhang, J.; Miao, X.; Chen, Z. Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 2022, 9, 727487. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorff, J.; Fallenberg, E.M.; Solbach, C.; Gerber-Schäfer, C.; Rancsó, C.; von Fritschen, U. Breast Implant-Associated Lymphoma. Dtsch Arztebl Int. 2018, 115, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Park, J.-U.; Chang, H. Comprehensive Evaluation of the Current Knowledge on Breast Implant Associated-Anaplastic Large Cell Lymphoma. Arch. Plast. Surg. 2022, 49, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; McCarthy, C.; Dabic, S.; Polanco, T.; Chilov, M.M.; Mehrara, B.J.; Disa, J.J. BIA-ALCL and Textured Breast Implants: A Systematic Review of Evidence Supporting Surgical Risk Management Strategies. Plast. Reconstr. Surg. 2021, 147, 7S–13S. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 74; Surgical implants and other foreign bodies. IARC Monogr Eval Carcinog Risks Hum; International Agency for Research on Cancer: Lyon, France, 1999; Volume 74, pp. 1–409. [Google Scholar]
- Costelloe, C.M.; Murphy, W.A.; Gladish, G.W.; Rozner, M.A. Radiography of Pacemakers and Implantable Cardioverter Defibrillators. Am. J. Roentgenol. 2012, 199, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Panunzio, A.; Sartori, P. Lung Cancer and Radiological Imaging. Curr. Radiopharm. 2020, 13, 238–242. [Google Scholar] [CrossRef]
- Hricak, H.; Abdel-Wahab, M.; Atun, R.; Lette, M.M.; Paez, D.; Brink, J.A.; Donoso-Bach, L.; Frija, G.; Hierath, M.; Holmberg, O.; et al. Medical imaging and nuclear medicine: A Lancet Oncology Commission. Lancet Oncol. 2021, 22, e136–e172. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Liguori, C.; Frauenfelder, G.; Massaroni, C.; Saccomandi, P.; Giurazza, F.; Pitocco, F.; Marano, R. Emerging clinical applications of computed tomography. Med. Devices Evid. Res. 2015, 8, 265–278. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Weenink, C.; Horeweg, N.; Prokop, M.; Yousaf-Khan, U. Reduced Lung-cancer mortality with volume CT screening in a random-ized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team. Reduced Lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Middleton, W.D.; Allison, S.J.; Dahiya, N.; Lee, K.S.; Levine, B.D.; Lucas, D.R.; Murphey, M.D.; Nazarian, L.N.; Siegel, G.W.; et al. Ultrasonography of Superficial Soft-Tissue Masses: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2022, 304, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.G.; Oudsema, R.; Masseaux, J.A.; Rosenberg, H.K. US of Pediatric Superficial Masses of the Head and Neck. RadioGraphics 2018, 38, 1239–1263. [Google Scholar] [CrossRef]
- Keel, S.B.; Jaffe, K.A.; Nielsen, G.P.; Rosenberg, A.E. Orthopaedic Implant-Related Sarcoma: A Study of Twelve Cases. Mod. Pathol. 2001, 14, 969–977. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).